Abstract
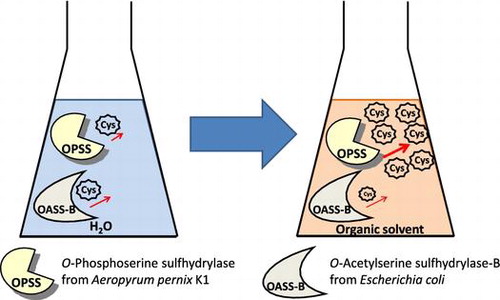
O-phospho-l-serine sulfhydrylase (OPSS) from archaeon Aeropyrum pernix K1 is able to synthesize l-cysteine even at 80 °C. In this article, we compared thermal stability and reactivity in organic solvent of OPSS with those of O-acetyl-l-serine sulfhydrylase B (OASS-B) from Escherichia coli. As a result, the thermostability of OPSS was much higher than that of OASS-B. Moreover, the activity of OPSS increased in the reaction mixture containing the organic solvent, such as N, N′-dimethyl formamide and 1,4-dioxane, whereas that of OASS-B gradually decreased as the content of organic solvent increased. From the crystal structural analysis, the intramolecular electrostatic interactions of N-terminal domain in OPSS seemed to be correlated with the tolerance of OPSS to high temperature and organic solvent. These results indicate that OPSS is more superior to OASS-B for the industrial production of l-cysteine and unnatural amino acids that are useful pharmaceuticals in the presence of organic solvent.
OPSS from hyperthermophilic archaeon is able to synthesize l-cysteine more efficiently in organic solvent than OASS-B from Escherichia coli.
The hyperthermophilic aerobic archaeon Aeropyrum pernix K1, whose genome sequence has been analyzed,Citation1) has an open reading frame (APE1586) assigned to a putative O-acetyl-l-serine sulfhydrylase (OASS; EC 2.5.1.47) that synthesizes l-cysteine from O-acetyl-l-serine (OAS) and sulfide in bacteria and higher plants. We have cloned and overexpressed the recombinant enzyme by using Escherichia coli and analyzed its function.Citation2−4) Unexpectedly, we found that this enzyme has an extremely high ability to convert O-phospho-l-serine (OPS) and sulfide into l-cysteine.Citation3) This was the first report of an enzyme catalyzing an OPS sulfhydrylation reaction and then, we concluded that this is a new enzyme that should be named O-phospho-l-serine sulfhydrylase (OPSS; newly assigned as EC 2.5.1.65). The three-dimensional (3D) structure of OPSS has also been determined recently and its substrate recognition mechanism is discussed.Citation5,6)
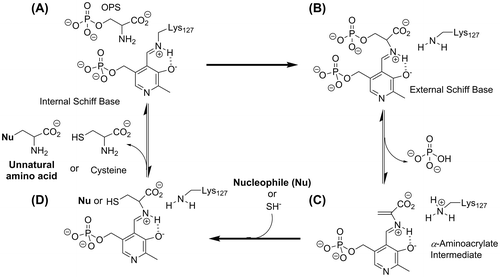
Both the OPS and OAS sulfhydrylation reactions for OPSS follow the ping-pong bi-bi mechanism as observed in other known OASS (Fig. ).Citation3,7−9) The active site of OPSS contains pyridoxal 5′-phosphate (PLP), as a cofactor, linked to lysine (K127) as an internal Schiff base (Fig. (A)). Binding of OPS displaces the lysine and forms an external Schiff base (Fig. (B)), initiating the first half-reaction that yields an α-aminoacrylate intermediate linked to PLP (Fig. (C)). The second half-reaction involves addition of sulfide to the intermediate, and generates an external Schiff base with l-cysteine (Fig. (D)). The active-site lysine reacts with this intermediate, releasing l-cysteine and regenerating the internal Schiff base with K127.Citation6)
OASS has been demonstrated to produce novel β-substituted l-amino acids when offered unnatural nucleophiles instead of sulfide.Citation10,11) Interestingly, this property is useful in producing pharmaceuticals, such as mucolytic agent l-carbocisteine and building blocks for the synthesis of pharmaceuticals and agrochemicals.Citation12) Like other OASSs, OPSS is able to catalyze the synthetic reactions of various unnatural amino acids from OPS and nucleophiles that can substitute for sulfide (Fig. (C) and (D), Nakamura, T. and Ishikawa, K., unpublished results). Due to its thermal stability, OPSS can be used for a more efficient production of unnatural amino acids compared with that of E. coli.
