Abstract
Human claudin-3 (CLDN3) is a tetraspanin transmembrane protein of tight junction structures and is known to be over-expressed in some malignant tumors. Although a specific monoclonal antibody (MAb) against the extracellular domains of CLDN3 would be a valuable tool, generation of such MAbs has been regarded as difficult using traditional hybridoma techniques, because of the conserved sequence homology of CLDN3s among various species. In addition, high sequence similarity is shared among claudin family members, and potential cross-reactivity of MAb should be evaluated carefully. To overcome these difficulties, we generated CLDN3-expressing Chinese hamster ovary and Sf9 cells to use an immunogens and performed cell-based screening to eliminate cross-reactive antibodies. As a result, we generated MAbs that recognized the extracellular loops of CLDN3 but not those of CLDN4, 5, 6, or 9. Further in vitro studies suggested that the isolated MAbs possessed the desired binding properties for the detection or targeting of CLDN3.
Graphical Abstract
Epitope analysis revealed that the isolated MAbs recognized various extracellular domains of human CLDN3.
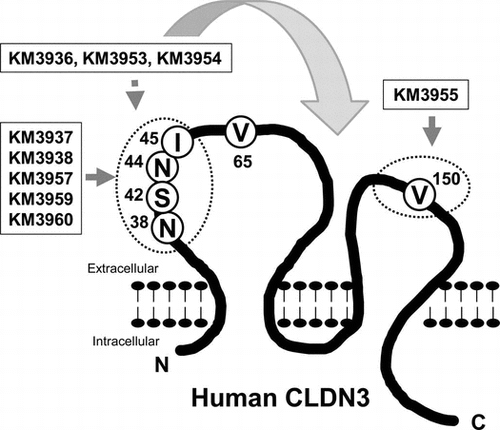
Human claudin-3 (CLDN3) is a tetraspanin transmembrane protein consisting of 220 amino acids and members of the claudin (CLDN) family that comprises 27 members in mammals.Citation1) The protein structure of CLDN3 has been predicted to consist of cytoplasmic N- and C-termini, four transmembrane domains, and two extracellular loops, EL1 (27–76) and EL2 (140–159) from the N-terminus.Citation2) CLDN members undergo homo- or hetero-binding between adjacent cells and generate tight junctions, which are key elements of the epithelial barrier and have defense functions.Citation1) In addition, CLDN3, along with CLDN4, has been well characterized as a functional receptor of cytotoxic Clostridium perfringens enterotoxin (CPE).Citation3)
Several studies have indicated that CLDN3 is highly expressed in a variety of carcinomas, and its expression is related to poor prognosis in some of these.Citation4) The role of CLDN3 in carcinogenesis is now under debate, because several studies reached contradictory conclusions.Citation4–7) However, its high expression on the cell surface of carcinoma cells is expected to make CLDN3 an attractive target for diagnosis and treatment. In fact, targeting of CLDN3 using CPE or an antibody has shown a promising anti-tumor efficacy in vitro and in vivo.Citation3,4,8,9)
It is clear that a specific antibody against CLDN3 would be an invaluable tool for studying CLDN biology or therapeutic applications. However, most antibodies recognize the intracellular C-terminal domains of CLDN3 and are useful only for the detection of CLDN3 expression in fixed or denatured tumor tissue, but not in intact cells in vitro and in vivo. In this study, we aimed to generate a specific monoclonal antibody (MAb) that recognizes the extracellular domains of CLDN3 via traditional mouse hybridoma techniques. To this end, we had to consider two potential difficulties. One was that there is a high degree of conservation among CLDN proteins in various species, so immunization of peptide antigens may be not enough to elicit immunoresponses to generate an objective hybridoma.Citation2) To avoid this problem, Offner et al. immunized chickens with synthetic peptides of the extracellular domains of CLDN3 and succeeded in the generation of a polyclonal antibody bound to CLDN3.Citation2) In addition, Romani et al. utilized a phage display method and isolated a human single-chain fragment variable against EL2 of CLDN3.Citation10) The other difficulty is that the CLDN family shares high sequence similarity among its family members,Citation1,2,8) so the cross-reactivity of a MAb should be evaluated carefully during the isolation steps.
