Abstract
Ginsenosides, also known as ginseng saponins, are the principal bioactive ingredients of ginseng, which are responsible for its diverse pharmacological activities. The present work aimed to assess skin anti-photoaging properties of ginsenoside Rb2 (Rb2), one of the predominant protopanaxadiol-type ginsenosides, in human epidermal keratinocyte HaCaT cells under UV-B irradiation. When the cultured keratinocytes were subjected to Rb2 prior to UV-B irradiation, Rb2 displayed suppressive activities on UV-B-induced reactive oxygen species elevation and matrix metalloproteinase-2 expression and secretion. However, Rb2 at the used concentrations was unable to modulate cellular survivals in the UV-B-irradiated keratinocytes. In brief, Rb2 possesses a protective role against the photoaging of human keratinocyte cells under UV-B irradiation.
Graphical Abstract
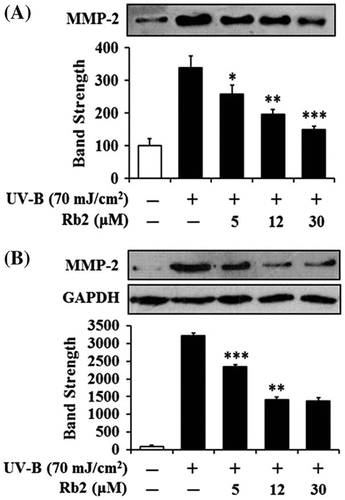
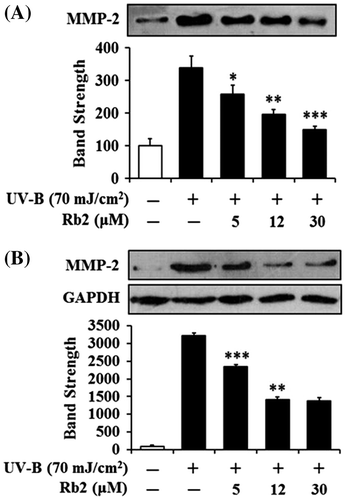
Suppression of UV-B-induced matrix metalloproteinase-2 protein levels by ginsenoside Rb2 in conditioned medium (A) and cellular lysate (B)
Ginseng, the root of Panax ginseng C.A. Meyer (family Araliaceae), is a popular phytomedicinal remedy which has been widely used in the Oriental countries, including Korea, China, and Japan, over the past few hundred years. Among various ingredients identified from ginseng so far, ginsenosides have been believed to be responsible for most pharmacological activities of ginseng. Ginsenosides are a class of steroidal glycosides which are exclusively found in the genus Panax and classified into three categories, such as protopanaxadiol (PPD)-, protopanaxatriol-, and oleanolic acid-type ginsenosides, based upon the chemical structure of their aglycones (sapogenins). Ginsenoside Rb2 (Rb2) (Fig. ) is a major PPD-type ginsenoside that is most abundantly found in ginseng.
Rb2 in pure form has been used to verify its pharmacological efficacies, especially antidiabetic, antimetastatic, fibrinolytic, antiviral, and antioxidative properties. Rb2 suppresses hepatic gluconeogenesis in hyperlipidemic conditions, known as an in vitro model of type 2 diabetes, through AMP-activated protein kinase-induced orphan nuclear receptor small heterodimer partner by relieving endoplasmic reticulum stress, a cause of gluconeogenesis, which may be partly responsible for the use of ginsenosides as antidiabetic treatments.Citation1) It was also identified to diminish glycosemia in streptozotocin-induced diabetic rats.Citation2) Rb2 diminishes invasiveness to the basement membrane of endometrial cancer cells through down-regulation of the expression and activity of matrix metalloproteinase-2 (MMP-2) without altering the expression of tissue inhibitors of metalloproteinase-1 and metalloproteinase-2, indicating its plausible use for inhibition of secondary spreading of uterine endometrial cancers.Citation3) Rb2 inhibits angiogenesis and improves wound healing via enhancing epidermal cell proliferation by up-regulating the expression of protein factors related to cell proliferation, such as epidermal growth factor and its receptor, fibronectin and its receptor, keratin 5/14, and collagen 1.Citation4) The invasion of rat lung endothelial cells into the reconstituted basement membrane, an essential event in tumor neovascularization, is suppressed by Rb2, whereas it does not inhibit the haptotactic migration of endothelial cells to fibronectin substrate.Citation5) The down-regulation of tumor-associated angiogenesis by Rb2 might be partly responsible for the inhibition of lung tumor metastasis.Citation5) Rb2 enhances the cellular plasminogen activator activity as well as the surface plasmin activity in bovine aortic endothelial cells, implying that Rb2 has an enhancing role on the fibrinolytic activity of endothelial cells.Citation6) Rb2 was also shown to be the most effective among ginsenosides from red ginseng to protect the lethal infection of haemagglutinating virus of Japan in mice, suggesting that it is a promising candidate as a mucosal immunoadjuvant to strengthen antiviral activity.Citation7) Rb2 decreases the activities of single calcium channel and markedly antagonizes the enhancement of free radical contents induced by xanthine–xanthine oxidase.Citation8) The SOD1 gene encoding Cu/Zn-superoxide dismutase, one of the major antioxidant enzymes, is significantly activated by Rb2 through transcription factor AP2, involved in the response to oxidative stress, binding sites and its induction.Citation9)