For producing l-cysteine and unnatural amino acids, it is very important for OPSS to be stable to stresses, such as heat and organic solvent. Here, we examined the stability of OPSS activity to l-cysteine synthesis toward the above stresses, comparing it to that of an OASS from E. coli. There are two kinds of l-cysteine synthase OASS-A and OASS-B in bacteria and plant. In this study, we selected OASS-B from E. coli for comparison with OPSS because E. coli OASS-A and OASS-B are very useful enzymes for unnatural amino acid synthesisCitation12) and the residues near the active site of OASS-B is more similar to OPSS than that of OASS-A. For example, OPSS has an arginine (Arg297) near the entrance of its active site and this residue is suggested to be very important for OPS recognition. OASS-B has the corresponding arginine near the active site (Arg210), but OASS-A does not have it.Citation13) In this paper, we purified histidine-tagged OASS-B (His-OASS-B) and OPSS and then, their stability to heat and reactivity in organic solvent were investigated.
Materials and methods
Materials
OPS was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan); OAS from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan) and MP Biomedicals Inc. (Irvine, CA, USA); PLP from Nacalai Tesque Inc. (Kyoto, Japan); and Ethylenediaminetetraacetic acid (EDTA) from Dojindo Laboratories (Kumamoto, Japan). Restriction enzymes and kits for genetic manipulation were purchased from Takara Bio Inc. (Shiga, Japan), Toyobo Co., Ltd. (Osaka, Japan), Merck (Darmstadt, Germany), and GE Healthcare UK, Ltd. (Buckinghamshire, UK). The synthetic primers used were from Sigma Genesis (Hokkaido, Japan). All other reagents were of analytical grade and obtained from Nacalai and Wako.
Plasmid construction
OASS-B gene was amplified by PCR with DNA from E. coli strain W3110 as a template, KOD plus from Toyobo and the following primer pairs, 5′-TTT GGA TCC ATG AGT ACA TTA GAA CAA ACA-3′ and 5′-TTT CTG CAG TTA AAT CCC CGC CCC CT-3′. Underlined sequences indicate the restriction enzyme site, BamHI and PstI, respectively. The composition of PCR reaction solution was according to the procedure recommended by the manufacturer except MgSO4 concentration modified to 1.2 mM. PCR was performed by using these primers for 30 cycles of denaturation at 94 °C for 15 s, annealing at 41 °C for 30 s, and extension at 68 °C for 1 min. The PCR product was digested with PstI and BamHI, and the 0.91-kb fragment containing the full-length OASS-B sequence was ligated into pUC19 (Takara Bio Inc.) digested with PstI and BamHI. The resultant plasmid was designated as pUC19/cysM. The E. coli JM109 (Toyobo) was transformed with the pUC19/cysM. The sequence of cloned OASS-B gene (cysM) was confirmed by the dideoxy chain termination method with an automated DNA sequencer (ABI PRISM 3100 Genetic Analyzer, Applied Biosystems, CA), compared with the known cysM (Accession number: NC_007779, Gene site:2544118 -2545029, Gene ID:12930332). A plasmid having the best sequencing result was digested with PstI and BamHI, and then ligated into pQE80L (QIAGEN, Hilden, Germany). The resultant plasmid was again confirmed by DNA sequencing and designated as pQE80L/cysM, and the pQE80L/cysM was transformed into the E. coli BL21 (DE3) pLysS (Merck).
