Abstract
We investigated the effect of daidzein feeding and estradiol treatment on food intake in cholecystokinin-1 receptor (CCK1R) deficiency, leptin receptor (ObRb) deficiency rats and their wild-type rats. These rats underwent an ovariectomy or a sham operation. For the 5 week experiment, each rat was divided in three groups: control, daidzein (150 mg/kg diet), and estradiol (4.2 μg/rat/day) groups. In both CCK1R+ and CCK1R− rats, daidzein feeding and estradiol treatment significantly decreased food intake. Daidzein feeding significantly reduced food intake in ovariectomized ObRb− rats, although not in ObRb+ rats. Estradiol treatment significantly lowered food intake in ovariectomized ObRb+ and ObRb− rats. In the ovariectomized rats, estradiol treatment significantly increases uterine weight, while daidzein feeding did not change it, suggesting that daidzein might have no or weak estrogenic effect in our experiment. These results suggest that CCK1R and ObRb signalings were not essential for the daidzein- and estradiol-induced anorectic action.
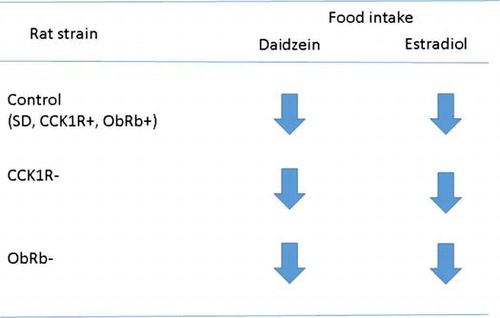
Graphical abstract
CCK1R and ObRb signaling did not mediate the daidzein- and estradiol- induced anorectic action.
Structures of the soybean isoflavone, daidzein are known to be analogous to 17β-estradiol. The isoflavone has affinity for estrogen receptors and induces estrogen-activated factors.Citation1−4) The increase in 17β-estradiol level causes a decrease in food intake and consequently a decrease in body weight gain.Citation5–8) Administration of 17β-estradiol to female rats lowered food intake immediately after administration.Citation9) We have supposed that isoflavone might decrease food intake by its estrogenic property. In our previous study, we found that the administration of isoflavone aglycone-rich fermented soybean extract (FSBE), rich in daidzein, decreased food intake in female rats with and without ovariectomy but not in male rats and FSBE administration increased the serum concentration of equol, which is metabolized from daidzein by intestinal bacteria and has strong estrogenic properties relative to other isoflavones.Citation10) However in same study, FSBE did not affect uterine weight even in ovariectomized (OVX) rats; dietary FSBE level might not be enough high to induce estrogenic property. In addition, 17β-estradiol treatment reduced food intake in male and OVX rats but not in female rats.Citation10) The contribution of estrogenic property of soy isoflavone to its effect on food intake is not simple.
Some reviews suggest that the anorexia of estrogen is induced by leptin and cholecystokinin (CCK) signaling in hypothalamus,Citation8,11,12) although the obvious mechanism such as receptor function and the signaling in hypothalamus and gastrointestinal tract has not been unknown yet. Whether soy isoflavone decreases food intake via these two major factors or not is very important to verify contribution of estrogenic property in detail. Otsuka Long–Evans Tokushima Fatty rat, which lacks the CCK-1 receptor CCK1R due to a 6.8 kb deletion of the CCK1R gene,Citation13–15) is highly susceptible to hyperphagia-induced obesity.Citation16–18) CCK is a brain–gut peptide that acts as a peripheral satiety signal, which binds to the CCK1R distributed in the brain and gastrointestinal tract.Citation19–21) In CCK1R deficiency rats and mice, administration of CCK did not affect food intake,Citation22,23) suggesting that the effect of CCK on ingestive behavior is caused via CCK1R. Obese Zucker fa/fa rat, which mutates the leptin receptor B (ObRb) gene, also develops hyperphagia-induced obesity. Leptin is secreted from white adipose tissue and bound to ObRb, which in turn regulated appetite, energy expenditure and body energy balance.Citation24) It is well known that leptin suppresses food intake.Citation25) In addition, it was reported that CCK interacts with leptin to decrease short-term food intake.Citation26,27) In this study, we examined the contribution of CCK and leptin signaling on the anorectic function of daidzein using the Otsuka Long–Evans Tokushima Fatty, CCKR1 deficient rats and the obese Zucker fa/fa rats, ObRb deficient rats. OVX animals are estrogen-deficiency model, resulting in hyperphagia and obesity. Continuous estradiol treatment in OVX rats has been shown to ameliorate the estrus cycle, leading to reduce food intake and body weight.Citation28) Estrogenic property could be evaluated more distinctly in OVX rats. In this study, we investigated the effect of daidzein on food intake in OVX female rats, in addition to normal female rats.