From the above background, we generated CLDN3-expressing Chinese hamster ovary (CHO) and Sf9 cells, in which CLDN3 was expressed using a strong promoter and enhancer, and used these transformants to immunize BXSB mice. Using intact cells as an immunogen, we expected direct stimulation of immune cells to produce antibodies against the cell surface antigen and to circumvent the suppression mechanism of the autoimmune response in mice. In addition, to isolate a specific MAb against CLDN3, candidates were subjected to negative selection against other human CLDN (CLDN4, 5, 6 and 9)-expressing transformants, which possess high sequence similarity to CLDN3.Citation8) This article describes the procedure used to isolate objective MAbs that specifically recognized the extracellular domains of CLDN3 and characterize their binding properties.
Materials and methods
Mice and cell lines
BXSB mice were purchased from Japan SLC (Shizuoka, Japan) and maintained in pathogen-free conditions. All experiments were performed in conformity with institutional guidelines and in accordance with national laws and policies.
A CHO cell line, DG44, was kindly provided by Dr Lawrence Chasin (Columbia University). Murine myeloma cell line P3-U1 (CRL-1597), insect cell line Sf9 (CRL-1711), breast cancer cell line MCF7 (HTB-22), T-lymphoid leukemia cell line Jurkat (TIB-152), and Burkitt’s lymphoma Daudi (CRL-213) were purchased from the American Type Culture Collection.
Establishment of CLDN/CHO cells
CLDN/CHO cells were generated as described previously.Citation8,11) In brief, a myc/his tag sequence was connected to the 3’ end of the CLDN open reading frame by polymerase chain reaction (PCR), and then the myc/his-tagged cDNA was cloned into an expression vector.Citation12) The expression vector was introduced into DG44 cells via electroporation. G418-resistant clones were selected, and a high-producing cell was isolated by single-cell cloning. Appropriate expression of the transfected gene was confirmed by reverse transcriptase-PCR and using anti-tag antibodies.Citation11)
To generate mouse–human chimeric CLDN3-expressing CHO cells, the chimeric cDNA was generated by site-directed mutagenesis of the human CLDN3 cDNA using a QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) and then subjected to the above procedure.
Expression of CLDN3 in Sf9 insect cells
In order to generate recombinant baculovirus and express human CLDN3 in Sf9 insect cells, we used a Bac-to-Bac Baculovirus Expression System (Invitrogen, Tokyo, Japan). In brief, the above-mentioned myc/his-tagged human CLDN3 cDNA was subcloned into pFASTBac1 vector (Invitrogen), and the purified plasmid was then used to transform Escherichia coli DH10Bac competent cells (Invitrogen). Recombinant bacmid DNA was obtained from transformed DH10Bac cells and used to recover recombinant baculovirus after transfection of Sf9 cells in accordance with the manufacturer’s instructions. The recombinant baculovirus was used to infect Sf9 cells at a multiplicity of infection of 0.1, and gene expression was confirmed using anti-tag antibodies. The infected cell pellet was harvested, washed with PBS, and then used as an immunogen.
Isolation of MAb against CLDN3
Six-week-old male BXSB mice (Japan SLC) were immunized four times with 1 × 107 transformants with Bordetella pertussis adjuvant. The spleen was removed 3 days after the final injection of antigen, and 2 × 108 splenocytes fused with 2 × 107 P3-U1 in the presence of polyethylene glycol 1000 (Junsei, Tokyo, Japan). Cultured hybridoma cells in wells showing anti-CLDN3 MAb activity were screened using an 8200 cellular detection system (Applied Biosystems, Tokyo, Japan) using CLDN3/CHO as the target cell. After cloning twice with limited dilution, a stable clone was obtained. The immunoglobulin class and subclass were determined using anti-mouse isotype-specific antibodies (Zymed, San Francisco, CA, USA).