Purification of enzymes
The recombinant E. coli BL21 (DE3) pLysS cells that express His-OASS-B were grown in 200 mL of lysogeny broth medium containing 50 μg mL−1 carbenicillin, with 130 strokes min−1 reciprocal shaking at 37 °C. The incubation was allowed to proceed until the absorbance of the culture broth at 600 nm reached 0.6, and then, carbenicillin and isopropyl-β-d-thiogalactopyranoside were added to the culture at a final concentration of 50 μg mL−1 and 1 mM, respectively. The culture was incubated for a further 4 h and the cells were collected by centrifugation at 5500 × g for 5 min at 4 °C. The collected cells were resuspended in binding/wash buffer (50 mM potassium phosphate (KPi), 0.3 M NaCl, 5 mM imidazole, and 0.2 mM PLP, pH 7.0) and sonicated with ultrasonic generator US-150T (NIHONSEIKI KAISHA Ltd., Tokyo, Japan). The suspension was centrifuged at 18,000 × g for 20 min at 4 °C.
The expressed His-OASS-B was purified from the culture supernatant by Ni2+ attachment to immobilized metal affinity chromatography (IMAC) resins (Bio-Rad, CA, USA) according to the batch-mode procedure recommended by the manufacturer. The resins were washed by the binding/wash buffer and His-OASS-B was eluted by elution buffer (50 mM KPi, 0.3 M NaCl, 0.5 M imidazole, and 0.2 mM PLP, pH 8.0). Each fraction was verified by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The His-OASS-B fractions were desalted by PD-10 columns (GE healthcare) and buffer was changed into 50 mM KPi buffer (pH 7.0) with 0.1 M PLP and 10% (w/v) glycerol. The solution was concentrated by Amicon Ultra-15 (Millipore, MA, USA) and stored at −80 °C. The amount of protein was determined using Coomassie brilliant blue solution for protein assay (Nacalai) with bovine serum albumin (Thermo Fisher Scientific Inc. IL, USA) as a standard.
The recombinant A. pernix OPSS was prepared by a PET system (Merck) and purified as described previously.Citation2)
Effect of thermal incubation on the stability of OPSS and His-OASS-B
The enzyme was incubated for 1 h at 0–70 °C for His-OASS-B (0.38 mg mL−1), and at 0–100 °C for OPSS (0.49 mg mL−1). Then the remaining activity of OPSS and His-OASS-B was measured. In the case of OPSS, the amount of OPSS and final concentrations of OPS and Na2S were 150 ng (18 nM) and 2.5 mM, respectively. The final volume of the reaction mixture was 180 μL. The reaction mixture was incubated at 80 °C for 3–5 min to maintain the conversion within 10%. In the case of His-OASS-B, the same protocol was used except 50 mM KPi (pH 6.7), and it was used as the reaction buffer instead of 50 mM KPi (pH 7.5) at 37 °C for 3 min. Both the buffers contained 0.2 mM PLP, 1 mM EDTA, and 1 mM dithiothreitol. Moreover, 50 ng (7.7 nM) of His-OASS-B and 2.5 mM OAS was used instead of OPSS and OPS. The amount of l-cysteine in the reaction mixture was determined according to the method of GaitondeCitation14) with slight modification and the specific activity was determined. A portion of the reaction mixture with the reagent was added to a 96-well plate and the absorbance at 560 nm was measured with VersaMAX (Molecular Devices Inc., CA, USA). Enzyme activity (1 U) was defined as the amount of enzyme required to produce 1 μmol of l-cysteine per 1 min.
Activity measurement in the presence of organic solvent
Dimethylformamide (DMF) and 1,4-dioxane were used as an organic solvent because these are aprotic polar solvent and have high boiling point, that is 153 and 101 °C, respectively. The activities of OPSS and His-OASS-B were measured in the presence of the organic solvent by a similar method without preincubation as mentioned above. The content of the organic solvent was 5, 10, 20, 30, 40 and 50% (v/v). In the case of OPSS, the amount of OPSS and final concentrations of OPS and Na2S were 140–560 ng (17–68 nM) and 2.5 mM, respectively. The final volume of the reaction mixture was 180 μL. The reaction mixture was incubated at 80 °C for 3 min to maintain the conversion within 10%.
