Abstract
A hyperthermophilic archaeon was isolated from a terrestrial hot spring on Kodakara Island, Japan and designated as Thermoproteus sp. glucose dehydrogenase (GDH-1). Cell extracts from cells grown in medium supplemented with glucose exhibited NAD(P)-dependent glucose dehydrogenase activity. The enzyme (TgGDH) was purified and found to display a strict preference for d-glucose. The gene was cloned and expressed in Escherichia coli, resulting in the production of a soluble and active protein. Recombinant TgGDH displayed extremely high thermostability and an optimal temperature higher than 85 °C, in addition to its strict specificity for d-glucose. Despite its thermophilic nature, TgGDH still exhibited activity at 25 °C. We confirmed that the enzyme could be applied for glucose measurements at ambient temperatures, suggesting a potential of the enzyme for use in measurements in blood samples.
Graphical abstract
The glucose dehydrogenase which we obtained (TgGDH) has both high thermal stability and high substrate specificity.
Glucose dehydrogenase (GDH) catalyzes the oxidization of d-glucose to d-glucono-1,5-lactone, and has been extensively applied for measuring glucose concentrations in blood. Three types of GDH enzymes have been reported so far: flavin adenine dinucleotide (FAD)-dependent GDH, pyrroloquinoline quinone (PQQ)-dependent GDH, and NAD(P)-dependent GDH. FAD-dependent GDH from Aspergillus oryzae was reported in 1967.Citation1,2) The enzyme is also found in bacteria, and the FAD-dependent GDH from Burkholderia cepacia has been extensively characterized.Citation3,4) The bacterial enzyme is a heterotrimer, consisting of a catalytic subunit (α subunit),Citation3) an electron transfer subunit (β subunit),Citation5) and a γ subunit functioning as a chaperone,Citation6) whereas the GDH from A. oryzae is an oligomer of a single protein.Citation2) PQQ-dependent GDHs have been found only in several genera of bacteria, including Klebsiella,Citation7) Acinetobacter,Citation8,9) Pseudomonas,Citation10) and Escherichia.Citation11) The NAD(P)-dependent GDH enzymes, which are described below, are found in both bacteria and archaea.
When considering the application of an enzyme for diagnostic purposes, such as glucose measurements, one of the most important properties of the enzyme is its substrate specificity. Relying on a GDH that recognizes saccharides other than glucose would lead to an overestimation of the glucose level in blood, and thus the substrate specificity of GDH for glucose must be strict. PQQ-dependent GDHs react not only toward d-glucose but also recognize other sugars including maltose, d-galactose, and d-xylose.Citation8) The FAD-dependent GDH from A. oryzae displays relatively high substrate specificity, but still acts on d-xylose.Citation12)
In addition to substrate specificity, the stability of the enzyme is also an important factor, as enzyme inactivation during storage will also result in inaccurate and inconsistent measurements of substrate. It is presumed that heat-resistant enzymes are stable for long periods during storage. It is, therefore, not surprising that several NAD(P)-dependent GDHs from hyperthermophilic archaea have been characterized. In some hyperthermophilic archaea, such as Sulfolobus solfataricusCitation13) and Thermoproteus tenax,Citation14) or the thermoacidophilic archaeon Thermoplasma acidophilum,Citation15) Picrophilus torridus,Citation16) and Thermoplasma volcanium,Citation17) NAD(P)-GDH represents the first enzyme in the modified Entner-Doudoroff pathway.Citation18) Although these enzymes displayed high levels of (thermo)stability, most of the enzymes characterized thus far have exhibited relatively broad substrate specificity compared to their bacterial counterparts such as those from Bacillus subtilisCitation19) and Bacillus megaterium.Citation20) When NADP+ is used as a cofactor, S. solfataricus GDH-1 (SsGDH1) shows promiscuous substrate specificity, and acts on d-galactose or d-xylose more strongly than on d-glucose at a substrate concentration of 40 mM.Citation13) When NAD+ is used as the cofactor, specificity of SsGDH-1 for glucose increased, but its activity toward d-xylose was still high, at about 26% of that toward d-glucose. When NADP+ is used as a cofactor, the activity of T. acidophilum GDH (TaGDH) toward d-galactose is 70% compared to that toward d-glucose.Citation15) The other GDHs from the thermoacidophiles P. torridus GDH (PtGDH) and T. volcanium GDH (TvGDH) also display high levels of activity towards d-galactose.Citation16,17) The T. tenax GDH (TtGDH) exhibits high activity towards d-xylose.Citation14) Until now, only one enzyme, the GDH-2 from S. solfataricus (SsGDH2), seems promising in terms of both substrate specificity and stability. The enzyme can utilize both NAD+ and NADP+, is specific for glucose, and displays high thermostability.Citation21)
Here, we report the enzymatic properties of a novel NAD(P)-GDH (TgGDH) from a hyperthemophilic archaeon, Thermoproteus sp. GDH1. The gene was cloned, and a soluble, active protein was successfully produced in Escherichia coli. The purified recombinant TgGDH showed both high thermal stability and high specificity for d-glucose. The properties of the enzyme are compared with those of previously studied GDH enzymes.