Although reactive oxygen species (ROS) at physiological concentrations are in close association with a variety of crucial cellular functions, including redox regulation and intracellular signaling, excessive ROS, which are generated during abnormal metabolic reactions, result in various kinds of damages to macromolecules, leading to physiological dysfunction, genetic mutation, and, ultimately, cell death.Citation10) The intracellular ROS level is also elevated in the living cells exposed to oxidative stress-inducing agents. When the cellular defense systems cannot thoroughly cope with exogenously added stress-inducing agents, the cells undergo oxidative stress, which adversely affect many different cellular processes and functions.
In the present work, we report a novel skin anti-photoaging property of Rb2 through evaluating a scavenging activity on ROS and a diminishing activity on MMP-2 in the human epidermal keratinocytes under UV-B irradiation.
Materials and methods
Reagents
Ginsenoside Rb2, purity ≥98% was obtained from Ambo Institute (Seoul, Korea). Bovine serum albumin (BSA), gelatin, Bradford reagent, dimethyl sulfoxide, sodium dodecyl sulfate (SDS), sodium nitrite, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), rhodamine 123, 2′,7′-dichlorofluorescein diacetate (DCFH-DA), and Griess reagent were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO, USA). Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), and penicillin–streptomycin were from HyClone Laboratories Inc. (Logan, UT, USA). Cell lysis buffer was from Promega Korea (Seoul, Korea). All other chemicals used in this work were of the highest grade commercially available.
Cell culture
An immortalized human keratinocyte cell line, HaCaT (ATCC, Manassas, VA, USA), was grown in DMEM containing 10% heat-inactivated FBS, 100 U/mL penicillin and 100 μg/mL streptomycin in a humidified atmosphere with 5% CO2 at 37 °C. Prior to the treatments, the 1 × 105 HaCaT cells were typically seeded on 24-well plates, cultured overnight, washed twice with 1 mL phosphate-buffered saline (PBS), and replaced with 1 mL FBS-free medium. After UV-B irradiation and/or Rb2 treatment, the mammalian cells were grown under the same culture conditions described above.
UV-B irradiation
As a UV-B source, an ultraviolet lamp (peak, 312 nm; model VL-6M, Vilber Lourmat, Marine, France) was used. A radiometer (model VLX-3W, Vilber Lourmat, Marine, France) with a sensor (bandwidth, 280–320 nm; model CX-312, Vilber Lourmat, Marine, France) was used to monitor the radiation intensity. The mammalian cells used in this work were irradiated with 70 mJ/cm2 UV-B radiation at 25 °C.
Preparation of cellular lysates
For preparation of cellular lysates, adherent cells were washed twice with PBS and stored on ice for 5 min. The cells were harvested by scraping off the bottom of the dish with a cell scraper and centrifuged at 15,000 rpm for 10 min. The cell pellets were resuspended in cell lysis buffer [25 mM Tris-phosphate (pH 7.8), 2 mM CDTA, 2 mM DTT, 10% glycerol, 1% Trition X-100] and stored for 30 min on ice. Cellular lysates were taken by centrifugation at 15,000 rpm for 15 min.
Protein contents in cellular lysates were measured according to the procedure of BradfordCitation11) using BSA as a standard.
Determination of intracellular ROS
To fluorometrically determine the intracellular ROS in cultured keratinocytes, a redox-sensitive fluorescent probe DCFH-DA, which produces the fluorescent 2′,7′-dichlorofluorescein (DCF; λexcitation = 485 nm, λemission = 530 nm) upon enzymatic reduction and subsequent oxidation by ROS, was used as previously described.Citation12) After the treatment with Rb2 and/or 20 μM DCFH-DA for 30 min at 37 °C, the cells were twice washed with 1 mL FBS-free medium. The cells were resuspended in 1 mL FBS-free medium and irradiated with 70 mJ/cm2 UV-B. The intracellular ROS levels were immediately quantitated by Multi-Mode Microplate Reader (Synergy™ Mx, BioTek Instruments, Winooki, VT, USA).