Flow cytometry
For the analysis of isolated MAb binding to cell surface molecules, cells were detached with 0.02% EDTA solution (Nacalai Tesque, Kyoto, Japan) and then stained with 0.5 µg mL−1 of isolated MAb or control mouse IgG (Dako, Tokyo, Japan). To detect the expression of transfected CLDN-myc/his, cells pretreated with 70% ethanol were incubated with 1 µg mL−1 of anti-myc antibody (MBL, Nagoya, Japan) or control mouse IgG1 (Dako). These reactivities were detected using fluorescein isothiocyanate-conjugated (FITC-conjugated) goat anti-mouse Ig antibody (Dako). Stained cells were then analyzed using an EPICS XL-MCL Flow Cytometer (Beckman Coulter, Tokyo, Japan).
Western blotting
Cells were lysed in lysis buffer containing 50 mM Tris-HCl (pH 7.2), 150 mM NaCl, 2 mM MgCl2, 2 mM CaCl2, 0.1% NaN3, 5 mM phenylmethylsulfonyl fluoride, 100 mM dithiothreitol, 1% Triton X-100, 50 mM N-ethylmaleimide, and 1 mg mL−1 leupeptin. The lysate (5 × 104 cell equivalents) was solubilized in loading buffer containing 0.1 M Tris–HCl (pH 6.8), 2% sodium dodecyl sulfate (SDS), 10% glycerol, and 5% 2-mercaptoethanol; boiled for 5 min; and separated by 5–20% SDS-polyacrylamide gel electrophoresis. The proteins were electrophoretically transferred onto a polyvinylidene difluoride (PVDF) membrane, and then, the membrane was blocked with 10% bovine serum albumin (BSA) and incubated with 2 µg mL−1 of rabbit anti-CLDN3 polyclonal antibody (Novus Biologicals, Littleton, CO, USA) or isolated MAb. After washing, the membrane was exposed to horseradish peroxidase-conjugated anti-mouse IgG (Zymed) and binding was visualized using ECL Western Blotting Detection Reagents (GE Healthcare, Buckinghamshire, UK).
Immunoprecipitation
Two-hundred µL of PBS containing rabbit anti-mouse Ig polyclonal antibody (Dako) was dispensed into each well of a 96-well ELISA plate (Greiner Japan, Tokyo, Japan) and kept at 4 °C overnight to allow the antibody to be absorbed. After blocking with 1% BSA-PBS, 200 µL of 1% BSA-PBS containing 1 µg mL−1 of rabbit anti-CLDN3 polyclonal antibody (Novus Biologicals) or isolated MAb was added to each well and kept at 4 °C overnight. After incubation, the plate was washed with PBS. One-hundred µL of cell lysate (5 × 106 cell equivalents) prepared with the above procedure was added to each well and kept at 4 °C overnight. After washing each well by PBS containing 0.05% Tween 20, 200 µL of loading buffer was added and kept at room temperature for 2 h. Twenty µL of the resultant mixture was subjected to Western blotting, as described earlier, to visualize the precipitated CLDN3 protein.
Immunohistochemistry
Frozen sections of human ovarian cancers and normal ovary were purchased from Biochain Institute, Inc (Hayward, CA, USA). The sections were fixed with ice-cold acetone for 5 min, air-dried, and washed with PBS. After inactivation of endogenous peroxidase activity with 0.3% H2O2 in methanol at room temperature for 5 min, the sections were preincubated with 1% BSA in PBS for 10 min and then incubated with 1 µg mL−1 of anti-CLDN MAb or control mouse IgG2a (Dako) for 1 h. Antibody binding was detected using Dako EnVision + System-HRP (Dako), and the signals were visualized using 3,3’-diaminobenzidine deposition. Nuclei were counterstained with hematoxylin.