Materials and methods
Micro-organisms, media, and growth conditions
Thermoproteus sp. strain GDH-1 was isolated from a hot spring mud sample collected at Kodakara Island in Kagoshima prefecture, Japan. The mud sample was inoculated to a medium composed of 0.5% tryptone, 0.5% yeast extract, 0.5% sodium chloride, resazurin (5 mg/L) as an indicator of dissolved oxygen, and an electron acceptor. The electron acceptors were added in the following concentrations: 0.2% of elemental sulfur, or 0.3% of sodium sulfate, sodium thiosulfate, or sodium nitrate. Cultures were performed under anaerobic conditions at 85 and 97 °C. Significant cell growth was observed in medium supplemented with thiosulfate (YT-TS medium). After several rounds of cultivation in liquid medium, 200 μL were plated on a solid YT-TS medium supplemented with 1% Gelrite, 1.25 mM CaCl2, and 5.0 mM MgSO4. Single colonies were isolated and grown in liquid YT-TS medium. Single colony isolation and liquid cultures were repeated three times to ensure isolation. After isolation, strain GDH-1 was routinely grown in a medium composed of 0.5% tryptone, 0.5% yeast extract, 0.5% d-glucose, 0.5% sodium thiosulfate, 0.5% sodium chloride, 0.005% sodium sulfide, and 5 mg/L resazurin. Oxygen was removed from the medium by repeatedly purging with nitrogen gas prior to cell inoculation. Routine cultures were performed at 85 °C for 3 days.
General DNA manipulations
The kits for genomic DNA isolation (MagExtractor -Genome-), DNA ligation (Ligation High), site-directed mutagenesis (KOD -Plus- Mutagenesis kit), PCR (KOD –plus-, Blend Taq -Plus-), and TA cloning (TArget Clone Plus) were manufactured by TOYOBO Co., Ltd (Osaka, Japan). The plasmid miniprep kit (Quantum Prep mini-prep kit) was manufactured by Bio-Rad Laboratories (Hercules, CA, USA). Purification of amplified DNA fragments was performed with Wizard SV Gel and PCR Clean-up System (Promega, Madison, WI, USA). Chemicals and equipment for DNA sequence analysis (BigDye Terminator ver.3.1 Cycle Sequencing kit, 3730 DNA Analyser) were provided by Applied Biosystems (Foster City, CA, USA).
Determination of 16S rRNA sequences
Genomic DNA of Thermoproteus sp. GDH-1 was extracted and purified from cultivated cells using MagExtractor -Genome-. The 16S rRNA region was amplified with the primer pairs 5′-TAGGGCTAAGCCATGCGAGTC-3′ and 5′-CACTCTGGGTTGCCCCA-3′, or 5′-CGGCAAGTCGCTCCTGAAATC-3′ and 5′-TTCCCCTACGGCTACCTTGTTACGA-3′. Amplified DNA fragments were directly sequenced.
Glucose dehydrogenase activity measurements
The enzyme reaction was carried out in a solution containing 90 mM bicine (pH 8.0), 5.0 mM β-NADP+, and 150 mM d-glucose at 60 °C. The reaction was initiated by adding enzyme, and the absorbance at 340 nm was monitored. The amount of reduced β-NADP+ was determined using a molar extinction coefficient value of 6.22 μM−1cm−1 at 340 nm. One unit (U) is defined as the amount of enzyme that reduces 1 micromole of cofactor per minute in the presence of substrate. The NAD+-dependent activity was measured in the same way by replacing β-NADP+ with β-NAD+. Protein concentrations were measured with Bio-Rad Protein Assay (Bio-Rad) using a calibration curve constructed with varying concentrations of bovine serum albumin.
Purification of native and recombinant TgGDH
Cells were harvested and disrupted by sonication in 20 mL of 50 mM potassium phosphate buffer (pH 7.0). After ultracentrifugation (100,000 × g, 1 h), ammonium sulfate was added to the supernatant at a final concentration of 30%. After centrifugation (20,000 × g, 30 min), ammonium sulfate was added to the supernatant at a final concentration of 48%, and the precipitate, which included GDH, was collected by centrifugation (20,000 × g, 30 min). The GDH fraction was dissolved in 20 ml of 50 mM potassium phosphate buffer (pH 7.0), and loaded onto a Resource Q column (GE Healthcare, Uppsala, Sweden). The flow-through fraction was collected, ammonium sulfate was added to a final concentration of 22.8%, and the solution was applied to a Resource ISO column (GE Healthcare). GDH was eluted with a linear gradient of ammonium sulfate (22.8–0%), and fractions with GDH activity were pooled. The fractions were further applied to a Superdex 200 column (GE Healthcare) with 50 mM Tris-HCl (pH 7.0) and 0.15 mM NaCl as an elution buffer.
In order to purify the recombinant TgGDH protein, E. coli cells were harvested by centrifugation and resuspended in 50 mM Tris-HCl buffer (pH 8.0) containing 0.1 M NaCl. Cells were disrupted by sonication and cell extracts were subjected to ultracentrifugation (100,000 × g, 1 h). The supernatant was incubated at 85 °C for 30 min and centrifuged (20,000 × g, 30 min) to remove most proteins from the host cell. The supernatant was then loaded onto a Resource Q column, followed by procedures described for the purification of the native enzyme.