In confocal microscopic analysis, the cells were treated with Rb2 and/or 20 μM rhodamine 123 for 30 min at 37 °C, irradiated with 70 mJ/cm2 UV-B, and immediately analyzed using Confocal Laser Scanning Microscope (Fluoview-FV300, Olympus, Tokyo, Japan). The assays were repeated at least three times.
Cell viability assay
To determine the cellular survivals of the keratinocytes in the presence of Rb2, the cell viability was determined using MTT assay which is used to assess metabolic activity.Citation13) The cells were treated with Rb2 for 30 min. After removing the medium by suction, the cells were treated with 5 μg/mL MTT in medium for 4 h. The cells were then lysed with dimethyl sulfoxide, and the amount of formazan, generated from the reduction of MTT by the mitochondria of living cells, was quantitated by the absorbance at a wavelength of 540 nm.
Gelatin zymography
The gelatinolytic activity of MMP-2 in culture supernatants was determined using zymographic analysis as previously described.Citation14) The cells were further incubated for 24 h at 37 °C and twice washed with 1 mL PBS. The cells in 1 mL FBS-free medium were treated with Rb2 for 30 min and irradiated with 70 mJ/cm2 UV-B. The culture supernatants, taken from the irradiated culture incubated for 24 h at 37 °C, were fractionated on 10% (w/v) SDS-PAGE gel impregnated with 1 mg/mL gelatin under a non-reducing condition. The proteins in the gel were renatured by shaking with 2.5% Triton X-100 at room temperature for 30 min, which was repeated two times, and incubated in incubation buffer (50 mM Tris buffer, pH 7.8, 5 mM CaCl2, 0.15 M NaCl, 1% Triton X-100) for 24 h. After the gel was stained with 0.1% Coomassie Brilliant Blue R-250, gelatin-degrading enzyme activities were convinced as clear zones against a blue background. MMP-2 activity band was identified in accordance with its molecular mass, which was estimated by molecular mass markers.
Western blotting analysis
In order to detect MMP-2 in conditioned medium and cellular lysate, Western blotting analysis was performed using anti-MMP-2 (ALX-210-753, Enzo Life Sciences, Farmingdale, NY, USA) and anti-GAPDH (LF-PA0212, Young In Frontier, Seoul, Korea) antibody as primary antibodies. Both culture supernatants and cellular lysates were run on 10% (w/v) SDS-PAGE and electrotransferred to PVDF membranes. The membranes were blocked with blocking buffer (2% BSA in 1× TBS-Tween 20), probed with primary antibody overnight at 4 °C, incubated with secondary antibody (goat anti-rabbit IgG-pAb-HRP-conjugate; ADI-SAB-300, Enzo Life Sciences, Farmingdale, NY, USA) for 1 h at room temperature, and developed with the use of an enhanced West-save up™ (AbFrontier, Seoul, Korea).
Statistical analysis
The results were represented as mean ± SD. Comparisons between experimental groups were statistically analyzed using unpaired Student’s t-test. A p value less than 0.05 was considered statistically significant.
Results
Cellular survival
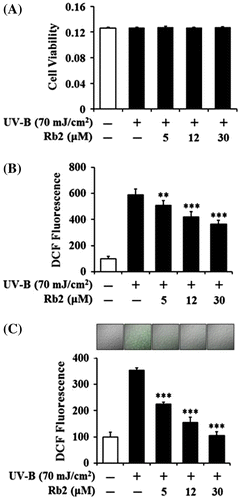
To determine whether UV-B radiation at the used intensity and Rb2 at the used concentrations display cytotoxicity on HaCaT cells or not, their effects on the viability of HaCaT cells were determined using MTT assay. As shown in Fig. (A), UV-B irradiation only could not make a change in the viability of the HaCaT cells, and their viabilities remained to be similar to that of the non-irradiated cells. Rb2 was not able to alter the viability of the HaCaT cells under UV-B irradiation (Fig. (A)). Collectively, UV-B irradiation and Rb2, at the used conditions, exhibit no significant cytotoxicity on keratinocytes.
Fig. 2. Effects of Rb2 on cellular viability (A) and ROS generation (B, C) in the human HaCaT keratinocytes under irradiation with 70 mJ/cm2 UV-B.