Results
Isolation of MAbs against extracellular domains of human CLDN3
First, we generated CLDN3-expressing transformants derived from CHO/DG44 and Sf9 cells to use as an immunogen. A CLDN3-expressing CHO cell was generated through transformation with an expression vector for CLDN3-myc/his containing a G-418 resistance gene followed by single-cell cloning with high drug concentration. The expression of CLDN3-myc/his on the cell surface was confirmed by confocal microscopy analysis.Citation11) In addition, CLDN3-expressing Sf9 cells were prepared through infection with a recombinant baculovirus to express CLDN3-myc/his, and bulk-infected cells were harvested to use as an immunogen. The expression of CLDN3 was confirmed using an anti-tag antibody by Western blotting and with KM3907, which is a cross-reactive antibody against CLDN3 and CLDN4Citation8) by flow cytometry (FCM) analysis (data not shown).
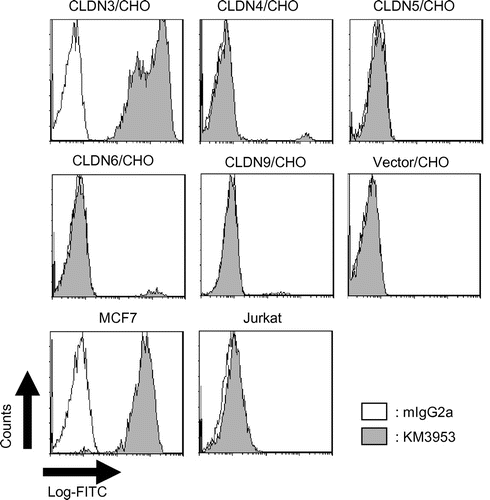
After immunization of BXSB mice with CLDN3-expressing transformants, we selected several mice with antiserum activity against CLDN3/CHO and then established hybridomas. Through cell-based screening using an 8200 cellular detection system, 14 MAbs were isolated as binders to CLDN3/CHO. These MAbs also showed binding to a CLDN3-positive breast cancer cell line, MCF7, but not to CLDN3-negative cell lines, Jurkat and Daudi, in the FCM analysis (Table ). Next, to select a specific mAb against CLDN3, we conducted a second screen to evaluate binding to CLDN4-, CLDN5-, CLDN6-, and CLDN9-expressing CHO cells. As a result, 5 MAbs (KM3933, KM3939, KM3952, KM3956, and KM3958) showed cross-reactivity and 9 MAbs (KM3936, KM3937, KM3938, KM3953, KM3954, KM3955, KM3957, KM3959, and KM3960) showed objective binding specificity for CLDN3 (Table and Fig. ). Among these, KM3936, KM3937, KM3938, and KM3939 were derived from immunized mice with Sf9 transfectants and the others originated from CLDN3/CHO.
Table 1. Characterization summary of isolated MAbs.
Fig. 1. FCM analysis of KM3953.