Identification of the TgGDH gene
The purified native TgGDH solution (10 μL) was subjected to SDS-PAGE with a 12.5% polyacrylamide gel, followed by CBB staining using CBB Stain One (NACALAI TESQUE, Kyoto, Japan). The main band in the sample was cut out from the stained gel, and its peptide sequence was analyzed by a tandem mass spectrometer (oMALDI-qQ-TOF MS/MS QSTAR Pulsar i, Applied Biosystems) at Hitachi High-Technologies (Hitachinaka, Japan). Based on the obtained amino acid sequences, degenerate PCR primers containing mixed bases were designed, and a PCR reaction was performed using genomic DNA as a template. The amplified DNA fragments were subcloned into pTA2 of TArget Clone Plus, using the TA cloning method, and sequenced. Based on the determined sequence, the regions of the 5′- and 3′-ends of the GDH gene were amplified using LA PCR in vitro Cloning Kit (TAKARA BIO, Otsu, Japan), and the complete gene sequence was determined.
Expression of TgGDH gene in E. coli
Primers 5′-AGATATACATATGAAGGCGGTAACGGTCACCCCAG-3′ and 5′-CCAGGATCCTTATAAGTGTTGTAGCACTAGAACCG-3′ (underlined sequences represent NdeI and BamHI sites) were used to amplify the entire coding region of the TgGDH gene from genomic DNA. The amplified DNA fragments were inserted into the cloning vector pTA2. Site-directed mutagenesis using mismatch primers 5′-AGCACGGCATTTGGGGGCTCC-3′ and 5′-GGAGCCCCCAAATGCCGTGCT-3′ was carried out in order to disrupt the NdeI site (CATATG) present inside the TgGDH gene without changing the amino acid sequence of the protein. The plasmid was then digested using NdeI and BamHI, and the gene was inserted into the expression vector pET21a. The resulting plasmid (pET21aTGDH1) was introduced into E. coli BL21 (DE3). Transformants were grown in LB medium (800 ml) containing 100 μg/mL of ampicillin at 37 °C for 3 h, to an optical density of 0.6 at 600 nm. The culture was then supplemented with IPTG (0.1 mM) to induce the expression of the TgGDH gene and cells were grown for a further 4 h at 37 °C.
Enzyme characterization
The kinetic analysis of the TgGDH reaction was carried out at 60 °C and pH 8.0. Activity levels with varying concentrations of NAD(P)+ were examined in the presence of 1 M d-glucose. Activity levels with varying concentrations of d-glucose were examined in the presence of 30 mM NAD+ or 5 mM NADP+.
The thermostability of TgGDH was examined by incubating a solution (0.1 M NaCl, 50 mM Tris-HCl, and pH 8.0) containing the enzyme at temperatures ranging from 50 to 95 °C for 30 min. Activity levels before and after incubation were compared. For stability towards various pH, the enzyme was incubated in 0.1 M citric acid buffer (pH 4.3–6.2), 0.1 M potassium phosphate buffer (pH 6.1–8.1), 0.1 M bicine buffer (pH 7.9–8.8), or 0.1 M glycine buffer (pH 8.8–10.6) at 25 °C for 24 h. Activity levels before and after incubation were compared.
The effect of temperature on enzyme activity was examined by measuring GDH activity at 25, 30, 37, 50, 60, 70, 80, or 85 °C. The effect of pH was examined by measuring GDH activity in 90 mM potassium phosphate buffer (pH 6.5–7.9), 90 mM bicine buffer (pH 7.9–8.8), 90 mM CHES buffer (pH 8.6–9.7), or 90 mM glycine buffer (pH 9.8–10.2).
In order to examine the substrate specificity, the activity of TgGDH was examined with eight saccharides: d-glucose, d-galactose, d-mannose, d-xylose, d-sorbitol, maltose, lactose, and sucrose, each at a concentration of 150 mM. The activity was measured using 5 mM of NADP+ or NAD+ as a cofactor as described above.
Determination of glucose concentration
For quantification of glucose in sample solution, 900 μL of a reagent for glucose quantification (0.1 M bicine, 0.5 M KCl, 20 mM NAD+, and 10 U/ml recombinant TgGDH) was placed in a quartz cell, and incubated at 25 °C (units were calculated based on the activity levels at 60 °C and pH 8.0). One hundred microliters of glucose solution was added to the reagent, and the reaction was carried out at 25 °C for 3 min to monitor the change in absorbance at 340 nm. The increase in absorbance per minute was calculated from the linear portion of the absorbance. A calibration curve was prepared using samples with defined glucose concentrations and measuring the rate of increase in absorbance at 340 nm.
Nucleotide sequence accession number
The sequences of the 16S ribosomal RNA gene and the TgGDH gene are available under the accession Nos. LC003040 and LC003234 in the GenBank/EMBL/DDBJ databases, respectively.