For l-cysteine synthesis by His-OASS-B and OAS, similar protocol was used. Instead of 50 mM KPi buffer (pH 7.5), 50 mM KPi (pH 6.7) was used as the reaction buffer at 37 °C for 2 min (DMF) or 3 min (1,4-dioxane). The amounts of enzymes were 25 ng (3.8 nM) for DMF experiment. In the case of 1,4-dioxane, 25 ng of His-OASS-B was used for 5 and 10% of 1,4-dioxane (v/v) experiment; otherwise, 50 ng (7.7 nM) of the enzyme was used.
Investigation of solvent accessible surface area and electrostatic interaction in OPSS and OASS-B
The investigation of solvent accessible surface area (SASA) was performed by ProtSA server (HTML, http://webapps.bifi.es/protsa).Citation15,16) Intramolecular ionic interaction in OPSS and OASS-B was investigated using Protein Contacts panel in Molecular Operating Environment software (MOE, ver.2011.10; Chemical Computing, Group, Montreal Canada). The atomic coordinates of OPSS and OASS-B were obtained from the Protein Data Bank and the ID numbers of OPSS and OASS-B were 3VSA and 2V03, respectively.Citation6,17) Chain A of OPSS was used for the contact analysis. An ionic bond contact is defined by proximity, the distance between basic nitrogen atoms to acidic oxygen atom (distance cut-off 4.5 Å).
Results
Purification of His-OASS-B and comparison of the activity with other OASS-Bs
His-OASS-B overexpressed in E. coli BL21 (DE3) pLysS was purified by affinity chromatography and 38 mg of His-OASS-B was obtained from 200 mL of the culture medium (Fig. ). The activity of His-OASS-B to l-cysteine synthesis was compared with other E. coli OASS-Bs reported previously to investigate the effect of histidine tag to the activity. As a result, the activity of His-OASS-B was same to that of Strep-tag II® OASS-B reported by Maier,Citation12) which has additional eight amino acid residues that can bind strongly to Strep-Tactin®, and about 90% of OASS-B without tag reported by Zhao et al.Citation18) (Table ).
Fig. 2. Purification of His-OASS-B.
Notes: Samples at various stages during the purification procedure were subjected to SDS–PAGE (the result of 12.5% polyacrylamide gel). Lane 1, molecular weight marker; Lane 2, crude cellular lysate supernatant; Lane 3, the fraction of wash buffer; Lane 4, the fraction after the cell lysate supernatant was passed through the column; Lane 5, the fraction of elution buffer.
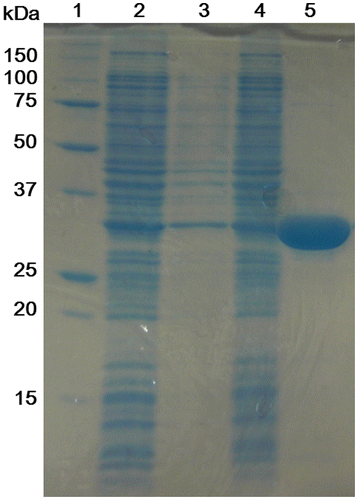
Table 1. Comparison of histidine-tagged OASS-B (His-OASS-B) with other reported OASS-B.
Comparison of thermal stability between His-OASS-B and OPSS
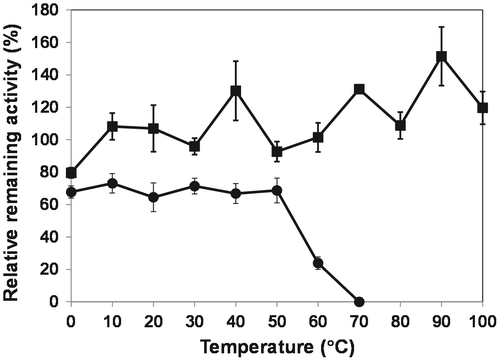
Subsequently, we compared the thermal stability of OPSS with His-OASS-B. The activity of OPSS slightly increased with a rise in temperature from 0 to 100 °C (Fig. ). Thus, our result is consistent with the previous report, where OPSS retained around 90% of its activity after the enzyme solutions had been incubated for 6 h at 100 °C and pH 6.1 or 6.7.Citation2) On the other hand, His-OASS-B was stable in the temperature range of 0–50 °C. However, when it was incubated at 60 or 70 °C, the precipitate was observed and the activity was completely lost at 70 °C (Fig. ). Therefore, our result indicates that OPSS is more thermostable than OASS-B.