Epitope analysis of isolated anti-CLDN3 MAbs
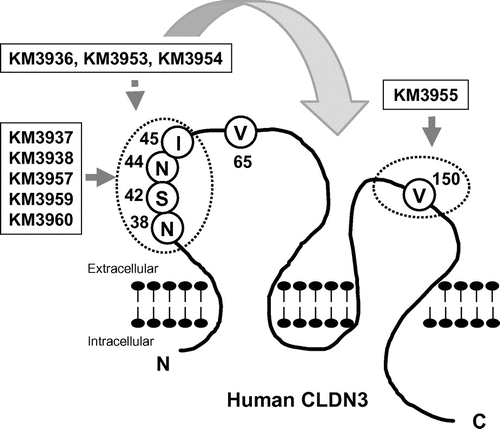
Next, we aimed to identify the epitope of the isolated MAbs. To this end, we constructed myc/his-tagged mouse–human chimeric CLDN3, on which the extracellular domains of human CLDN3 were partially exchanged for counterparts from the mouse CLDN3 sequence (Fig. (A)) and generated CHO transfectants. An anti-myc antibody bound similarly to all transfectants, including control CLDN3/CHO (Fig. (B)). Through FCM analysis, KM3955 showed binding to extracellular loop 1 mutants: mCL3-EL1, mCL3-EL1-N, and mCL3-EL1-C, but not to extracellular loop 2 mutants: mCL3-EL1&2 and mCL3-EL2 (Fig. (B)). In contrast, the binding of KM3937, KM3938, KM3957, KM3959, and KM3960 was detectable on mCL3-EL2 and mCL3-EL1-C, but negative on mCL3-EL1&2, mCL3-EL1, and mCL3-EL1-N (Figs. (B) and ). According to KM3936, KM3953, and KM3954, a partial decrease in its binding was detected against mCL3-EL1 and mCL3-EL1-N, but binding was detected qualitatively to all mutants. These results indicated that V150 in the EL2 domain was a major contributor for the binding of KM3953. On the other hand, N38/S42/N44/I45 of the EL1 domain was for KM3937, KM3938, KM3957, KM3959 and KM3960 (Fig. ). Interestingly, we could not determine the precise epitope of KM3936, KM3953, and KM3954 using this method. Because these MAbs showed cross-reactivity against mouse CLDN3-expressing CHO cells (data not shown), we believe that these MAbs partially recognized not only N38/S42/N44/I45 in the EL1 domain, but also other extracellular domains of CLDN3 (Fig. ).
Fig. 2. Construction of mouse–human chimeric CLDN3 and FCM analysis of anti-CLDN3 MAbs.
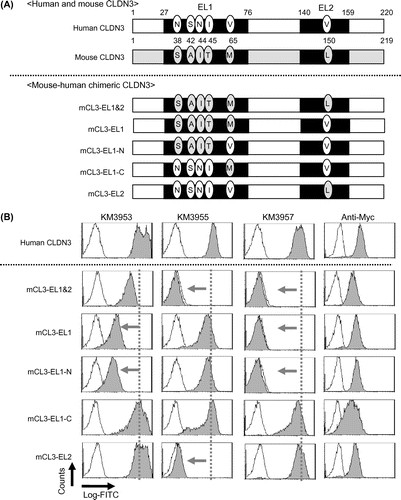
Fig. 3. Schematic representation of epitopes recognized by anti-CLDN3 MAbs.
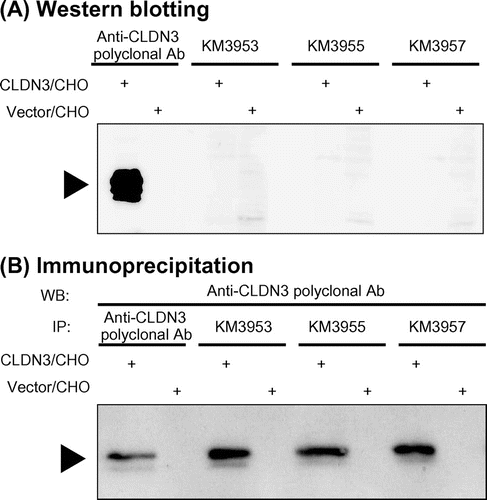
Western blotting and immunoprecipitation assay
Five MAbs (KM3937, KM3938, KM3953, KM3955, and KM3957) were subjected to Western blotting and immunoprecipitation assays. We prepared cell lysates from CLDN3/CHO and Vector/CHO cells and then conducted Western blotting under denaturing conditions with SDS. A positive control antibody, which recognized the intra-cellular C-terminus of CLDN3, specifically visualized CLDN3 protein in transfectants at a size of 20–25 kDa but not isolated MAbs (Fig. (A) and Table ). Next, to evaluate antibody binding under non-denaturing conditions, immunoprecipitation assays with isolated MAbs were performed. As a result, all MAbs showed binding to the CLDN3 protein (Fig. (B) and Table ). These results suggested that KM3937, KM3938, KM3953, KM3955, and KM3957 recognized the conformational structure of the CLDN3 protein and did not bind to denatured target.