Results and discussion
Isolation of the hyperthermophilic archaeon, Thermoproteus sp. GDH-1
As a source for isolating hyperthermophiles, we collected mud samples from terrestrial hot springs on Kodakara Island in Kagoshima prefecture, Japan. Samples were inoculated into a nutrient-rich medium containing tryptone and yeast extract along with electron acceptors such as sulfur, sulfate, thiosulfate, or nitrate, and grown under anaerobic conditions at high temperatures. In the medium supplemented with thiosulfate (YT-TS medium), we observed significant growth of cells with homogenous morphology at 97 °C. The strain was subjected to serial dilution (1%) and cultivation for several rounds, and then inoculated onto solid medium based on YT-TS medium, followed by single colony isolation (see Materials and Methods). The isolated strain was designated as strain GDH-1. Several representative properties of the isolated strain are shown in Table . GDH-1 cells were long rods of about 0.5 μm in diameter with a length of 10–30 μm. Specific growth rates at 80, 85, 90, and 95 °C were 0.0287, 0.0458, 0.0527, and 0.0489 h−1, respectively, indicating an optimal growth temperature of approximately 90 °C. Cell growth was not observed at temperatures below 80 °C. The 16S rRNA sequence (2133 bp) of this strain was determined and compared with 16S rRNA sequences of other organisms available in the NCBI database. As a result, strain GDH-1 was closely related to members of the genus Thermoproteus, with a 98.9% identical sequence to that of Thermoproteus tenax. The growth characteristics and morphology of GDH-1 cells also support that this organism is a member of Thermoproteus, and was thus designated as Thermoproteus sp. GDH-1.
Table 1. Morphological and biochemical properties of strain GDH-1.
Glucose dehydrogenase activity in cell-free extracts of Thermoproteus sp. GDH-1 cells
Cell-free extracts of GDH-1 cells grown in standard growth medium supplemented with d-glucose harbored GDH activity. In order to identify and carry out an initial examination of the protein exhibiting this GDH activity (TgGDH), the native enzyme was purified to apparent homogeneity by ammonium sulfate precipitation, hydrophobic interaction chromatography, and gel filtration chromatography. The purified enzyme showed significant levels of activity for d-glucose in the presence of NAD+ as a cofactor. The enzyme did not show notable activity for d-galactose, d-mannose, d-xylose, d-sorbitol, maltose, lactose, and fructose. As the enzyme displayed a surprisingly strict substrate specificity for d-glucose, we set out to clone the gene and carry out a detailed biochemical characterization on the recombinant enzyme.
Gene cloning of TgGDH and its primary structure
To determine the peptide sequence of native TgGDH, we tried to further remove trace contaminant proteins by carrying out an additional gel filtration step. TgGDH eluted faster than all other contaminating proteins, suggesting that the protein forms large, possibly aggregated, complexes in buffer solution at room temperature. It has been reported that the molecular mass of the NAD(P)-dependent GDH from T. tenax (TtGDH), estimated by gel filtration, was very high (approx. 600 kDa) without cofactor at room temperature.Citation14) The purified native TgGDH was digested with trypsin, and partial peptide sequences were predicted by tandem mass spectrometry. Based on the peptide sequences, we constructed degenerate primers. After amplifying a partial fragment of the TgGDH gene, the complete open reading frame was cloned and sequenced by genome walking. The complete TgGDH gene was composed of 1047 bp, and encoded a 348 amino acid polypeptide. The estimated molecular mass of the monomer protein was approximately 36.9 kDa.
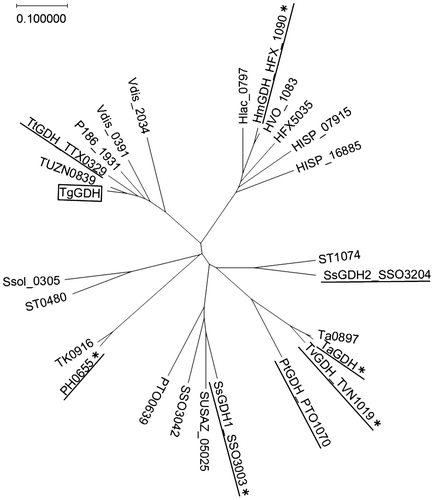
TgGDH displayed similarity to members of the zinc-dependent medium-chain alcohol dehydrogenase (MDR) superfamily, and showed the highest amino acid sequence identity to TtGDH (79%). A BLASTP search for archaeal sequences with similarity to TgGDH was performed and representative sequences were chosen to construct a phylogenetic tree (Fig. ). Sequences of archaeal GDH proteins that have been biochemically or structurally examined are included in the tree. The topology of the tree indicates that TgGDH is closely related with TtGDH, and relatively distant from other characterized archaeal NAD(P)-GDH proteins.
Fig. 1. A phylogenetic tree of archaeal NAD(P)-GDHs.
Notes: A phylogenetic tree of the archaeal NAD(P)-GDHs and their homologous sequences available in the NCBI database was constructed using ClustalW. The sequence of a protein which has been characterized as a threonine dehydrogenase (PH0655) is included. The GDH proteins which have been biochemically characterized are underlined. The asterisks denote the GDH proteins whose structures have been solved.