Materials and methods
Animals. Female Long–Evans Tokushima Otsuka (CCK1R+) and Otsuka Long–evans Tokushima fatty (CCK1R−) rats (Tokushima Research Institute, Otsuka Pharmaceutical, Tokushima, Japan, respectively), and Zucker +/+ (ObRb+) and Zucker fa/fa (ObRb−) rats (Japan SLC, Hamamatsu, Japan, respectively), at 6 weeks old, were raised in stainless wire mesh cages in a room controlled by a 12-h light–dark cycle (dark phase: 15:00–3:00) and constant temperature (23 ± 1°C). They were housed separately for two days for acclimatization to the environment. Animals were fed regular tap water and a commercial solid diet (MF; Oriental Yeast, Osaka, Japan) ad libitum. This study was conducted in accordance with the ethical guidelines of the Ehime University Animal Experimentation Committee and was in complete compliance with the National Institutes of Health: Guide for the care and use of laboratory animals. All efforts were made to minimize the number of animals used and to limit experimentation to what was necessary to produce reliable scientific information.
Diets and isoflavones. The daidzein were purchased from the LC laboratories, MA, USA. 7-week-old CCK1R+, CCK1R−, ObRb+ and ObRb− rats were used in our experiment. These rats were given an AIN-76 control (C) diet, which is formulated from 200 g/kg of casein, 350 g/kg of α-corn starch, 350 g/kg of sucrose, 50 g/kg of corn oil, 40 g/kg of AIN-76 mineral mixture, and 10 g/kg of AIN-76 vitamin mixture, or a diet supplemented with daidzein (D) (150 mg/kg diet). The addition of daidzein was performed at the expense of half amount of cornstarch and sucrose. This supplement dose of daidzein was sufficient to decrease food intake of female rats in our previous study.Citation10)
Experimental protocols. After two days of acclimatization, the CCK1R−, CCK1R+, ObRb+, and ObRb− rats (n = 36, respectively) were divided into two groups, respectively. In one group (n = 18), bilateral ovariectomy was performed under sodium pentobarbital anesthesia (Nembutal, Dainippon Pharmaceutical, Osaka, Japan, 30 mg/kg body weight, intraperitoneal injection), and in the other group (n = 18), a sham operation was performed. All rats recovered for 5 days after the operation. Each group was divided into three groups (n = 6, respectively). Two groups were given free access to the experimental diets; C and D diets, respectively (corresponding to the C and D groups, respectively). Third group was given the C diet after a subcutaneous implantation of 17β-estradiol-benzoate at 4.2 μg/rat/day (17β-estradiol 3-benzoate, 0.25 mg/pellet, 60 day release, SE-281, Innovative Research of America, Toledo, OH) (Es group), as previously described.Citation10) Throughout the duration of the experiment for 5 weeks, food intake and body weight were recorded every morning for each of the animals, and each diet was replenished. The rats were killed by decapitation, and a blood sample corresponding to a non-fasting state was collected from the neck at 20:00 h on the last day of the experimental period. The blood was centrifuged at 1500 × g at 4 °C for 10 min to separate the serum and then stored at −50 °C until analysis. The uterine were removed and weighed.