Fig. 4. Western blotting and immunoprecipitation assay with anti-CLDN3 MAbs.
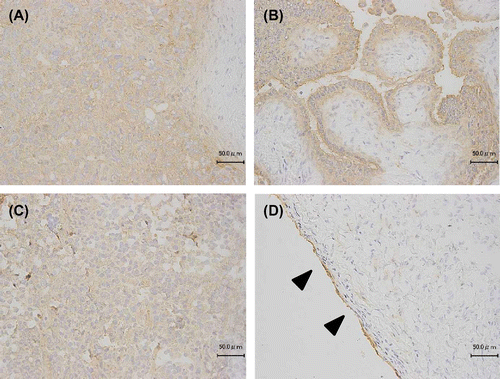
Immunohistochemical analysis of KM3953 and KM3957 using human ovarian cancers and normal ovary
In mammalian ovaries, the expression of CLDN3 is weak but detectable on the ovarian surface epithelium (OSE) and its high expression on ovarian cancers has been described previously.Citation13,14) We, therefore, conducted immunohistochemical studies on frozen human ovary sections using KM3953 and KM3957. Before testing, the visualization process was consolidated using CLDN3/CHO and Vector/CHO xenografts (data not shown). Fig. shows the results with epithelial ovarian cancers: serous adenocarcinoma (A), serous cystoadenocarcinoma (B), mucinous cystoadenocarcinoma (C), and normal ovary (D) with KM3953. Positive signals were clearly detected on ovarian cancer cells and the OSE in a normal ovary with KM3953 and KM3957; in contrast, the negative control antibody did not show signals (data not shown).
Fig. 5. Immunohistochemical staining using KM3953 for ovarian carcinomas and normal ovary tissue.
Discussion
In this study, we succeeded in generating nine MAbs (KM3936, KM3937, KM3938, KM3953, KM3954, KM3955, KM3957, KM3959, and KM3960) that recognize the extracellular domains of CLDN3, but not CLDN4, 5, 6, or 9, which possess high sequence similarity to CLDN3.Citation8) The isolated MAbs also showed binding to a CLDN3-expressing cell line, MCF7, but not CLDN3-negative cells in FCM analysis. Immunoprecipitation assays suggested that most of the isolated MAbs recognized the conformational structure of CLDN3 protein. In addition, epitope analysis using mouse–human chimeric CLDN3-expressing transformants revealed that a variety of MAbs have been generated, which recognized various extracellular domains of CLDN3. Moreover, the binding of KM3953 and KM3957 was detected with the OSE and cancer cells by immunohistochemical staining. These results suggested that the resultant MAbs, especially KM3953 and KM3957, possessed desirable binding properties for detecting CLDN3 expression. To the best of our knowledge, this is a first report to isolate a MAb against extracellular domains of CLDN3 with the above binding properties.
We believe that our approach, using CLDN3-expressing transformants as an immunogen and excluding cross-reactive antibodies with a cell-based assay, was effective for isolating the desired MAbs via a traditional hybridoma technique. Historically, using a target-expressing intact cell as an immunogen has been an effective method for the generation of MAbs against extracellular domains of target proteins. The celebrated example is anti-CD20 MAbs.Citation15,16) CD20 is a tetraspanin transmembrane protein expressed in B-cell hematopoietic lineages, and the targeting MAbs, rituximab, and others are indispensable for therapeutic applications in oncology and immunology.Citation15,16) Most therapeutic anti-CD20 MAbs were originally generated through conventional hybridoma techniques, in which a CD20-expressing cell line or transformant was used as an immunogen.Citation16–18) These facts strongly encouraged us to adopt the above strategy to induce an adequate immunological response to generate hybridomas producing a specific MAb against the extracellular domains of CLDN3. In addition, excluding cross-reactive MAbs against other CLDNs was also an important step, because 5 of 14 MAbs showed undesired cross-reactivity against other CLDNs (Table ).