Expression and purification of recombinant TgGDH
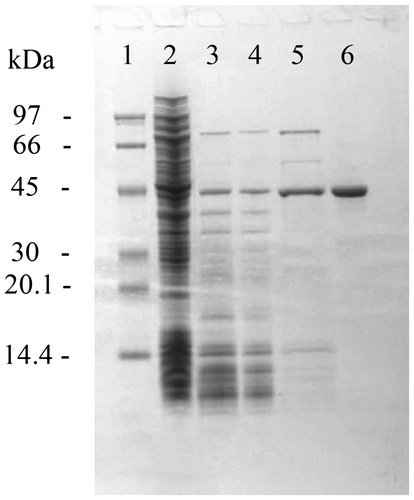
A TgGDH expression vector was constructed and introduced into E. coli BL21 (DE3). The recombinant protein was produced in a soluble form, and TgGDH was purified to apparent homogeneity (Fig. ). In a previous report, the GDH from T. tenax was purified in the presence of dithiothreitol.Citation14) During the purification steps in this study, reducing agents such as dithiothreitol or 2-mercaptoethanol were not added. As we did not observe large decreases in activity, TgGDH does not seem to be sensitive to oxygen or oxidative stress under ambient aerobic conditions.
Fig. 2. SDS-PAGE of crude and purified recombinant TgGDH.
Notes: Lanes are molecular weight marker (M), crude extract after sonication (1), supernatant fraction after heat treatment (2), fractions containing TgGDH after RESOURCE Q (3), RESOURCE ISO (4), and gel filtration (5) column chromatography steps.
Characterization of recombinant TgGDH
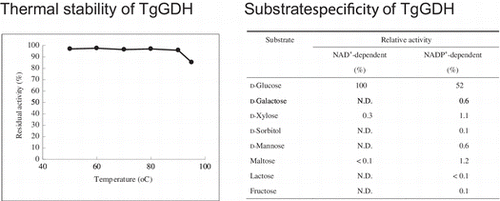
We examined the enzymatic features of the recombinant TgGDH. The substrate specificity of the recombinant enzyme was strict towards d-glucose, as observed for the native enzyme (Table ). In particular, when NAD+ was used as a cofactor, we did not observe activity with any of the examined sugars other than d-glucose.
Table 2. Substrate specificity of recombinant TgGDH.
Kinetic parameters of the purified TgGDH are shown in Table . Although TgGDH utilized both NAD+ and NADP+ as cofactors, the maximum velocity (Vmax) was higher when NAD+ was used as the cofactor. However, in the presence of NAD+, the Km value for d-glucose was approximately 10-fold higher than that in the presence of NADP+, indicating that the affinity of the enzyme for d-glucose is much higher in the presence of NADP+. These properties resemble those of TtGDH. It was reported that TtGDH also preferred NADP+ to NAD+ as cofactor, and the Vmax value in the presence of NAD+ was higher than that in the presence of NADP+.Citation14) According to previous reports, other archaeal GDHs, such as SsGDH, TaGDH, PtGDH, TvGDH, and HmGDH, also preferred NADP+ to NAD+.Citation13,15–17,22) It, therefore, seems that archaeal GDHs commonly prefer NADP+ as cofactor.
Table 3. Kinetic parameters of recombinant TgGDH.
The thermal stability of the recombinant TgGDH is shown in Fig. (A). Purified enzyme was incubated at various temperatures for 30 min, and activity was compared with that observed prior to incubation. Inactivation of TgGDH was not observed with this heat treatment at temperatures up to 90 °C. In addition, the enzyme was stable in a pH range from 6.0 to 9.0 (Fig. (B)). These properties indicate that the enzyme can most likely withstand heat treatment for its purification, which would be effective in industrial-scale preparation of the enzyme, and that long-term storage in diagnostic reagents should also pose no problems.
Fig. 3. Biophysical properties of recombinant TgGDH.
Notes: (A) Effect of temperature on the stability of recombinant TgGDH. GDH solution was incubated at various temperatures for 30 min, immediately cooled, and residual activity was measured at pH 8.0 and 60 °C, (B) Effect of pH on the stability of recombinant TgGDH. Enzyme was incubated at 25 °C for 24 h in citrate buffer (closed diamonds), potassium phosphate buffer (open squares), bicine buffer (closed triangles), and glycine buffer (open circles), and residual activity was measured at pH 8.0 and 60 °C, (C) Effect of pH on the activity of recombinant TgGDH. Measurements were carried out in potassium phosphate buffer (closed circles), bicine buffer (closed squares), CHES-NaOH buffer (open triangles), and glycine buffer (open circles), and (D) Effect of temperature on the activity of recombinant TgGDH. Activity was measured at various temperatures from 25 to 85 °C.