Serum analysis. Serum estradiol level was determined using a commercially available enzyme-linked immunosorbent assay detection kit. Serum daidzein and equol levels were carried out as previously described.Citation29) Briefly, frozen plasma samples from rats were thawed and 100 μl aliquots were mixed with 100 μL of hydrolysis buffer (0.1 mol/L sodium acetate pH 5 with 0.1% (wt/vol) ascorbic acid and 0.01% (wt/vol) EDTA, 8 μL of glucuronidase and 4 μL of sulfatase. The reaction mixture was allowed to hydrolyze to glucuronide and sulfate metabolites at 37 °C for at least 15 h. Subsequently, 10 μL of an internal standard (formononetin, 5 μg/mL in dimethylsulfoxide), 120 μL of water, 75 μL of ammonium acetate buffer (1 mmol/L, pH 7), and 83 μL of triethylammonium sulfate buffer (3 mol/L, pH 7) was added, and the samples were then heated to 60 °C for 10 min to facilitate the dissociation of isoflavones from plasma proteins; the mixture was then centrifuged. The deproteinized samples were passed over 0.5 g Sep-Pak C-18 cartridges (Nihon Waters, Tokyo, Japan) that had been previously washed with 10 mL of chloroform, 10 mL of methanol, and 20 mL of water. The cartridges were washed with 5 mL of ammonium acetate buffer (10 mmol/L, pH 5) and 5 ml of water, at room temperature. The absorbed isoflavones were eluted with 1.5 mL of methanol. The methanol effluent was evaporated to dryness under a gentle stream of nitrogen at 45 °C, dissolved in 100 μL of (40:60 vol/vol) methanol/aqueous acetic acid (1%), and stored at −20 °C until HPLC analysis. A 30 μL aliquot of the sample was applied to a reverse phase HPLC column (4.6 × 150 mm, CAPCELL PAK C18, particle size 5 μm, Shiseido, Tokyo, Japan). The mobile phase was potassium phosphate buffer containing 40% of a mixture of methanol and acetonitrile (3:2, vol/vol) at 40 °C, and the flow rate was 1.0 ml/min. Daidzein and equol were detected using an electrochemical detector (electrochemical detector 3005, Shiseido, Tokyo, Japan) under the following conditions: working electrode, glassy carbon; applied voltage, 800 mV. Formononetin was detected simultaneously using a UV–vis detector (SPD-10AV, Shimadzu Corporation, Kyoto, Japan) at 254 nm.
Statistical analysis. Data are expressed as the mean ± standard errors (SE). Data from weekly food intake and weekly body weight gain were analyzed by three way repeated measures analysis of variance (ANOVA) and Student’s t-test with Bonferroni corrections. Data from total food intake, total body weight gain, serum estradiol and isoflavones levels, and uterine weight were analyzed by three-way ANOVA followed by Bonferroni post hoc tests. Statistical significance was defined as p < 0.05.
