Abstract
The receptor for diuretic hormone 31 (DH31R) was identified in the silkworm Bombyx mori. A heterologous expression system revealed that an orphan G-protein coupled receptor, BNGR-B1, responded to DH31 and upregulated the intracellular cAMP level. DH31R (BNGR-B1) was predominantly expressed in the anterior silk gland, midgut, and ovary, whereas DH31 was predominantly expressed in the central nervous system and midgut.
The coordination of the balance between water and ions is essential for insect survival. Several types of diuretic hormones (DHs) have been identified/predicted in many insect species, and their importance for regulating fluid secretion has been reported.Citation1) In Bombyx, four DHs (DH41, DH34, DH45, and DH31) have been identified, and their localization was studied in the central nervous system (CNS).Citation2) The DHs are classified into the following two categories: corticotropin releasing factor (CRF)-type and calcitonin-type. DH41, DH34, and DH45 are classified as CRF-type DHs, and DH31 is the only calcitonin-type DH. The first DH31 was isolated from the cockroach, Diploptera punctata, and it upregulates fluid secretion rates in Malpighian tubules.Citation3) Various DH31 homologs have been predicted in many species using genome information, whereas little information is available about their distribution and ligand–receptor binding. The interaction of DH31 and DH31R has been reported in the fruit fly Drosophila melanogasterCitation4) and in the kissing bug Rhodnius prolixus.Citation5) One DH31R gene with three splicing variants has been reported in Drosophila, and two DH31R genes with two and three splicing variants have been reported in Rhodnius.Citation5) Conversely, Bombyx DH31R has not yet been identified.
To determine the receptor for DH31 in B. mori, protein BLAST analysis was performed in B. mori using the deduced amino acid sequence of Dorosophila DH31R isoform A (DH31R-A, GenBank accession number: AAN16138). The protein BLAST analysis showed a 29–55% identity to BNGR-B1, BNGR-B2, BNGR-B3, and BNGR-B4 (GenBank accession numbers: NP_001127732, NP_001127733, NP_001127734, and NP_001127735, respectively), and Bombyx DHR with >75% query coverage. These candidate receptors were aligned with Drosophila DH31R-A, DH44R isoform 1 (GenBank accession number: AAF58250), and pigment dispersing factor receptor isoform A (PDFR-A, GenBank accession number: AAF45788) as well as Rhodnius DH31R1-B, and DH31R2-B (GenBank accession numbers: KC660149 and KF446640, respectively). The calcitonin receptor (CR) in Mus musculus (GenBank accession number: AAK56132) was used as an outgroup. The sequence alignment and phylogenetic tree generation (neighbor-joining method, with a bootstrap analysis of 1000 trials, excluding gap position) were performed with ClustalX. The phylogenetic analysis of these candidates showed that BNGR-B1 and BNGR-B4 have a high possibility of an affinity for DH31 in Bombyx (Fig. (A)). In Drosophila, PDFR showed a weak affinity to DH31,Citation6) whereas Bombyx DH31 did not show any affinity to Bombyx PDFR, BNGR-B2.Citation7) In addition, the receptor of DH41 (DHR) has been reported in Manduca sexta,Citation8) and the homologous receptor was identified in B. mori.Citation9) Thus, BNGR-B2 (PDFR) and DHR were excluded from subsequent analysis, which focused on the three remaining orphan G-protein coupled receptors (GPCRs): BNGR-B1, B3, and B4. These BNGRs were identified by the global analysis of neuropeptide GPCR genes in B. mori,Citation10) but their ligands and functions have not been fully investigated.
Fig. 1. Screening of candidate DH31R.