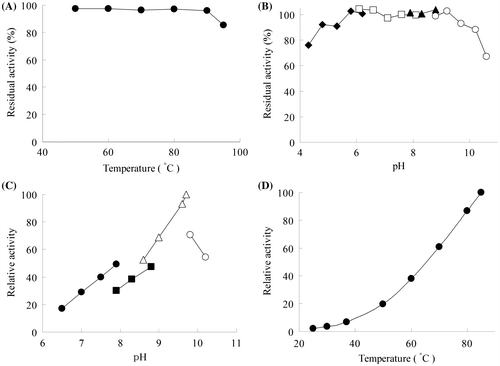
The pH and temperature dependency of the recombinant GDH is shown in Fig. (C) and (D), respectively. The optimum pH was 9.7, and the optimum temperature seemed to be higher than 85 °C. Activity at temperatures below 40 °C was less than 10% of the activity at 85 °C. An Arrhenius plot of the data indicated that the activation energy of the reaction was 67.6 kJ/mol.
The relatively low activity levels of TgGDH at ambient temperatures may give the impression that the enzyme is not suitable for diagnostic application. Measurements of blood glucose are usually carried out at temperatures below 40 °C. However, we have found that TgGDH still exhibits sufficient levels of activity at temperatures below 40 °C. We applied the recombinant enzyme for measurements at 25 °C, and found that glucose concentrations in buffer could be accurately quantified with TgGDH at least in the range of 2–15 mM. This indicates that the thermophilic nature of TgGDH does not necessarily rule out the possibilities of its use for diagnostic purposes. One drawback of TgGDH may be its poor affinity for NAD+, as NAD+ is more stable and cheaper than NADP+. There are a number of reports in which the cofactor specificity of a given oxidoreductase has been altered by mutagenesis, including the malate dehydrogenase from Thermus flavus,Citation23) the lactate dehydrogenase from T. thermophilus,Citation24) and the glutamate dehydrogenase from Clostridium symbiosum.Citation25) The strategies used in these studies may provide clues for further enhancing the capacity of TgGDH for its use in glucose sensors. The relatively high Km value for d-glucose in the presence of NAD+ may be considered as a disadvantage in the application of TgGDH, as the Km value is much higher than the normal levels of glucose found in blood (approximately 5.5 mM or lower). At present, however, a number of enzymes exhibiting higher Km values than the usual glucose levels in blood are being applied for blood glucose measurements. These include the FAD-dependent glucose oxidase from Aspergillus niger with a Km of 33 mM for d-glucoseCitation26) and the FAD-dependent glucose dehydrogenase from A. oryzae, with a Km of 25 mM.Citation12) In addition, when the rate assay method is adopted, substrate concentrations lower than the Km value can be measured accurately. There is no doubt that if the Km value for d-glucose were lowered, glucose measurements in blood samples would be considerably enhanced. We are now engaged in modifying the TgGDH protein in order to improve these properties.
Alignment of amino acid sequences
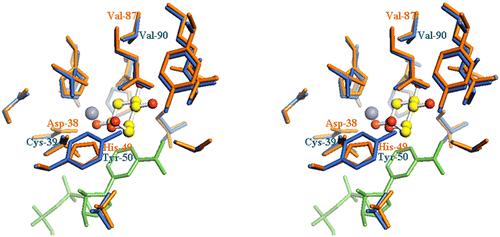
Until now, X-ray crystal structures have been determined for several NAD(P)-dependent GDH proteins from archaea, including SsGDH1,Citation27) TaGDH,Citation28) TvGDH,Citation17) and the enzyme from Haloferax mediterranei (HmGDH).Citation29) The primary structure of TgGDH is 34.0, 33.1, 31.8, and 36.9% identical with those of SsGDH1, TaGDH, TvGDH, and HmGDH, respectively. Judging from the sequence alignment with HmGDH, many amino acid residues important for recognition of substrate and formation of ligands to the Zn2+ are conserved in TgGDH (Fig. ). Baker et al. reported that residues His-49, Glu-114, Glu-150, and Asn-303 of HmGDH form hydrogen bonds to d-glucose.Citation29) Three of them are conserved in TgGDH as Glu-110, Glu-146, and Asn-291. HmGDH residues that interact with the Zn2+, His-63, Glu-64, and Glu-150, are also conserved in TgGDH as His-64, Glu-65, and Glu-146, respectively. Differences among these GDHs are that the residue equivalent to Asp-38 of HmGDH, which binds to Zn2+, is substituted by a cysteine residue (Cys-39), and that His-49, which forms a hydrogen bond with the C6-hydroxyl group of d-glucose in HmGDH, is substituted by a tyrosine residue (Tyr-50) in TgGDH (Fig. ). Although Cys-39 is highly conserved among members of the MDR superfamily,Citation30) Tyr-50 is the characteristic of Thermoproteus GDHs. All of the residues of TgGDH described above are conserved in TtGDH. Although TtGDH shows significant amino acid sequence homology with TgGDH (79% identical), its activity for d-xylose is higher than that for d-glucose. The key residues that lead to such a difference in substrate recognition should reside in the 21% different residues. Haferkamp et al. described that SsGDH2, which is strictly specific for d-glucose, may have a weaker hydrogen bond to the C3-hydroxyl group than that of SsGDH1, which exhibits less specificity.Citation21) In this case, SsGDH2 maintains affinity for glucose, whereas affinity for xylose is lost because xylose has no C6-hydroxyl group. They described that the transition of Asn-89 in SsGDH1 to Val-93 in SsGDH2 would probably cause a loss of a hydrogen bond to the C3-hydroxyl group. Such a hypothesis is supported by Kanoh et al.,Citation17) where they describe that the change from Asp-89 in SsGDH1 to Val-93 in TvGDH results in a loss of a hydrogen bond to the C3-hydroxyl group of d-glucose. The decreased number of interactions between the C3-hydroxyl group of the sugar and the enzyme are likely to be responsible for the lack of reactivity toward d-xylose. However, this Val residue is conserved not only in TgGDH (Val-90), but can also be found in TtGDH (Val-90) and HmGDH (Val-87). Therefore, Val-90 in TgGDH does not seem to be the main factor responsible for the low activity towards d-xylose. In comparison with HmGDH, Baker et al. reported that Asn-303, which is equivalent to Asn-291 in TgGDH, forms a hydrogen bond to the C3-hydroxyl group of sugars. Again, however, the Asn and its neighboring residues are found in both TgGDH and TtGDH. Therefore, at present it is difficult to explain the differences in substrate recognition among these GDH enzymes simply by comparing their primary structures.