Results
Food intake and body weight
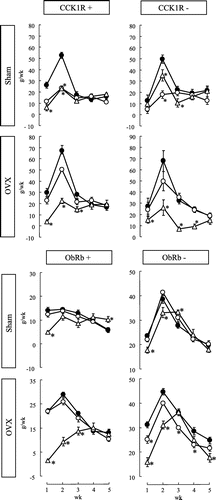
Fig. shows the changes in food intake for 5 weeks in CCK1R+, CCK1R−, ObRb+, and ObRb− rats, which operated sham and OVX. In week two, daidzein feeding and estradiol treatment significantly decreased food intake in the CCK1R+ and CCK1R− rats; in other weeks until 4 week, similar significant and insignificant trend were observed. Daidzein feeding significantly reduced total food intake in the CCK1R+ and CCK1R− rats underwent sham operation, but not OVX (Table ). In contrast, estradiol treatment significantly decreased total food intake in the OVX CCK1R+ and CCK1R− rats and tended to decrease it in the sham CCK1R+ rats (p = 0.055, Student’s t-test with Bonferroni corrections) (Table ). During experimental period, daidzein feeding significantly decreased food intake and estradiol treatment also decreased food intake except in weeks 3 and 4 in the OVX ObRb− rats (Fig. ), resulting in a decreased total food intake (Table ). Daidzein feeding tended to decrease food intake in the OVX ObRb+ rats in week three (p = 0.097, Student’s t-test with Bonferroni corrections). Estradiol treatment significantly decreased food intake in weeks 1, 2 and 5 (Fig. ) and total food intake in the OVX ObRb+ rats (Table ). However, there was no significant difference in food intake among groups in sham-operated ObRb+ and ObRb− rats (Fig. and Table ).
Fig. 1. Weekly changes in the amount of food intake in CCK1R+, CCK1R−, ObRb+, and ObRb− rats.
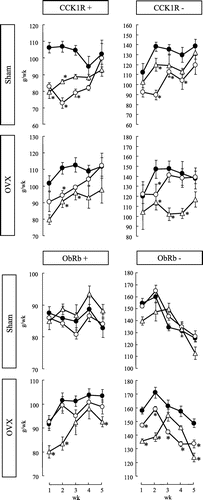
Table 1. Effects of dietary daidzein and estradiol treatment on total food inteke, total body weight, serum estradiol and isoflavone levels and uterine weight in CCK1R+ and CCK1R− rats.
Table 2. Effects of dietary daidzein and estradiol treatment on total food intake, total body weight, serum estradiol and isoflavone levels and uterine weight in ObRb+ and ObRb− rats.
Fig. shows the changes in body weight gain for 5 weeks in CCK1R+, CCK1R−, ObRb+, and ObRb− rats, which operated sham and OVX. Weekly (Fig. ) and total (Tables and ) body weight gain reflected the amount of food intake. White adipose tissue weight also reflected the amount of food intake (data not shown).
Fig. 2. Weekly changes in the amount of body weight gain in CCK1R+, CCK1R−, ObRb+, and ObRb− rats.
Serum estradiol and isoflavones levels and uterine weight
Tables and show the effect of daidzein and estradiol on serum estradiol and isoflavone levels, and uterine weight in Long–Evans strain (CCK1R+ and −) rats (Table ) and Zucker strain (ObRb+ and −) rats (Table ), which operated sham and OVX. There was no significant difference among groups in serum estradiol level and uterine weight in the sham-operated CCK1R+ rats (Table ). There was also no significant difference among groups in uterine weight in the sham-operated CCK1R− rats, but estradiol treatment significantly increased serum estradiol level in the sham-operated CCK1R− rats (Table ). There was no significantly change among groups in serum estradiol level in the ObRb+ and ObRb− rats (Table ). However, estradiol treatment significantly increased uterine weight in the OVX ObRb+ and ObRb− rats (Table ). There were no significant difference in serum estradiol levels and uterine weight in either rat strains fed daidzein diet (Tables and ). Daidzein feeding significantly increased serum daidzein and equol levels in all rats (Tables and ). In OVX rats, daidzein levels were higher, and equol levels were lower when compared with the sham rats (Tables and ).
Discussion
In this study, daidzein feeding decreased food intake and body weight gain with or without CCK1R (Figs. and and Table ). These results suggest that CCK-CCK1R signaling does not play a key role in the daidzein-induced anorectic action. Also, the daidzein feeding significantly decreased food intake and body weight gain in OVX ObRb− rats (Figs. and and Table ), suggesting that leptin-ObRb signaling were not essential for the daidzein-induced anorectic action. Findings that two major appetite regulating factors rarely contribute to the mechanism are of significance.