Notes: (A) A phylogenetic tree was generated based on the amino acid sequences of the following receptors: Drosophila DH31R, DH44R, and PDFR; Rhodnius DH31R-1B and DH31R-2B; Bombyx DHR and DH31R candidates (BNGR-B1, B2, B3, and B4). Phylogenetic analysis was performed with the neighbor-joining method using the ClustalX multiple alignment program and a bootstrap value of 1000 trials for each branch position. The indicated numbers are the bootstrap values as a percentage of 1000 replicates, and the scale bar indicates 0.05 changes per residue. Bootstrap values greater than 50% are indicated. The Mus musculus CR was used as an outgroup. (B) DH31-binding analysis of DH31R candidate receptors by examining the changes in the intracellular cAMP levels. BNGR-B1, B3, or B4-expressing HEK293 cells were treated with 1 μM DH31 and 0.5 mM IBMX. Each datum point represents the mean ± SEM (n = 3). Statistically significant differences were evaluated by Student’s t-test (***p < 0.001). (C) The dose response of DH31 was evaluated by the change in the intracellular cAMP levels in the BNGR-B1-expressed HEK293 cells. The cells were treated with 10−12 to 10−5 M of DH31 and 0.5 mM IBMX. Each datum point represents the mean ± SEM (n = 3).
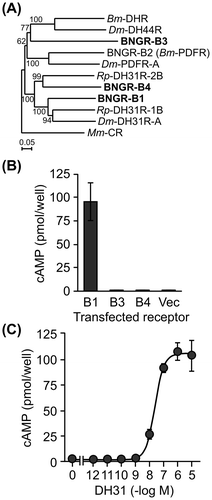
Ligand–receptor interactions were evaluated using a heterologous expression system Citation11) with synthetic Bombyx DH31 mature peptide (AFDLGLGRGYSGALQAKHLMGLAAANFAGGP-NH2). An empty pME18S vector or the full-length ORF of the candidate receptor (BNGR-B1, B3 or B4) inserted into the pME18S vector was transfected into HEK293 cells using Lipofectamine LTX with PLUS reagent (Invitrogen, Carlsbad, CA, USA) as previously described.Citation7) The transfected cells were treated with 1 μM DH31 in the presence of 0.5 mM 3-isobutyl-1-methylxanthine (IBMX) at 37 °C for 30 min. The amount of intracellular cAMP was measured using the cAMP-Screen Chemiluminescent Immunoassay System (Applied Biosystems Foster City, CA, USA) with a Wallac ARVO SX 1420 Multilabel Counter (PerkinElmer, Waltham, MA, USA) as previously described.Citation7) The BNGR-B1-expressed cells only responded to DH31, and a significant increase in the intracellular cAMP was observed (Fig. (B)). In addition, the dose response to DH31 (10−12 to 10−5 M) was investigated in the BNGR-B1-expressed HEK293 cells using the change in the intracellular cAMP level. DH31 showed the highest activity at a concentration of 10−6 M, and the EC50 was 24.2 nM (Fig. (C)). Because the EC50 values of DH31 reported in other insect species were 0.7–116 nM,Citation3,4,12) this result is consistent with previous studies.
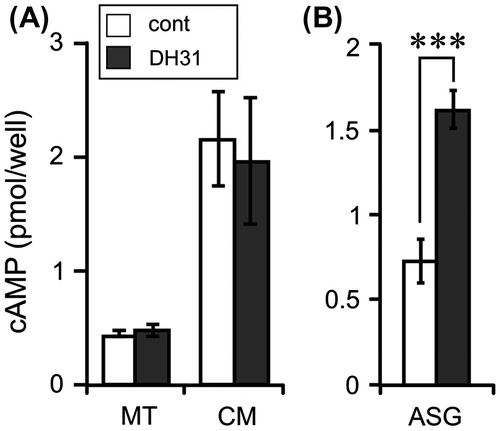
The expression levels of DH31 and BNGR-B1 were investigated by RT-PCR with GoTaq (Promega, Madison, WI, USA). The PCR products were separated on an agarose gel and visualized with ethidium bromide. The larvae of silkworm (Kinshu × Showa) were reared on an artificial diet (SilkMate PS: Nihon Nosan Kogyo) at 25 °C under a 16-h light: 8-h dark photoperiod. The first feeding day was designated as day 0, and gut purge occurred at day 7 of the fifth instar in our rearing conditions. The distribution of all the genes was evaluated on day 0 after gut purged (G0) larvae. cDNA was prepared from 10 selected tissues (BR: brain, SA: salivary gland, PG: prothoracic gland, ASG: anterior silk gland, FB: fat body, MT: Malpighian tubules, EP: epidermis, MG: midgut, TE: testis, and OV: ovary) and the CNS (BR, SOG: suboesophageal ganglion, TG: thorax ganglia, and AG: abdominal ganglia) using a High Pure RNA Tissue Kit (Roche, Indianapolis, IN, USA) and Superscript III reverse transcriptase (Invitrogen). The expression of DH31 has been reported in some species, but the distribution of DH31 was different among these species. DH31 is expressed in the CNS and midgut in Drosophila,Citation13,14) whereas it is only expressed in the CNS in Rhodnius.Citation15) Consequently, the expression of DH31 was investigated in B. mori, and DH31 was predominantly expressed in the BR and MG (Fig. (A)). Furthermore, DH31 expression in the CNS was investigated, and it was detected in all of the selected regions, with particularly high DH31 expression being observed in the terminal part of the abdominal ganglia (Fig. (B)). BNGR-B1 was expressed in many tissues, and high expression was observed in the ASG, MG, and OV, whereas there was almost no expression in the MT and EP (Fig. (A)). Because DH31 upregulates the fluid secretion rates in D. punctata MT,Citation3) BNGR-B1 expression was expected in the MT of B. mori. In R. prolixus, the expression of another DH31R (DH31R-2) was reported in the MT.Citation5) The Rhodnius DH31R-2 is not orthologous to Drosophila DH31R, but is orthologous to an orphan receptor CG4395. The Rhodnius DH31R-2 showed high similarity with BNGR-B4 (Fig. (A)), whereas DH31 could not upregulate the intracellular cAMP level via BNGR-B4 in our experimental conditions (Fig. (B)). In Diploptera and Drosophila, DH31 induces tubule secretion in the MT via an intracellular cAMP increaseCitation3,12); therefore, the intracellular and secreted cAMP levels were investigated in Bombyx MT. Bombyx DH31 did not show any increase in the intracellular and secreted cAMP levels (Fig. (A)). However, the intracellular cAMP level of ASG was significantly upregulated by DH31 (Fig. (B)). Thus, non-diuretic function(s) of DH31 is suggested in the BNGR-B1 expressed tissues, whereas the details remain to be elucidated. In conclusion, the BNGR-B1 is DH31R in B. mori and cAMP is the second messenger of the receptor.
Fig. 2. Gene expression analysis of DH31 and BNGR-B1.
Notes: (A) The tissue distributions of DH31 and BNGR-B1 were evaluated by standard RT-PCR in the selected tissues of G0 larvae. BR: brain, SA: salivary gland, PG: prothoracic gland, ASG: anterior silk gland, FB: fat body, MT: Malpighian tubules, EP: epidermis, MG: midgut, TE: testis, and OV: ovary. (B) The distribution of DH31 was evaluated by standard RT-PCR in the CNS of G0 larvae. BR: brain, SOG: suboesophageal ganglion, TG1-3: thorax ganglion 1-3, AG1-8: abdominal ganglion 1-8. ((A) and (B)) Ribosomal protein L3 (RpL3) was used as an internal standard. The PCR primer sets used for the expression analysis were as follows: DH31: Fw, GTGCTGTGCTCCTGATCGTC, Rv, TGCGTTCCATCTGAATGAGG; BNGR-B1: Fw, TTCAACAATCGGACCTTGCC, Rv, GGTTCACGTCATCCTCCTCG; and RpL3: Fw, AGCACCCCGTCATGGGTCTA, Rv, TGCGTCCAAGCTCATCCTGC.
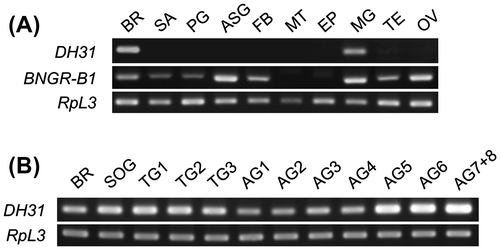
Fig. 3. Effects of DH31 on the cAMP levels in the Malpighian tubules and anterior silk gland.