Fig. 3. Thermal stability of OPSS (squares) and His-OASS-B (circles).
Notes: The enzymes were incubated for 1 h at temperatures from 0 to 100 °C for OPSS, and from 0 to 70 °C for His-OASS-B, respectively. The relative remaining activities were determined from the activity without incubation as 100. Results are expressed as the mean ± standard deviation from three replicates.
Comparison of the reactivity in organic solvent between His-OASS-B and OPSS
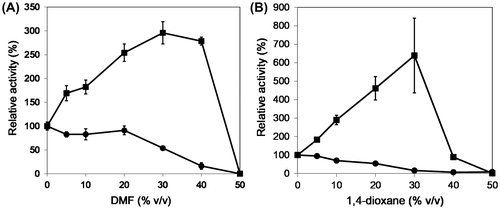
To use OPSS for the production of various unnatural amino acids, the problem that some nucleophiles, such as thiophenol for S-phenyl-L-cysteine, do not dissolve in the reaction buffer containing OPSS and OPS should be solved. OPSS would be very useful if it works even in the reaction mixture containing organic solvent that can dissolve hydrophobic nucleophile. Moreover, it is preferable for the organic solvents to have high boiling point because the reaction temperature of unnatural amino acid synthesis by OPSS can be set to around 80 °C, due to its high activity and thermostability as shown above. Therefore, we selected two kinds of organic solvents, DMF and 1,4-dioxane. Another reason for the selection of DMF and 1,4-dioxane is that these are aprotic solvent and do not decrease the nucleophilicity of nucleophiles, such as sulfide that attack the amino acrylate intermediate in the second half-reaction (Fig. ). Thus, the activity of OPSS and His-OASS-B to l-cysteine synthesis was measured in the various concentrations of these organic solvents. As a result, the activity of OASS-B decreased as the content of organic solvent increased. On the other hand, the activity of OPSS increased by three- to six-fold as the content of the organic solvent increased by 30% (v/v) (Fig. ). These results showed not only that OPSS is more thermostable than His-OASS-B, but also that it has higher reactivity than His-OASS-B in organic solvent. These properties are advantageous to synthesis of unnatural amino acids from hydrophobic nucleophile.
Fig. 4. The reactivity of OPSS and His-OASS-B in the presence of polar and aprotic solvent, such as (A) N, N′-dimethylformamide (DMF) and (B) 1,4-dioxane.
Notes: Filled squares and circles show the relative remaining activity of OPSS and His-OASS-B, respectively. The enzymes were incubated for 3 min at temperatures at 80 °C for OPSS and 30 °C for His-OASS-B in the solution containing the aprotic solvent by 0–50% (v/v). The relative activities were determined from the activity without organic solvent as 100. Results are expressed as the mean ± standard deviation from three replicates.
Discussion
His-OASS-B was purified by affinity chromatography with nickel column in this paper, and compared to the specific activity and thermal stability with previously reported OASS-B (Table , Fig. ).Citation18) As a result, it was found that our data were comparable to those reported previously. Thus, histidine tag in our study seemed to have little effect on the activity of OASS-B. However, His-OASS-B was less thermostable than OASS-B without His-tag that retained its activity at 60 °C and decreased by 40% at 70 °C compared to that without incubation (Fig. ).Citation18) The activity of OPSS increased slightly as the temperature increased and never decreased even at over 70 °C (Fig. ). Therefore, nothing is changed in that OPSS is more thermostable than OASS-B whether OASS-B is histidine-tagged or not, although the specific activity of His-OASS B (150 U/mg) without heat treatment was higher than that of OPSS (80 U/mg). OPSS should be useful in the production of cysteine and unnatural amino acids in the condition, where OASS-B is not able to maintain its activity such as at over 70 °C.