It has been reported that estrogen interacts CCK signaling.Citation8) The effect of estradiol on an increase in intraduodenal intralipid-induced satiation disappeared after injection of a CCK1R antagonist.Citation30) Satiation by CCK was abolished in ER-α knockout mice.Citation31) Estradiol treatment increased CCK-induced c-Fos expression in the brain of OVX rats.Citation32,33) In addition, estradiol treatment inhibited gastric emptying and gastrointestinal transit in OVX rats by CCK stimulation and CCK1R activation.Citation34) From these reports, we have hypothesized that estrogen-induced anorexia would be mediated by CCK1R. However, our present results suggest that the signaling via CCK1R does not appear to be key mechanism on anorectic action of estradiol (Fig. and Table ). There is no other report that CCK1R is not involved in the mechanism of estrogen-induced anorexia. In addition, we have hypothesized that estrogen-induced anorexia would be mediated by ObRb, because some reviews suggest that estradiol-induced anorexia is regulated by hypothalamic leptin signaling.Citation11,12) However, in this study, in both ovariectomized ObRb+ and ObRb− rats, estradiol treatment significantly decreased food intake (Fig. and Table ). Some reports showed that estradiol treatment decreased food intake in rat and mouse of ObRb−, in accordance with our present study.Citation35–37) Rocha et al. reported that estradiol treatment did not change leptin concentrations in the serum and the cerebrospinal fluid.Citation38) These findings and our results suggest that anorectic action of estradiol is independent of leptin signaling. Findings that two major appetite regulating factors did not play key roles in the mechanism of estradiol on decreasing food intake are interesting and of novelty, although we fail to elucidate the different mechanism of isoflavone and estradiol.
The daidzein feeding did not decrease food intake and body weight gain in OVX or sham-operated ObRb+ rats (Figs. and and Table ). For ObRb+ rats, the food intake and body weight gain were low even in OVX rats, when compared to CCK1R+ rats, or SD rats in the previous study,Citation10) although body weight gain of our sham ObRb+ rats was similar to that of ObRb+ rats observed in previous study.Citation39,40) Appetite is regulated to maintain energy balance by multiple mechanisms, including leptin signaling. The anorexic effect of daidzein feeding may be affected by energy state in animals. It seems to be difficult that the daidzein feeding decreases the food intake and body weight gain beyond such low level.
The daidzein feeding did not decrease food intake and body weight gain in also sham-operated ObRb− rats (Figs. and and Table ). We speculate that the absence of anorectic effect might associate with serum equol level. In this study, we observed that the serum level of equol was lower than that of daidzein (Table ). Equol is metabolized from daidzein by intestinal bacteria, possessing antioxidant activity, anti-cancer, and anti-osteoporosis effects.Citation41,42) Rachon et al. reported that dietary equol at 400 mg/kg diet decreased food intake and body fat in OVX rats.Citation43) Wu et al. reported that the preventive effects of the isoflavones on fat accumulation in early postmenopausal women depend on an individual’s equol-producing capacity.Citation44) These results may indicate that equol may be bioactive substance of anorectic action. Further investigation will elucidate the mechanism of equol-induced anorectic action. We showed that Zucker strain (ObRb+ and −) rats had lower serum equol level (Table ) when compared with Long–Evans strain (CCK1R+ and −) rats (Table ) and Sprague–Dawley (SD) rats.Citation10) Our results suggested that the serum equol levels in Zucker strains were lower to exert the decreasing effect on food intake and body weight gain for sham rats, but sufficient for OVX rats. Despite low level of serum equol, food intake was reduced in OVX ObRb− rats, presumably causing high sensitivity to equol. However, it remains unclear why OVX ObRb− rats were highly sensitive to equol.