Notes: In (A), the viable cell number, represented as the relative percentages, was determined using MTT assay. The intracellular ROS levels were determined using DCFH-DA in a microplate fluorometer (B) and using rhodamine 123 followed by confocal laser scanning microscopic analysis (C). In the lower panel of (C), the ROS-associated fluorescent signals, expressed as percentage control, were quantified using Adobe Photoshop software (Adobe Systems, Mountain View, CA). The ROS level was represented as DCF fluorescence, an arbitrary unit. **p < 0.01; ***p < 0.001 vs. the non-treated control (UV-B irradiation only).
Scavenging activity on the UV-B-induced ROS in keratinocytes
The cultured HaCaT cells were pretreated with varying concentrations (0, 5, 12, or 30 μM) of Rb2 prior to the irradiation with 70 mJ/cm2 UV-B. As shown in Fig. (B), the UV-B irradiation, in the absence of Rb2 pretreatment, could give rise to about 5.9-fold enhancement in the ROS level over that in the non-irradiated control HaCaT cells. Rb2 attenuated the UV-B-induced ROS enhancement in a concentration-dependent manner (Fig. (B)). Pretreatment with Rb2 at the concentrations of 5, 12, and 30 μM reduced the UV-B-induced ROS enhancement to 86.5, 71.4, and 62.9%, respectively (Fig. (B)). This reducing activity of Rb2 on the UV-B-induced ROS was also ascertained by confocal microscopic analysis using rhodamine 123. When the cultured HaCaT cells were pretreated with Rb2 at the concentrations of 5, 12, and 30 μM in the confocal microscopic analysis, the ROS levels were reduced to 63.8, 44.0, and 29.8%, respectively, of the UV-B-induced ROS level (Fig. (C)). As shown in Fig. (C), fluorescence intensities on the microscopic patterns were serially weakened as Rb2 concentrations went up. Taken together, Rb2 plays a scavenging role on the ROS levels of keratinocytes under UV-B irradiation.
Diminishing effects on MMP-2
Since UV irradiation is capable of enhancing expression of certain MMP family members, such as MMP-9, MMP-2, and MMP-1, which degrade collagen and other extracellular matrix proteins that compose the dermal connective tissue, chronic exposure to UV radiation impairs normal architecture of the skin, leading to skin photoaging.Citation15) Rb2 attenuated the UV-B-enhanced MMP-2 gelatinolytic activity in a concentration-dependent manner in keratinocytes (Fig. ). Since Rb2 appeared to diminish the UV-B-enhanced MMP-2 enzyme activity, MMP-2 protein levels in both conditioned medium and cellular lysate of keratinocytes were examined using Western blotting analysis (Fig. ). As expected, the UV-B radiation significantly enhanced the MMP-2 protein levels in both conditioned medium and cellular lysate (Fig. ). Rb2, at the concentrations of 5, 12, and 30 μM, could reduce the UV-B-enhanced MMP-2 protein levels in conditioned medium in a concentration-dependent manner (Fig. (A)). As shown in Fig. (B), Rb2, at the same concentrations, was able to diminish the UV-B-enhanced MMP-2 protein levels in cellular lysates. Collectively, Rb2 is able to down-regulate the UV-B-enhanced MMP-2 of HaCaT cells through diminishing production and subsequent secretion of MMP-2 protein.
Fig. 3. Effect of Rb2 on MMP-2 activity in the conditioned media prepared from the human HaCaT keratinocytes under irradiation with 70 mJ/cm2 UV-B.
Notes: (A) Mammalian cells were subjected to fresh media with the indicated concentrations (0, 5, 12, or 30 μM) of Rb2 for 30 min before the irradiation. The gelatinolytic activity of MMP-2 in the conditioned medium was detected using gelatin zymography. The relative band strength was determined with densitometry using the ImageJ software which can be downloaded from the NIH website. (B) The equal loading of conditioned media was shown by silver staining. *p < 0.05; **p < 0.01; ***p < 0.001 vs. the non-treated control (UV-B irradiation only).
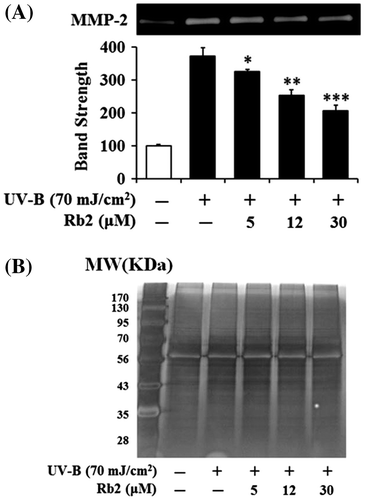
Fig. 4. Effects of Rb2 on MMP-2 protein levels in the conditioned media (A) and cellular lysates (B) prepared from the human HaCaT keratinocytes under irradiation with 70 mJ/cm2 UV-B.