Through epitope analysis, we were surprised that the isolated MAbs recognized various epitopes of CLDN3, because only small portions of CLDN3 are predicted to be exposed on cell surface. The isolated MAbs were categorized into three groups according to their epitope (Fig. ). First, KM3937, KM3938, KM3957, KM3959, and KM3960 were persumed to recognize N38/S42/N44/I45 in the EL1 domain. Before this study, we succeeded in the isolation of a cross-reactive MAb against CLDN3 and CLDN4, KM3907, which recognized the EL1 domain of both CLDN3 and CLDN4.Citation8) Interestingly, KM3907 showed the same binding properties against a mouse–human chimeric CLDN3 as KM3937 (data not shown). Further analysis of the difference between KM3907 and isolated MAbs will be necessary, but we believe that N38/S42/N44/I45 in the CLDN3 sequence might be important amino acids for the difference in conformational structure between CLDN3 and CLDN4. Second, KM3955 was characterized as having binding specificity against V150 in the EL2 domain. In this study, only KM3955 was identified as recognizing EL2, and it showed lower avidity compared with the other isolated MAbs in dose-dependent assays using FCM analysis (data not shown). These results may indicate that the EL2 domain of CLDN3 shows low immunogenicity with the present method. Finally, KM3936, KM3953, and KM3954, for which the epitopes were not clearly identified, were classified into the third group. Because its binding against mCL3-EL1 and mEL3-EL1-N decreased slightly compared with other isolated MAbs, the epitope might include N38/S42/N44/I45 in EL1. However, major epitopes might be shared between human and mouse CLDN3, so we could not determine the precise epitope in this study. Further studies will be needed, but the third group of MAbs might be highly useful for understanding therapeutic applications of anti-CLDN3 MAb from other points of view. Because targeting CLDN3 has some concerns regarding adverse effects on CLDN3-expressing normal tissues, such as kidney, liver, and pancreas,Citation1) these MAbs were utilized for toxicological evaluations in a mouse model to elucidate the potential concerns.
In conclusion, we succeeded in the generation of specific MAbs against the extracellular domains of CLDN3 via mouse hybridoma technique. We hope that our procedures and results will be a good basis for the generation of specific MAbs against other CLDNs. It is well known that the CLDN family consists of many members in various species and they have roles in homo- and hetero-binding in tight junction structures and paracellular pore formation in epithelial tissues.Citation1) Moreover, several CLDNs were identified in conjunction with human diseases and might play a cancer-promoting or tumor-suppressing role in carcinogenesis.Citation4) For further understanding of CLDN biology and their potential as therapeutic targets, specific MAbs to detect or target CLDN in intact cells will be a powerful tool for future studies.
Acknowledgment
We thank So Ohta for helpful comments and discussions.
References
- Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013;93:525–569.10.1152/physrev.00019.2012
- Offner S, Hekele A, Teichmann U, Weinberger S, Gross S, Kufer P, Itin C, Baeuerle PA, Kohleisen B. Epithelial tight junction proteins as potential antibody targets for pancarcinoma therapy. Cancer Immunol. Immunother. 2005;54:431–445.10.1007/s00262-004-0613-x
- English DP, Santin AD. Claudins over expression in ovarian cancer: potential targets for Clostridium perfringens enterotoxin (CPE) based diagnosis and therapy. Int. J. Mol. Sci. 2013;14:10412–10437.10.3390/ijms140510412
- Kwon MJ. Emerging roles of claudins in human cancer. Int. J. Mol. Sci. 2013;14:18148–18180.10.3390/ijms140918148
- Okugawa T, Oshima T, Chen X, Hori K, Tomita T, Fukui H, Watari J, Matsumoto T, Miwa H. Down-regulation of claudin-3 is associated with proliferative potential in early gastric cancers. Dig. Dis. Sci. 2012;57:1562–1567.10.1007/s10620-012-2043-5
- Shang X, Lin X, Alvarez E, Manorek G, Howell SB. Tight junction proteins claudin-3 and claudin-4 control tumor growth and metastases. Neoplasia 2012;14:974–985.