Although rats had potent equol-producing activity when compared with human and pig,Citation45) there is no report about the difference of serum equol level among rat strains. Interestingly, our results suggest that the equol-producing activity was different between the two strains. Although we cannot explain clearly why the biotransformation capacities from daidzein to equol were different between Zucker and Long–Evans strains, we have speculated that the differences in the intestinal microflora or those of internal dynamics of daidzein/equol after phase I and II metabolism would relate to the biotransformation capacities.
We showed here that β-estradiol treatment increased uterine weight in OVX rats of both strains and serum estradiol level in OVX Long–Evans strain (CCK1R+ and −) rats (Tables and ). It remains to be unknown why estradiol treatment did not increase serum estradiol level in Zucker strain. On the other hand, there was no effect of daidzein on uterine weight and serum estradiol levels in OVX rats (Tables and ). It is also reported that 17β-estradiol treatment increased uterine weight in OVX rats until the same weight in sham-operated rats, while dietary administration of isoflavone (mixture, daidzein, or equol) did not improve it.Citation10,46) These data may indicate that the estrogenic effect of daidzein/equol is little referable to anorexia by daidzein at 150 mg/kg diet, although we have speculated that daidzein feeding might suppress appetite by interacting with estrogen or by yielding its own estrogenic effect.
In conclusion, our results showed that the anorectic effect of daidzein is independent of CCK-CCK1R and leptin-ObRb signaling. Some researchers showed that leptin and CCK signaling related to the anorexia of estrogen. However, our results suggested that leptin-ObRb and CCK-CCK1R signaling were not essential for the anorectic effect of estradiol.
Funding
This research was supported by the Uehara Memorial Foundation, the Fuji Foundation for Protein Research, and a Grant-in-Aid for Scientific research from Ministry of Education, Science, Sports, and Culture of Japan.
Notes
Abbreviations: CCK1R, cholecystokinin-1 receptor; ObRb, leptin receptor; FSBE, isoflavone aglycone-rich fermented soybean extract; OVX rats, ovariectomized rats; CCK1R+, Long-Evans Tokushima Otsuka; CCK1R−, Otsuka Long-Evans Tokushima Fatty; ObRb+, Zucker +/+; ObRb−, Zucker fa/fa; C, AIN-76 control; D, daidzein; Es group, the group given the C diet after a subcutaneous implantation of 17-estradiol-benzoate at 4.2 g/rat/day.
References
- Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann. Med. 1997;29:95–120.10.3109/07853899709113696
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263.
- Gutendorf B, Westendorf J. Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicology. 2001;166:79–89.10.1016/S0300-483X(01)00437-1
- Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001;131:1362S–1375S.
- Lyons PM, Truswell AS, Mira M, Vizzard J, Abraham SF. Reduction of food intake in the ovulatory phase of the menstrual cycle. Am. J. Clin. Nutr. 1989;49:1164–1168.
- Gong EJ, Garrel D, Calloway DH. Menstrual cycle and voluntary food intake. Am. J. Clin. Nutr. 1989;49:252–258.
- Parker GC, McKee ME, Bishop C, Coscina DV. Whole-body metabolism varies across the estrous cycle in Sprague-Dawley rats. Physiol. Behav. 2001;74:399–403.10.1016/S0031-9384(01)00599-6
- Geary N. Estradiol, CCK and satiation. Peptides. 2001;22:1251–1263.10.1016/S0196-9781(01)00449-1
- Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. J. Comp. Physiol. Psychol. 1975;88:183–193.10.1037/h0076186
- Kishida T, Mizushige T, Ohtsu Y, Ishikawa S, Nagamoto M, Izumi T, Obata A, Ebihara K. Dietary soy Isoflavone–aglycone lowers food intake in female rats with and without ovariectomy. Obesity (Silver Spring). 2008;16:290–297.10.1038/oby.2007.68
- Gao Q, Horvath TL. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am. J. Physiol. Endocrinol. Metab. 2008;294:E817–E826.10.1152/ajpendo.00733.2007
- Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J. Steroid. Biochem. Mol. Biol. 2010;122:65–73.10.1016/j.jsbmb.2009.12.005
- Takiguchi S, Takata Y, Funakoshi A, Miyasaka K, Kataoka K, Fujimura Y, Goto T, Kono A. Disrupted cholecystokinin type-A receptor (CCKAR) gene in OLETF rats. Gene. 1997;197:169–175.10.1016/S0378-1119(97)00259-X
- Takiguchi S, Takata Y, Takahashi N, Kataoka K, Hirashima T, Kawano K, Miyasaka K, Funakoshi A, Kono A. A disrupted cholecystokinin A receptor gene induces diabetes in obese rats synergistically with ODB1 gene. Am. J. Physiol. 1998;274:E265–270.