Notes: Mammalian cells were subjected to fresh media with the indicated concentrations (0, 5, 12, or 30 μM) of Rb2 for 30 min before the irradiation. The MMP-2 proteins were determined using Western blotting analysis with anti-MMP-2 antibodies. In (B), GAPDH was used as a protein loading control. In the lower panels of both (A) and (B), the relative band strength was determined with densitometry using the ImageJ software which can be downloaded from the NIH website. The equal loading of conditioned media was shown in Fig. (B). *p < 0.05; **p < 0.01; ***p < 0.001 vs. the non-treated control (UV-B irradiation only).
Discussion
UV-B (280–315 nm) evokes a photooxidation reaction that disrupts the antioxidant status of the skin cells and enhances the cellular ROS level, sequentially accelerating skin photoaging and the development of malignant diseases.Citation16–19) Accordingly, UV-B is regarded as one of the well-known causes of adverse changes in the skin, such as wrinkle formation, coarseness, laxity, mottled pigmentation, degradation of matrix macromolecules, epidermal thickening, vascularization, and immunosuppression.Citation20,21) In keratinocytes, UV-B induces the immediate generation of superoxide radical, which is sequentially converted to other ROS species, such as hydrogen peroxide and hydroxyl radical.Citation22)
As people get older, their skins usually undergo photoaging, defined as premature aging of the skin due to repeated exposure to UV radiation from the sun and artificial UV source, in addition to chronological aging. UV-A and UV-B radiation from sunlight is responsible for roughly 90% of the symptoms of skin photoaging.Citation23,24) UV radiation, including UV-B, increases production of one or more MMPs and then gives rise to damages to the skin, leading to skin photoaging. UV-A activates MMP-1 through the involvement of oxidative stress, under which ROS are excessively produced and/or antioxidant functions are depleted in the skin cells such as keratinocytes and fibroblasts.Citation25) Several extracts of plant origin were previously shown to possess skin anti-photoaging activities. An extract of Butea monosperma (Lam.) Taub. flowers is capable of reducing the secretion of MMP-1, MMP-2, MMP-9, and MMP-10 in UV-B-treated normal human epidermis keratinocytes.Citation26) An ethanol extract of the red alga Bonnemaisonia hamifera protects HaCaT cells against UV-B-induced oxidative damage by scavenging ROS and absorbing UV-B photons.Citation27) An aqueous extract of Labisia pumila restores UV-B-suppressed collagen synthesis of human fibroblasts to normal levels and down-regulated UV-B-induced MMP-9 expression in HaCaT cells.Citation28) An ethanol extract of Gynura procumbens (Lour.) Merr. suppresses UV-B-induced MMP-1 expression, MMP-9 activity, and ROS production in human primary dermal fibroblasts.Citation29)
In the present work, UV-B irradiation at the intensity of 70 mJ/cm2 markedly enhanced the ROS levels in HaCaT cells, which was detected using both fluorometric and confocal microscopic analyses. The current results reconfirm that the cellular damages caused by UV-B irradiation might be mediated by intracellular ROS. Although Rb2 was identified to scavenge the UV-B-induced ROS in HaCaT cells, mechanism(s) responsible for the ROS-scavenging effect of Rb2 currently remain elusive. One possibility is that Rb2 inhibits the enzymatic activities responsible for the generation of ROS. Another possibility is that Rb2 directly reacts with ROS although seemingly improbable. A third possibility is that Rb2 activates or induces antioxidants which abolish the ROS species, such as glutathione and superoxide dismutase. This possibility might be supported by the previous finding that Rb2 greatly activates the SOD1 gene through enhancing the specific binding of transcription factor AP2 to the proximal promoter.Citation9) The low ROS levels, diminished by Rb2, might sequentially play a suppressive role in UV-B-induced MMP-2 enhancement in keratinocytes, implying a plausible skin anti-photoaging property of Rb2.