Fig. 4. Alignment of the amino acid sequences of TgGDH, TtGDH, HmGDH, SsGDH1, SsGDH2, TvGDH, and TaGDH.
Notes: Identical and similar amino acid residues are shaded in black and gray, respectively. Residues involved in the binding of Zn2+ and d-glucose in HmGDH are indicated with asterisks and open squares, respectively.
Fig. 5. Stereo view of the TgGDH active site region.
Notes: Homology modeling was performed using the software MODELLERCitation31), based on the structure of HmGDH (PDB ID: 2vwh). The program Pymol was used for molecular visualization. The colors of the residues are orange for HmGDH and blue for TgGDH. d-Glucose is shown in ball and stick, Zn2+ as a gray sphere, and NADP+ as green sticks.
References
- Bak TG, Sato R. Studies on the glucose dehydrogenase of Aspergillus oryzae. I. Induction of its synthesis by rho-benzoquinone and hydroquinone. Biochim. Biophys. Acta. 1967;139:265–276.10.1016/0005-2744(67)90031-9
- Bak TG. Studies on glucose dehydrogenase of Aspergillus oryzae. II. Purification and physical and chemical properties. Biochim. Biophys. Acta. 1967;139:277–293.10.1016/0005-2744(67)90032-0
- Sode K, Tsugawa W, Yamazaki T, Watanabe M, Ogasawara N, Tanaka M. A novel thermostable glucose dehydrogenase varying temperature properties by altering its quaternary structures. Enzyme Microb. Technol. 1996;19:82–85.10.1016/0141-0229(95)00170-0
- Tsuya T, Ferri S, Fujikawa M, Yamaoka H, Sode K. Cloning and functional expression of glucose dehydrogenase complex of Burkholderia cepacia in Escherichia coli. J. Biotechnol. 2006;123:127–136.10.1016/j.jbiotec.2005.10.017
- Yamazaki T, Tsugawa W, Sode K. Twentieth symposium on biotechnology for fuels and chemicals. Gatlinburg: Springer; 1999. p. 325–335.
- Yamashita Y, Ferri S, Huynh ML, Shimizu H, Yamaoka H, Sode K. Direct electron transfer type disposable sensor strip for glucose sensing employing an engineered FAD glucose dehydrogenase. Enzyme Microb. Technol. 2013;52:123–128.10.1016/j.enzmictec.2012.11.002
- Neijssel OM, Tempest DW, Postma PW, Duine JA, Jzn JF. Glucose metabolism by K+-limited Klebsiella aerogenes: evidence for the involvement of a quinoprotein glucose dehydrogenase. FEMS Microbiol. Lett. 1983;20:35–39.10.1111/fml.1983.20.issue-1
- Dokter P, Frank J, Duine JA. Purification and characterization of quinoprotein glucose dehydrogenase from Acinetobacter calcoaceticus L.M.D. 79.41. Biochem. J. 1986;239:163–167.
- Matsushita K, Shinagawa E, Adachi O, Ameyama M. Quinoprotein d-glucose dehydrogenases in Acinetobacter calcoaceticus LMD 79.41: purification and characterization of the membrane-bound enzyme distinct from the soluble enzyme. Antonie van Leeuwenhoek. 1989;56:63–72.10.1007/BF00822585
- Mitchell CG, Dawes EA. The role of oxygen in the regulation of glucose metabolism, transport and the tricarboxylic acid cycle in Pseudomonas aeruginosa. J. Gen. Microbiol. 1982;128:49–59.
- Hommes RW, Simons JA, Snoep JL, Postma PW, Tempest DW, Neijssel OM. Quantitative aspects of glucose metabolism by Escherichia coli, grown in the presence of pyrroloquinoline quinone. Antonie van Leeuwenhoek. 1991;60:373–382.10.1007/BF00430375
- Bak TG. Studies on glucose dehydrogenase of Aspergillus oryzae. 3. General enzymatic properties. Biochim. Biophys. Acta. 1967;146:317–327.10.1016/0005-2744(67)90218-5
- Giardina P, de Biasi MG, de Rosa M, Gambacorta A, Buonocore V. Glucose dehydrogenase from the thermoacidophilic archaebacterium Sulfolobus solfataricus. Biochem. J. 1986;239:517–522.