It is conventional idea that enzymes are denatured in organic solvent and they lose their native structures and thus catalytic activity, even though enzymes can work in organic solvents containing little or no water.Citation19,20) In fact, the activity of OASS-B decreased as the content of organic solvent increased (Fig. ). On the other hand, it has been reported that enzymes from hyperthermophiles display tolerance against organic solvents, although detailed mechanisms have not been elucidated.Citation21) Interestingly, our results showed the activity of OPSS increased as the content of the organic solvent increased by 30% (v/v) (Fig. ). This may be caused from the effect of aprotic solvent that retains polarity without decrease in nucleophilicity of the second substrate (sulfide). Enhancement of enzymatic activity in polar and aprotic solvent was also observed in the case of enzymatic uridine phosphorolysis.Citation22) It is to be noted that the specific activity of His-OASS-B without organic solvent (150 U/mg) was higher than that of OPSS (28 U/mg in the case of DMF and 20 U/mg in the case of 1,4-dioxane, respectively), as in the result of the thermal stability experiment. To examine the stability of OPSS in the organic solvent and to clarify the advantage of OPSS over the stresses, such as heat and organic solvent, we measured the activity of l-cysteine synthesis for 3 min at 80 °C after the pre-incubation of OPSS in the presence of 25% DMF or 1,4-dioxane at 80 °C for 30 min or 1 h. As a result, the specific activities are as follows: 84 U/mg for 30 min and 52 U/mg for 1 h in DMF, and 106 U/mg for 30 min and 69 U/mg for 1 h in 1,4-dioxane, respectively. Comparing to the specific activity without pre-incubation (61 U/mg), OPSS is at least stable in organic solvent at 80 °C for 1 h, where His-OASS B or OASS-B should be denatured as shown in Fig. . Hence, OPSS would be more useful than OASS-B to the production of unnatural amino acids that needs higher temperature and higher contents of organic solvent.
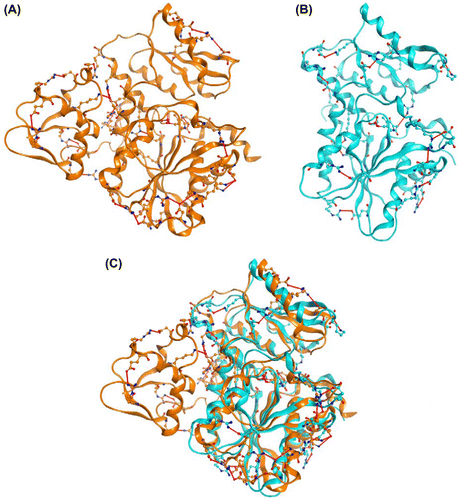
To clarify the reason why OPSS had more tolerance to high temperature and organic solvent than His-OASS-B, SASA was investigated by ProtSA server. We found that the ratio of the surface area of charged amino acids (Arg, Asp, Glu, His, and Lys) to that of whole protein was 42% in OASS-B and 49% in OPSS, respectively. Thus, OPSS has more charged residues on the surface than OASS-B. Moreover, the number of intramolecular electrostatic interaction was searched by the modeling software, MOE. OPSS had 26 electrostatic interactions, while OASS-B had 17 (Tables and ). It should be noted that N-terminal domain (residues 2–73), which is found in OPSS specifically and not found in OASS-B,Citation5) had six electrostatic interactions (Fig. ). Therefore, the tolerance of OPSS to high temperature and organic solvent may be well correlated to the intramolecular electrostatic interactions of this N-terminal domain. In the case of indole-3-glycerol phosphate synthase (IGPS), structures of orthologous proteins from hyperthermophilic and mesophilic organisms were compared, and it was inferred that N-terminal segment of thermophilic or hyperthermophilic IGPS may be important for thermostability from the ratios of B factors of N-terminal atoms to those of the (β/α)8-barrel core.Citation23)
Table 2. Electrostatic interaction in OPSS.