Matrix metalloproteinases (MMPs) are a large and complex family of zinc-dependent endopeptidases, which are capable of degrading essentially all components of extracellular matrix.Citation30) Skin photoaging is associated with deterioration of the dermal extracellular matrix due to increased MMP expression and decreased collagen synthesis under oxidative stress.Citation31,32) UV radiation also induces expression and secretion of MMP-1, MMP-2, MMP-3, MMP-9, and MMP-13 in the epidermis and dermis, which contribute to skin damage and photoaging.Citation33,34) Collagen degradation, considered as a cause of aging in both naturally aged and photoaged skin, is related to the induction of MMPs which are secreted from epidermal keratinocytes and dermal fibroblasts.Citation35)
MMP-2 (64 kD) is secreted as proMMP-2 (72 kD) and located on the cell surface and requires activation to exert its catalytic activation.Citation36) After proMMP-2 is recruited to the cell surface by interacting with tissue inhibitor of metalloproteinase-2 (TIMP-2) bound to membrane type 1 MMP (MT1-MMP) by forming a ternary complex, it is activated on the cell surface by free MT1-MMP closely located to the ternary complex.Citation37) MT1-MMP is currently known to be the most common physiological activator of proMMP-2.Citation38) Type 1 collagen stimulates proteolytic activation of constitutively secreted proMMP-2 on the cell surface.Citation39) The induction of TIMP-2 by cholesterol causes the conversion of proMMP-2 into active MMP-2 through the mediation of JNK and ERK in human dermal fibroblasts.Citation40) Independently of the MT1-MMP/TIMP-2 pathway, activation of proMMP-2 by snake venom proteinases is also known to be another mechanism in human fibroblasts.Citation41)
In this work, MMP-2 level is enhanced in HaCaT cells by UV-B irradiation and the enhanced MMP-2 level is down-regulated by Rb2. Analogously with this finding, the involvement of MMP-2 in the response to UV-B irradiation has been verified. Enhanced production of MMP-2 but not MMP-9 was found in human corneal fibroblasts in response to UV-B, especially in the acute phase after the irradiation.Citation42) A daily use cream with a photostable combination of UV-A and UV-B absorbers diminishes the MMP-2 mRNA induced in the buttock skin biopsies of healthy volunteers exposed to solar simulated radiation.Citation43) Sargachromenol, purified from Sargassum horneri, plays a down-regulating role in UV-B-induced MMP-2 mRNA level in dermal fibroblasts.Citation44)
In conclusion, the skin anti-photoaging activity of Rb2 was assessed in the HaCaT cells exposed to UV-B radiation. Rb2 suppresses the UV-B-induced ROS level and MMP-2 activity and expression. Rb2 might elicit its skin anti-photoaging property through the down-regulation of UV-B-induced MMP-2 by an ROS-dependent mechanism. Elucidation of precise mechanism(s) on these effects of Rb2 will require further experimental approaches. Rb2 would be a potential candidate used as a natural resource for manufacturing anti-photoaging cosmetics.
Author contribution
C.L. and K.K. designed the research and wrote the paper. S.O. performed the experiments.
Funding
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea [grant number A103017]; also by 2014 Research Grant from Kangwon National University [grant number 120140161].
Acknowledgment
The authors are grateful to Mr Sihyeong Lee for his technical assistance.
References
- Lee KT, Jung TW, Lee HJ, Kim SG, Shin YS, Whang WK. The antidiabetic effect of ginsenoside Rb2 via activation of AMPK. Arch. Pharm. Res. 2011;34:1201–1208.10.1007/s12272-011-0719-6
- Yokozawa T, Kobayashi T, Oura H, Kawashima Y. Studies on the mechanism of the hypoglycemic activity of ginsenoside-Rb2 in streptozotocin-diabetic rats. Chem. Pharm. Bull. 1985;33:869–872.10.1248/cpb.33.869
- Fujimoto J, Sakaguchi H, Aoki I, Toyoki H, Khatun S, Tamaya T. Inhibitory effect of ginsenoside-Rb2 on invasiveness of uterine endometrial cancer cells to the basement membrane. Eur. J. Gynaecol. Oncol. 2001;22:339–341.
- Choi S. Epidermis proliferative effect of the Panax ginseng ginsenoside Rb2. Arch. Pharm. Res. 2002;25:71–76.10.1007/BF02975265
- Sato K, Mochizuki M, Saiki I, Yoo YC, Samukawa K, Azuma I. Inhibition of tumor angiogenesis and metastasis by a saponin of Panax ginseng, ginsenoside-Rb2. Biol. Pharm. Bull. 1994;17:635–639.10.1248/bpb.17.635
- Liu JW, Wei DZ, Du CB, Zhong JJ. Enhancement of fibrinolytic activity of bovine aortic endothelial cells by ginsenoside Rb2. Acta Pharmacol. Sin. 2003;24:102–108.