Notes: (A) MT (approx. 1 cm long) was cultured in 100 μL of Grace’s insect culture medium (Gibco, Grand Island, NY) with 1 μM DH31 and 0.5 mM IBMX at 25 °C for 1 h. The amount of cAMP was measured in the MT and cultured medium (CM). Each datum point represents the mean ± SEM (n = 8). (B) ASG (approx. 2.5 cm long) was cultured in 300 μL of Grace’s insect culture medium with 1 μM DH31 and 0.5 mM IBMX at 25 °C for 30 min. Each datum point represents the mean ± SEM (n = 6). cAMP was extracted from the tissues by 100 μL of acidic ethanol (0.1% 10 N HCl, v/v), and the supernatant was used after evaporation. Statistically significant differences were evaluated by Student’s t-test (***p < 0.001).
Additional information
Funding
References
- Coast GM, Orchard I, Phillips JE, Schooley DA. Insect diuretic and antidiuretic hormones. Adv. Insect Physiol. 2002;29:279–409.10.1016/S0065-2806(02)29004-9
- Roller L, Yamanaka N, Watanabe K, Daubnerová I, Žitňan D, Kataoka H, Tanaka Y. The unique evolution of neuropeptide genes in the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 2008;38:1147–1157.10.1016/j.ibmb.2008.04.009
- Furuya K, Milchak RJ, Schegg KM, Zhang J, Tobe SS, Coast GM, Schooley DA. Cockroach diuretic hormones: characterization of a calcitonin-like peptide in insects. Proc. Natl. Acad. Sci. USA. 2000;97:6469–6474.10.1073/pnas.97.12.6469
- Johnson EC, Shafer OT, Trigg JS, Park J, Schooley DA, Dow JA, Taghert PH. A novel diuretic hormone receptor in Drosophila: evidence for conservation of CGRP signaling. J. Exp. Biol. 2005;208:1239–1246.10.1242/jeb.01529
- Zandawala M, Li S, Hauser F, Grimmelikhuijzen CJ, Orchard I. Isolation and functional characterization of calcitonin-like diuretic hormone receptors in Rhodnius prolixus. PLoS One. 2013;8:e82466.10.1371/journal.pone.0082466
- Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS, De Loof A, Schoofs L, Taghert PH. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron. 2005;48:213–219.10.1016/j.neuron.2005.09.009
- Iga M, Nakaoka T, Suzuki Y, Kataoka H. Pigment dispersing factor regulates ecdysone biosynthesis via Bombyx neuropeptide G protein coupled receptor-B2 in the prothoracic glands of Bombyx mori. PLoS One. 2014;9:e103239.10.1371/journal.pone.0103239
- Reagan JD. Expression cloning of an insect diuretic hormone receptor. A member of the calcitonin/secretin receptor family. J. Biol. Chem. 1994;269:9–12.
- Ha SD, Kataoka H, Suzuki A, Kim BJ, Kim HJ, Hwang SH, Kong JY. Cloning and sequence analysis of cDNA for diuretic hormone receptor from the Bombyx mori. Mol. Cells. 2000;10:13–17.10.1007/s10059-000-0013-9
- Yamanaka N, Yamamoto S, Žitňan D, Watanabe K, Kawada T, Satake H, Kaneko Y, Hiruma K, Tanaka Y, Shinoda T, Kataoka H. Neuropeptide receptor transcriptome reveals unidentified neuroendocrine pathways. PLoS One. 2008;3:e3048.10.1371/journal.pone.0003048
- Katada S, Nakagawa T, Kataoka H, Touhara K. Odorant response assays for a heterologously expressed olfactory receptor. Biochem. Biophys. Res. Commun. 2003;305:964–969.10.1016/S0006-291X(03)00863-5
- Coast GM, Webster SG, Schegg KM, Tobe SS, Schooley DA. The Drosophila melanogaster homologue of an insect calcitonin-like diuretic peptide stimulates V-ATPase activity in fruit fly Malpighian tubules. J. Exp. Biol. 2001;204:1795–1804.
- Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 2007;39:715–720.10.1038/ng2049
- Veenstra JA. Peptidergic paracrine and endocrine cells in the midgut of the fruit fly maggot. Cell Tissue Res. 2009;336:309–323.10.1007/s00441-009-0769-y
- Zandawala M, Paluzzi JP, Orchard I. Isolation and characterization of the cDNA encoding DH31 in the kissing bug Rhodnius prolixus. Mol. Cell. Endocrinol. 2011;331:79–88.10.1016/j.mce.2010.08.012