- de Souza WF, Fortunato-Miranda N, Robbs BK, de Araujo WM, de-Freitas-Junior JC, Bastos LG, Viola JP, Morgado-Díaz JA. Claudin-3 overexpression increases the malignant potential of colorectal cancer cells: roles of ERK1/2 and PI3K-Akt as modulators of EGFR signaling. PLoS ONE 2013;8:e74994.10.1371/journal.pone.0074994
- Kato-Nakano M, Suzuki M, Kawamoto S, Furuya A, Ohta S, Nakamura K, Ando H. Characterization and evaluation of the antitumour activity of a dual-targeting monoclonal antibody against claudin-3 and claudin-4. Anticancer Res. 2010;30:4555–4562.
- Li X, Iida M, Tada M, Watari A, Kawahigashi Y, Kimura Y, Yamashita T, Ishii-Watabe A, Uno T, Fukasawa M, Kuniyasu H, Yagi K, Kondoh M. Development of an anti-claudin-3 and -4 bispecific monoclonal antibody for cancer diagnosis and therapy. J. Pharmacol. Exp. Ther. 2014;351:206–213.10.1124/jpet.114.216911
- Romani C, Comper F, Bandiera E, Ravaggi A, Bignotti E, Tassi RA, Pecorelli S, Santin AD. Development and characterization of a human single-chain antibody fragment against claudin-3: a novel therapeutic target in ovarian and uterine carcinomas. Am. J. Obstet. Gynecol. 2009;201:e1–e9.
- Suzuki M, Nakano K-M, Kawamoto S, Furuya A, Abe Y, Misaka H, Kimoto N, Nakamura K, Ohta S, Ando H. Therapeutic antitumor efficacy of monoclonal antibody against claudin-4 for pancreatic and ovarian cancers. Cancer Sci. 2009;100:1623–1630.10.1111/cas.2009.100.issue-9
- Nakamura K, Tanaka Y, Fujino I, Hirayama N, Shitara K, Hanai N. Dissection and optimization of immune effector functions of humanized anti-ganglioside GM2 monoclonal antibody. Mol. Immunol. 2000;37:1035–1046.10.1016/S0161-5890(01)00021-9
- Zhu Y, Brännström M, Janson PO, Sundfeldt K. Differences in expression patterns of the tight junction proteins, claudin 1, 3, 4 and 5, in human ovarian surface epithelium as compared to epithelia in inclusion cysts and epithelial ovarian tumours. Int. J. Cancer 2006;118:1884–1891.10.1002/(ISSN)1097-0215
- Aravindakshan J, Chen X, Sairam MR. Differential expression of claudin family proteins in mouse ovarian serous papillary epithelial adenoma in aging FSH receptor-deficient mutants. Neoplasia 2006;8:984–994.10.1593/neo.06529
- Beers SA, Chan CH, French RR, Cragg MS, Glennie MJ. CD20 as a target for therapeutic type I and II monoclonal antibodies. Semin. Hematol. 2010;47:107–114.10.1053/j.seminhematol.2010.01.001
- van Meerten T, Hagenbeek A. CD20-targeted therapy: the next generation of antibodies. Semin. Hematol. 2010;47:199–210.10.1053/j.seminhematol.2010.01.007
- Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, Newman RA, Hanna N, Anderson DR. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994;83:435–445.
- Teeling JL, French RR, Cragg MS, van den Brakel J, Pluyter M, Huang H, Chan C, Parren PW, Hack CE, Dechant M, Valerius T, van de Winkel JG, Glennie MJ. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 2004;104:1793–1800.10.1182/blood-2004-01-0039