- Yoo YC, Lee J, Park SR, Nam KY, Cho YH, Choi JE. Protective effect of ginsenoside-Rb2 from Korean red ginseng on the lethal infection of haemagglutinating virus of Japan in mice. J. Ginseng Res. 2013;37:80–86.10.5142/jgr.2013.37.80
- Wang XM, Qi Y, Sun CW, Zhong GG, Jiang Y, Qiu YH. Single calcium channel analysis and electron spin resonance (ESR) spectral study on the myocardial effects of ginsenoside Rb2. Zhongguo Zhong Yao Za Zhi. 1994;19:621–624.
- Kim YH, Park KH, Rho HM. Transcriptional activation of the Cu, Zn-superoxide dismutase gene through the AP2 site by ginsenoside Rb2 extracted from a medicinal plant, Panax ginseng. J. Biol. Chem. 1996;271:24539–24543.
- Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001;31:1287–1312.10.1016/S0891-5849(01)00724-9
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254.10.1016/0003-2697(76)90527-3
- Royall JA, Ischiropoulos H. Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch. Biochem. Biophys. 1993;302:348–355.10.1006/abbi.1993.1222
- Freshney RI. Culture of animal cells: a manual of basic technique. 4th ed. New York (NY): Wiley-Liss Press; 1994.
- Kleiner DE, Stetler-Stevenson WG. Quantitative zymography: detection of picogram quantities of gelatinases. Anal. Biochem. 1994;218:325–329.10.1006/abio.1994.1186
- Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. Matrix-degrading metalloproteinases in photoaging. J. Invest. Dermatol. Symp. Proc. 2009;14:20–24.10.1038/jidsymp.2009.8
- Birch-Machin MA, Swalwell H. How mitochondria record the effects of UV exposure and oxidative stress using human skin as a model tissue. Mutagenesis. 2010;25:101–107.10.1093/mutage/gep061
- Barresi C, Stremnitzer C, Mlitz V, Kezic S, Kammeyer A, Ghannadan M, Posa-Markaryan K, Selden C, Tschachler E, Eckhart L. Increased sensitivity of histidinemic mice to UVB radiation suggests a crucial role of endogenous urocanic acid in photoprotection. J. Invest. Dermatol. 2011;131:188–194.10.1038/jid.2010.231
- Ikehata H, Ono T. The mechanisms of UV mutagenesis. J. Radiat. Res. 2011;52:115–125.10.1269/jrr.10175
- Peres PS, Terra VA, Guarnier FA, Cecchini R, Cecchini AL. Photoaging and chronological aging profile: understanding oxidation of the skin. J. Photochem. Photobiol. B. 2011;103:93–97.10.1016/j.jphotobiol.2011.01.019
- Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19:176–194.10.1096/fj.04-2079rev
- Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J. Am. Acad. Dermatol. 2006;55:1–19.10.1016/j.jaad.2005.05.010
- Aitken GR, Henderson JR, Chang SC, McNeil CJ, Birch-Machin MA. Direct monitoring of UV-induced free radical generation in HaCaT keratinocytes. Clin. Exp. Dermatol. 2007;32:722–727.10.1111/ced.2007.32.issue-6
- Jenkins G. Molecular mechanisms of skin ageing. Mech. Ageing Develop. 2002;123:801–810.10.1016/S0047-6374(01)00425-0
- Rittié L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002;1:705–720.10.1016/S1568-1637(02)00024-7
- Pygmalion MJ, Ruiz L, Popovic E, Gizard J, Portes P, Marat X, Lucet-Levannier K, Muller B, Galey JB. Skin cell protection against UVA by sideroxyl, a new antioxidant complementary to sunscreens. Free Radic. Biol. Med. 2010;49:1629–1637.10.1016/j.freeradbiomed.2010.08.009
- Krolikiewicz-Renimel I, Michel T, Destandau E, Reddy M, André P, Elfakir C, Pichon C. Protective effect of a Butea monosperma (Lam.) Taub. flowers extract against skin inflammation: antioxidant, anti-inflammatory and matrix metalloproteinases inhibitory activities. J. Ethnopharmacol. 2013;148:537–543.10.1016/j.jep.2013.05.001
- Piao MJ, Hyun YJ, Cho SJ, Kang HK, Yoo ES, Koh YS, Lee NH, Ko MH, Hyun JW. An ethanol extract derived from Bonnemaisonia hamifera scavenges ultraviolet B (UVB) radiation-induced reactive oxygen species and attenuates UVB-induced cell damage in human keratinocytes. Mar. Drugs. 2012;10:2826–2845.10.3390/md10122826