- Siebers B, Wendisch VF, Hensel R. Carbohydrate metabolism in Thermoproteus tenax: in vivo utilization of the non-phosphorylative Entner-Doudoroff pathway and characterization of its first enzyme, glucose dehydrogenase. Arch. Microbiol. 1997;168:120–127.10.1007/s002030050477
- Smith LD, Budgen N, Bungard SJ, Danson MJ, Hough DW. Purification and characterization of glucose dehydrogenase from the thermoacidophilic archaebacterium Thermoplasma acidophilum. Biochem. J. 1989;261:973–977.
- Angelov A, Futterer O, Valerius O, Braus GH, Liebl W. Properties of the recombinant glucose/galactose dehydrogenase from the extreme thermoacidophile, Picrophilus torridus. FEBS J. 2005;272:1054–1062.10.1111/ejb.2005.272.issue-4
- Kanoh Y, Uehara S, Iwata H, Yoneda K, Ohshima T, Sakuraba H. Structural insight into glucose dehydrogenase from the thermoacidophilic archaeon Thermoplasma volcanium. Acta. Crystallogr., D. Biol. Crystallogr. 2014;70:1271–1280.10.1107/S1399004714002363
- Ahmed H, Ettema TJ, Tjaden B, Geerling AC, van der Oost J, Siebers B. The semi-phosphorylative Entner-Doudoroff pathway in hyperthermophilic archaea: a re-evaluation. Biochem. J. 2005;390:529–540.
- Hilt W, Pfleiderer G, Fortnagel P. Glucose dehydrogenase from Bacillus subtilis expressed in Escherichia coli I: purification, characterization and comparison with glucose dehydrogenase from Bacillus megaterium. Biochim. Biophys. Acta. 1991;1076:298–304.10.1016/0167-4838(91)90281-4
- Mitamura T, Urabe I, Okada H. Enzymatic properties of isozymes and variants of glucose dehydrogenase from Bacillus megaterium. Eur. J. Biochem. 1989;186:389–393.10.1111/ejb.1989.186.issue-1-2
- Haferkamp P, Kutschki S, Treichel J, Hemeda H, Sewczyk K, Hoffmann D, Zaparty M, Siebers B. An additional glucose dehydrogenase from Sulfolobus solfataricus: fine-tuning of sugar degradation? Biochem. Soc. Trans. 2011;39:77–81.10.1042/BST0390077
- Bonete MJ, Pire C, LLorca FI, Camacho ML. Glucose dehydrogenase from the halophilic Archaeon Haloferax mediterranei: enzyme purification, characterisation and N-terminal sequence. FEBS Lett. 1996;383:227–229.10.1016/0014-5793(96)00235-9
- Nishiyama M, Birktoft JJ, Beppu T. Alteration of coenzyme specificity of malate dehydrogenase from Thermus flavus by site-directed mutagenesis. J. Biol. Chem. 1993;268:4656–4660.
- Tomita T, Kuzuyama T, Nishiyama M. Alteration of coenzyme specificity of lactate dehydrogenase from Thermus thermophilus by introducing the loop region of NADP(H)-dependent malate dehydrogenase. Biosci. Biotechnol. Biochem. 2006;70:2230–2235.10.1271/bbb.60170
- Griffin J, Engel PC. An examination by site-directed mutagenesis of putative key residues in the determination of coenzyme specificity in clostridial NAD-dependent glutamate dehydrogenase. Enzyme Res. 2011;2011:595793.
- Swoboda BE, Massey V. On the reaction of the glucose oxidase from Aspergillus niger with bisulfite. J. Biol. Chem. 1966;241:3409–3416.
- Milburn CC, Lamble HJ, Theodossis A, Bull SD, Hough DW, Danson MJ, Taylor GL. The structural basis of substrate promiscuity in glucose dehydrogenase from the hyperthermophilic archaeon Sulfolobus solfataricus. J. Biol. Chem. 2006;281:14796–14804.10.1074/jbc.M601334200
- John J, Crennell SJ, Hough DW, Danson MJ, Taylor GL. The crystal structure of glucose dehydrogenase from Thermoplasma acidophilum. Structure. 1994;2:385–393.10.1016/S0969-2126(00)00040-X
- Baker PJ, Britton KL, Fisher M, Esclapez J, Pire C, Bonete MJ, Ferrer J, Rice DW. Active site dynamics in the zinc-dependent medium chain alcohol dehydrogenase superfamily. Proc. Natl. Acad. Sci. USA. 2009;106:779–784.10.1073/pnas.0807529106
- Esclapez J, Britton KL, Baker PJ, Fisher M, Pire C, Ferrer J, Bonete MJ, Rice DW. Crystallization and preliminary X-ray analysis of binary and ternary complexes of Haloferax mediterranei glucose dehydrogenase. Acta. Crystallogr., Sect. F: Struct. Biol. Cryst. Commun. 2005;61:743–746.10.1107/S1744309105019949
- Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815.10.1006/jmbi.1993.1626
