Abstract
The cholesterol ester accumulates in macrophages in the early stage of atherosclerotic lesions, leading to the formation of foam cells. We examined the inhibitory effects of the crude extracts of 22 edible plants on foam cell formation and isolated nine chlorophyll derivatives as potent inhibitors from Chinese cabbage. The results of the present study suggest that the chlorophyll derivatives contained in edible plants may be useful for the prevention and treatment of atherosclerosis.
Graphical abstract
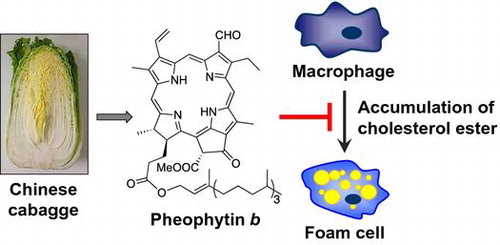
Nine chlorophyll derivatives were isolated from Chinese cabbage as potent inhibitors of the cholesterol ester accumulation in macrophages.
The formation of massive clusters of macrophage-derived foam cells, in which the cholesterol ester (CE) accumulates in subendothelial spaces is one of the characteristic features of the early stage of atherosclerotic lesions.Citation1) Foam cells produce various bioactive molecules, such as cytokines, growth factors, and proteases, which play critical roles in the development and progression of atherosclerosis.Citation1) Macrophages have been shown to incorporate modified low-density lipoproteins (LDL), such as oxidized LDL (Ox-LDL) and acetylated LDL (Ac-LDL), through their scavenger receptors.Citation2) The class A scavenger receptor,Citation3) class B scavenger receptor (CD36),Citation4) class B scavenger receptor type-I (SR-BI),Citation5) and lectin-like Ox-LDL receptor-1 (LOX-1)Citation6) were previously identified as scavenger receptors for the modified LDL. Since free cholesterol, which is incorporated with modified LDL, is toxic to cells, it is esterified to CE by acyl coenzyme A:cholesterol acyltransferase (ACAT), an intracellular enzyme located in the rough endoplasmic reticulum.Citation7) The intracellular accumulation of CE changes macrophages into foam cells. Since the formation of foam cells through these mechanisms plays an important role in the progression of early atherosclerotic lesions in vivo, the prevention and inhibition of foam cell formation are considered to be major targets for the treatment of atherosclerosis. Therefore, a number of anti-atherosclerotic agents possessing various functions, such as the prevention of LDL-oxidation, inhibition of scavenger receptor expression, inhibition of the uptake of modified LDL, and inhibition of ACAT expression and activity, have been explored to date. We recently isolated manzamine A from a marine spongeCitation8) and esculeogenin A and tomatidine from the tomatoCitation9,10) that inhibited foam cell formation by suppressing ACAT activity. They also suppressed hyperlipidemia and atherosclerosis in apoE-deficient mice, which suggested that they are promising lead compounds in the prevention or treatment of atherosclerosis. In the present study, we examined the inhibitory effects of the crude extracts of 22 edible plants on foam cell formation in human monocyte-derived macrophages (HMDMs) and identified new positive candidates for the prevention and treatment of atherosclerosis.
Materials and methods
General experimental procedures
1H NMR spectra were recorded on a JEOL JNM-ECX-400 NMR spectrometer in CDCl3. Chemical shifts were referenced to the residual solvent peak (δH 7.24). Mass spectra were measured on a JEOL JMS-700 mass spectrometer.
Materials
Penicillin G and streptomycin sulfate were purchased from Invitrogen (Tokyo, Japan). Ficoll-Paque and [9,10-3H]-oleate (4 Ci/mg) were purchased from GE Healthcare Bio-Sciences (Tokyo, Japan). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Wako (Saitama, Japan). Chlorophylls a and b were purchased from Sigma-Aldrich Japan (Osaka, Japan). All other chemicals were of the best grade available from commercial sources.
Preparation of screening samples from 22 edible plants
Commercially available edible plants were extracted with MeOH and the condensed aqueous extract was partitioned with EtOAc. The EtOAc and water fractions were dissolved in DMSO at the concentrations of 100 μg/mL.