- Choi HK, Kim DH, Kim JW, Ngadiran S, Sarmidi MR, Park CS. Labisia pumila extract protects skin cells from photoaging caused by UVB irradiation. J. Biosci. Bioeng. 2010;109:291–296.10.1016/j.jbiosc.2009.08.478
- Kim J, Lee CW, Kim EK, Lee SJ, Park NH, Kim HS, Kim HK, Char K, Jang YP, Kim JW. Inhibition effect of Gynura procumbens extract on UV-B-induced matrix-metalloproteinase expression in human dermal fibroblasts. J. Ethnopharmacol. 2011;137:427–433.10.1016/j.jep.2011.04.072
- Curran S, Murray GI. Matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 1999;189:300–308.10.1002/(ISSN)1096-9896
- Chae S, Piao MJ, Kang KA, Zhang R, Kim KC, Youn UJ, Nam KW, Lee JH, Hyun JW. Inhibition of matrix metalloproteinase-1 induced by oxidative stress in human keratinocytes by mangiferin isolated from Anemarrhena asphodeloides. Biosci. Biotechnol. Biochem. 2011;75:2321–2325.10.1271/bbb.110465
- Kim MS, Oh GH, Kim MJ, Hwang JK. Fucosterol inhibits matrix metalloproteinase expression and promotes type-1 procollagen production in UVB-induced HaCaT cells. Photochem. Photobiol. 2013;89:911–918.10.1111/php.12061
- Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997;337:1419–1428.10.1056/NEJM199711133372003
- Lee YM, Kang SM, Lee SR, Kong KH, Lee JY, Kim EJ, Chung JH. Inhibitory effects of TRPV1 blocker on UV-induced responses in the hairless mice. Arch. Dermatol. Res. 2011;303:727–736.10.1007/s00403-011-1153-9
- Lee YM, Kim YK, Kim KH, Park SJ, Kim SJ, Chung JH. A novel role for the TRPV1 channel in UV-induced matrix metalloproteinase (MMP)-1 expression in HaCaT cells. J. Cell. Physiol. 2009;219:766–775.10.1002/jcp.v219:3
- Ellenrieder V, Alber B, Lacher U, Hendler SF, Menke A, Boeck W, Wagner M, Wilda M, Friess H, Büchler M, Adler G, Gress TM. Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int. J. Cancer. 2000;85:14–20.10.1002/(ISSN)1097-0215
- Nagase H. Cell surface activation of progelatinase A (proMMP-2) and cell migration. Cell Res. 1998;8:179–186.10.1038/cr.1998.18
- Sato H, Takino T. Coordinate action of membrane-type matrix metalloproteinase-1 (MT1-MMP) and MMP-2 enhances pericellular proteolysis and invasion. Cancer Sci. 2010;101:843–847.10.1111/j.1349-7006.2010.01498.x
- Morley ME, Riches K, Peers C, Porter KE. Hypoxic inhibition of human cardiac fibroblast invasion and MMP-2 activation may impair adaptive myocardial remodelling. Biochem. Soc. Trans. 2007;35:905–957.
- Kim S, Oh JH, Lee Y, Lee J, Cho KH, Chung JH. Induction of tissue inhibitor of matrix metalloproteinase-2 by cholesterol depletion leads to the conversion of proMMP-2 into active MMP-2 in human dermal fibroblasts. Exp. Mol. Med. 2010;42:38–46.10.3858/emm.2010.42.1.004
- Saravia-Otten P, Frisan T, Thelestam M, Gutiérrez JM. Membrane independent activation of fibroblast proMMP-2 by snake venom: novel roles for venom proteinases. Toxicon. 2004;44:749–764.10.1016/j.toxicon.2004.08.002
- Koźák I, Klisenbauer D, Juhás T. UV-B induced production of MMP-2 and MMP-9 in human corneal cells. Physiol. Res. 2003;52:229–234.
- Seité S, Colige A, Piquemal-Vivenot P, Montastier C, Fourtanier A, Lapière C, Nusgens B. A full-UV spectrum absorbing daily use cream protects human skin against biological changes occurring in photoaging. Photodermatol. Photoimmunol. Photomed. 2000;16:147–155.10.1034/j.1600-0781.2000.160401.x
- Kim JA, Ahn BN, Kong CS, Kim SK. Protective effect of chromene isolated from Sargassum horneri against UV-A-induced damage in skin dermal fibroblasts. Exp. Dermatol. 2012;21:630–631.10.1111/j.1600-0625.2012.01535.x