Table 3. Electrostatic interaction in OASS-B from E. coli.
Fig. 5. Intramolecular electrostatic interaction of (A) OPSS (orange, PDB code 3vsa, and chain A) and (B) OASS-B (cyan, PDB code 2v03) and (C) superposition of (A) and (B).
Notes: Red line shows electrostatic interaction formed between amino acid residues shown in Tables and . Carbon, nitrogen, oxygen, and phosphorus atoms are shown as orange (OPSS) or cyan (OASS-B), blue, red, and pink balls, respectively. Illustration was created with MOE (ver.2011.10; Chemical Computing, Group, Montreal Canada).
In conclusion, our results indicate that OPSS is more stable toward heat and organic solvent, and easier to apply industrial production of unnatural amino acids than OASS-B. Biocatalyst is useful in the production of industrial materials without environmental pollutant compared to chemical synthesis. Especially, enzymes from hyperthermophilic archaeon are usually more stable to thermal and organic solvent stress than those from mesophile. Therefore, they are used in industrial application, such as DNA polymerase for PCR technology.Citation21,24,25) Developing the methodology of efficient amino acid synthesis, such as l-cysteine, and unnatural amino acid by OPSS is now in progress.
References
- Kawarabayasi Y, Hino Y, Horikawa H, Yamazaki S, Haikawa Y, Jin-no K, Takahashi M, Sekine M, Baba S, Ankai A, Kosugi H, Hosoyama A, Fukui S, Nagai Y, Nishijima K, Nakazawa H, Takamiya M, Masuda S, Funahashi T, Tanaka T, Kudoh Y, Yamazaki J, Kushida N, Oguchi A, Aoki K, Kubota K, Nakamura Y, Nomura N, Sako Y, Kikuchi H. Complete genome sequence of an aerobic hyper-thermophilic Crenarchaeon, Aeropyrum pernix K1. DNA Res. 1999;6:83–101.10.1093/dnares/6.2.83
- Mino K, Ishikawa K. Characterization of a novel thermostable O-acetylserine sulfhydrylase from Aeropyrum pernix K1. J. Bacteriol. 2003;185:2277–2284.10.1128/JB.185.7.2277-2284.2003
- Mino K, Ishikawa K. A novel O-phospho-l-serine sulfhydrylation reaction catalyzed by O-acetylserine sulfhydrylase from Aeropyrum pernix K1. FEBS Lett. 2003;551:133–138.10.1016/S0014-5793(03)00913-X
- Ishikawa K, Mino K, Nakamura T. New function and application of the cysteine synthase from archaea. Biochem. Eng. J. 2010;48:315–322.10.1016/j.bej.2009.10.015
- Oda Y, Mino K, Ishikawa K, Ataka M. Three-dimensional structure of a new enzyme, O-phosphoserine sulfhydrylase, involved in l-cysteine biosynthesis by a hyperthermophilic archaeon, Aeropyrum pernix K1, at 2.0 Å Resolution. J. Mol. Biol. 2005;351:334–344.10.1016/j.jmb.2005.05.064
- Nakamura T, Kawai Y, Kunimoto K, Iwasaki Y, Nishii K, Kataoka M, Ishikawa K. Structural analysis of the substrate recognition mechanism in O-phosphoserine sulfhydrylase from the hyperthermophilic archaeon Aeropyrum pernix K1. J. Mol. Biol. 2012;422:33–44.10.1016/j.jmb.2012.05.009
- Mino K, Yamanoue T, Sakiyama T, Eisaki N, Matsuyama A, Nakanishi K. Effects of bienzyme complex formation of cysteine synthetase from Escherichia coli on some properties and kinetics. Biosci. Biotechnol., Biochem. 2000;64:1628–1640.10.1271/bbb.64.1628
- Cook PF, Wedding RT. A reaction mechanism from steady state kinetic studies for O-acetylserine sulfhydrylase from Salmonella typhimurium LT-2. J. Biol. Chem. 1976;251:2023–2029.