- Funakoshi A, Miyasaka K, Shinozaki H, Masuda M, Kawanami T, Takata Y, Kono A. An animal model of congenital defect of gene expression of Cholecystokinin (CCK)-A receptor. Biochem. Biophys. Res. Commun. 1995;210:787–796.10.1006/bbrc.1995.1728
- Moran TH, Bi S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos. Trans. R Soc. Lond. B Biol. Sci. 2006;361:1211–1218.10.1098/rstb.2006.1857
- Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka long-evans Tokushima fatty) rat: a new NIDDM rat strain. Diabetes. Res. Clin. Pract. 1994;24:S317–S320.10.1016/0168-8227(94)90269-0
- Moran TH. Unraveling the obesity of OLETF rats. Physiol. Behav. 2008;94:71–78.10.1016/j.physbeh.2007.11.035
- Bi S, Moran TH. Actions of CCK in the controls of food intake and body weight: lessons from the CCK-A receptor deficient OLETF rat. Neuropeptides. 2002;36:171–181.10.1054/npep.2002.0895
- Rehfeld JF. Cholecystokinin as satiety signal. Int. J. Obes. 1981;5:465–469.
- Peikin SR. Role of cholecystokinin in the control of food intake. Gastroenterol. Clin. North Am. 1989;18:757–775.
- Kopin AS, Mathes WF, McBride EW, Nguyen M, Al-Haider W, Schmitz F, Bonner-Weir S, Kanarek R, Beinborn M. The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J. Clin. Invest. 1999;103:383–391.10.1172/JCI4901
- Miyasaka K, Kanai S, Ohta M, Kawanami T, Kono A, Funakoshi A. Lack of satiety effect of cholecystokinin (CCK) in a new rat model not expressing the CCK-A receptor gene. Neurosci. Lett. 1994;180:143–146.10.1016/0304-3940(94)90507-X
- Hamann A, Matthaei S. Regulation of energy balance by leptin. Exp. Clin. Endocrinol. Diabetes. 1996;104:293–300.10.1055/s-0029-1211457
- Kasiske BL, O’Donnell MP, Keane WF. The Zucker rat model of obesity, insulin resistance, hyperlipidemia, and renal injury. Hypertension. 1992;19:I110–I110.10.1161/01.HYP.19.1_Suppl.I110
- Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc. Natl. Acad. Sci. USA. 1997;94:10455–10460.10.1073/pnas.94.19.10455
- Wang L, Martı́nez V, Barrachina MD, Taché Y. Fos expression in the brain induced by peripheral injection of CCK or leptin plus CCK in fasted lean mice. Brain Res. 1998;791:157–166.10.1016/S0006-8993(98)00091-2
- Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol. Behav. 1999;67:141–147.10.1016/S0031-9384(99)00060-8
- Janning P, Schuhmacher US, Upmeier A, Diel P, Michna H, Degen GH, Bolt HM. Toxicokinetics of the phytoestrogen daidzein in female DA/Han rats. Arch. Toxicol. 2000;74:421–430.10.1007/s002040000149
- Asarian L, Geary N. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2007;148:5656–5666.10.1210/en.2007-0341
- Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–4757.
- Eckel LA, Houpt TA, Geary N. Estradiol treatment increases CCK-induced c-Fos expression in the brains of ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R1378–1385.
- Eckel LA, Geary N. Estradiol treatment increases feeding-induced c-Fos expression in the brains of ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R738–746.
- Wu CL, Hung CR, Chang FY, Pau KY, Wang PS. Involvement of Cholecystokinin receptor in the inhibition of gastrointestinal motility by estradiol in ovariectomized rats. Scand. J. Gastroenterol. 2002;37:1133–1139.10.1080/003655202760373326
- Gale SK, van Itallie TB. Genetic obesity: estrogenic influences on the body weight and food intake of lean and obese adult Zucker (fa/fa) rats. Physiol. Behav. 1979;23:111–120.10.1016/0031-9384(79)90130-6
- Shaw MA, Whitaker EM, Hervey E, Hervey GR. The effects of ovarian hormones on regulation of energy balance in Zucker rats. J. Endocrinol. 1983;98:165–171.10.1677/joe.0.0980165
- Tritos NA, Segal-Lieberman G, Vezeridis PS, Maratos-Flier E. Estradiol-induced anorexia is independent of leptin and melanin-concentrating hormone. Obes. Res. 2004;12:716–724.10.1038/oby.2004.84
- Rocha M, Grueso E, Puerta M. The anorectic effect of oestradiol does not involve changes in plasma and cerebrospinal fluid leptin concentrations in the rat. J. Endocrinol. 2001;171:349–354.10.1677/joe.0.1710349
- Alemzadeh R, Karlstad MD, Tushaus K, Buchholz M. Diazoxide enhances basal metabolic rate and fat oxidation in obese Zucker rats. Metabolism. 2008;57:1597–1607.10.1016/j.metabol.2008.06.017
- Comai K, Triscari J, Sullivan AC. Differences between lean and obese Zucker rats: the effect of poorly absorbed dietary lipid on energy intake and body weight gain. J. Nutr. 1978;108:826–835.
- Bayer T, Colnot T, Dekant W. Disposition and biotransformation of the estrogenic isoflavone daidzein in rats. Toxicol. Sci. 2001;62:205–211.10.1093/toxsci/62.2.205
- Minamida K, Tanaka M, Abe A, Sone T, Tomita F, Hara H, Asano K. Production of equol from daidzein by gram-positive rod-shaped bacterium isolated from rat intestine. J. Biosci. Bioeng. 2006;102:247–250.10.1263/jbb.102.247
- Rachoń D, Vortherms T, Seidlovä-Wuttke D, Wuttke W. Effects of dietary equol on body weight gain, intra-abdominal fat accumulation, plasma lipids, and glucose tolerance in ovariectomized Sprague-Dawley rats. Menopause. 2007;14:925–932.10.1097/gme.0b013e31802d979b
- Wu J, Oka J, Ezaki J, Ohtomo T, Ueno T, Uchiyama S, Toda T, Uehara M, Ishimi Y. Possible role of equol status in the effects of isoflavone on bone and fat mass in postmenopausal Japanese women. Menopause. 2007;14:866–874.10.1097/gme.0b013e3180305299
- Gu L, House SE, Prior RL, Fang N, Ronis MJ, Clarkson TB, Wilson ME, Badger TM. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J. Nutr. 2006;136:1215–1221.
- Mathey J, Mardon J, Fokialakis N, Puel C, Kati-Coulibaly S, Mitakou S, Bennetau-Pelissero C, Lamothe V, Davicco MJ, Lebecque P, Horcajada MN, Coxam V. Modulation of soy isoflavones bioavailability and subsequent effects on bone health in ovariectomized rats: the case for equol. Osteoporos. Int. 2007;18:671–679.10.1007/s00198-007-0351-y