- Tai CH, Nalabolu SR, Jacobson TM, Minter DE, Cook PF. Kinetic mechanisms of the A and B isozymes of O-acetylserine sulfhydrylase from Salmonella typhimurium LT-2 using the natural and alternative reactants. Biochemistry. 1993;32:6433–6442.10.1021/bi00076a017
- Ikegami F, Murakoshi I. In: Lea PJ, editor. Methods in plant biochemistry. London: Academic Press; 1993. p. 133–151.
- Ikegami F, Kusama-Eguchi K, Sugiyama E, Watanabe K, Lambein F, Murakoshi I. Interaction of some plant heterocyclic β-substituted alanines with rat brain N-methyl-d-aspartate (NMDA) receptors. Biol. Pharm. Bull. 1995;18:360–362.10.1248/bpb.18.360
- Maier TH. Semisynthetic production of unnatural L-α-amino acids by metabolic engineering of the cysteine-biosynthetic pathway. Nat. Biotechnol. 2003;21:422–427.10.1038/nbt807
- Claus MT, Zocher GE, Maier TH, Schulz GE. Structure of the O-acetylserine sulfhydrylase isoenzyme CysM from Escherichia coli. Biochemistry. 2005;44:8620–8626.10.1021/bi050485+
- Gaitonde MK. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 1967;104:627–633.
- Bernadó P, Blackledge M, Sancho J. Sequence-specific solvent accessibilities of protein residues in unfolded protein ensembles. Biophys. J. 2006;91:4536–4543.10.1529/biophysj.106.087528
- Estrada J, Bernado P, Blackledge M, Sancho J. ProtSA: a web application for calculating sequence specific protein solvent accessibilities in the unfolded ensemble. BMC Bioinf. 2009;10:104–111.10.1186/1471-2105-10-104
- Zocher G, Wiesand U, Schulz GE. High resolution structure and catalysis of O-acetylserine sulfhydrylase isozyme B from Escherichia coli. FEBS J. 2007;274:5382–5389.10.1111/j.1742-4658.2007.06063.x
- Zhao C, Kumada Y, Imanaka H, Imamura K, Nakanishi K. Cloning, overexpression, purification, and characterization of O-acetylserine sulfhydrylase-B from Escherichia coli. Protein Expression Purif. 2006;47:607–613.10.1016/j.pep.2006.01.002
- Zaks A, Klibanov AM. Enzymatic catalysis in organic media at 100 °C. Science. 1984;224:1249–1251.10.1126/science.6729453
- Klibanov AM. Improving enzymes by using them in organic solvent. Nature. 2001;409:241–246.10.1038/35051719
- Doukyu N, Ogino H. Organic solvent-tolerant enzymes. Biochem. Eng. J. 2010;48:270–282.10.1016/j.bej.2009.09.009
- Komissarov AA, Moltchan OK, Romanova DV, Debabov VG. Enzyme-catalyzed uridine phosphorolysis: SN2 mechanism with phosphate activation by desolvation. FEBS Lett. 1994;355:192–194.10.1016/0014-5793(94)01204-0
- Bagautdinov B, Yutani K. Structure of indole-3-glycerol phosphate synthase from Thermus thermophilus HB8: implications for thermal stability. Acta Crystallogr., Sect D: Biol. Crystallogr. 2011;67:1054–1064.10.1107/S0907444911045264
- Atomi H. Recent progress towards the application of hyperthermophiles and their enzymes. Curr. Opin. Chem. Biol. 2005;9:166–173.10.1016/j.cbpa.2005.02.013
- de Miguel Bouzas, T, Barros-Velazquez J, Villa TG. Industrial applications of hyperthermophilic enzymes: a review. Protein Pept. Lett. 2006;13:645–651.