Cell culture
Human monocytes were purified from the blood of healthy volunteers by Ficoll density gradient centrifugation.Citation11) Purified monocytes were suspended in DMEM and seeded onto 24-well plates (4 × 105/well). After a 1-h incubation for adherence, the medium was replaced by DMEM supplemented with 10% pooled human serum, streptomycin (100 μg/mL), and penicillin G (100 U/mL). Adherent monocytes were incubated for 7 days to induce their differentiation into macrophages. Cells contained >95% macrophages and were >98% viable under these conditions as determined by trypan blue staining. CHO cells stably overexpressing human ACAT-1 (hACAT-1 CHO cells) or human ACAT-2 (hACAT-2 CHO cells)Citation12) were cultured in Nutrient mixture F-12 HAM medium (Sigma-Aldrich, Japan) supplemented with 10% fetal calf serum, streptomycin (50 μg/mL), penicillin G (50 U/mL), and G418 (800 μg/mL). All experiments using the cells were performed under a humidified atmosphere containing 5% CO2 at 37 °C.
Measurement of CE formation
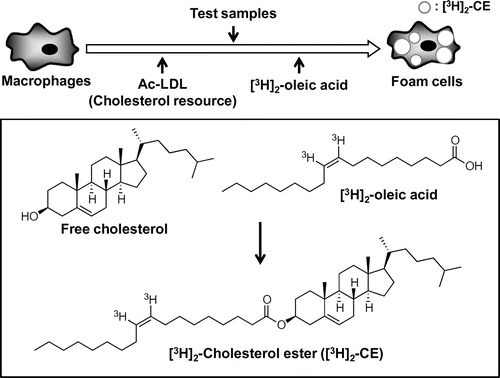
HMDM monolayers or ACAT CHO cells were incubated with Ac-LDL (50 μg/mL) in the presence of 0.1 mM [3H]2-oleate conjugated with BSA for 24 h, and cellular lipids were extracted to determine the radioactivity of cholesteryl-[3H]2-oleate (Fig. ), as described previously.Citation13)
Isolation of inhibitors of CE accumulation
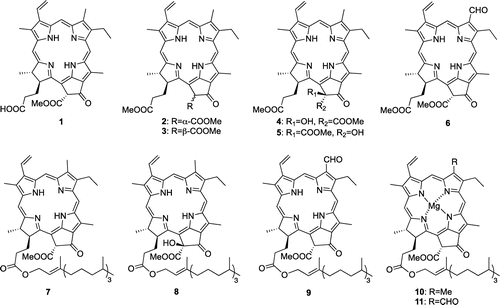
Chinese cabbage (CC) (45 kg) was extracted with MeOH and the condensed aqueous extract was partitioned with EtOAc. The EtOAc soluble fraction (36.4 g) was subjected to silica gel column chromatography with n-hexane/EtOAc (4:1, 2:1), CHCl3/MeOH (4:1), CHCl3/MeOH/H2O (6:4:1), and MeOH. Fraction 2 (3.9 g), which was eluted with n-hexane/EtOAc (2:1), was repeatedly purified by silica gel column chromatography with n-hexane/EtOAc (2:1), silica gel HPLC with CHCl3/MeOH (100:0, 99:1, 80:20), and gel-filtration HPLC with CHCl3/MeOH (9:1) to afford pheophorbide a (1, 3.7 mg), pheophytin a (7, 3.1 mg), and (132R)-132-hydroxypheophytin a (8, 2.6 mg) (Fig. ). Fraction 3 (328 mg), which was eluted with n-hexane/EtOAc (2:1), was purified by silica gel column chromatography with CHCl3/MeOH (50:1, 20:1, 9:1, 1:1) and silica gel HPLC with n-hexane/EtOAc (3:1) to afford pheophytin b (9, 3.9 mg). Fraction 4 (18.6 g), which was eluted with CHCl3/MeOH (4:1), was purified by silica gel column chromatography with n-hexane/EtOAc (3:1, 4:3, 1:2) to afford fraction 4–2 (351.4 mg) and fraction 4–3 (57.2 mg). The fraction 4–2, which was eluted with n-hexane/EtOAc (4:3), was purified by ODS HPLC with 95% MeOH-H2O and gel-filtration HPLC with CH2Cl2 to afford methyl pheophorbide a (2, 32.4 mg), methyl 13-epi-pheophorbide a (3, 2.6 mg), methyl (132R)-132-hydroxypheophorbide a (4, 0.67 mg), and methyl (132S)-132-hydroxypheophorbide a (5, 0.31 mg). The fraction 4–3, which was eluted with n-hexane/EtOAc (4:3), was purified by silica gel HPLC with n-hexane/EtOAc (3:1) and gel-filtration HPLC with CH2Cl2 to afford methyl pheophorbide b (6, 0.22 mg).
Statistical analysis
All experimental data are expressed as the mean ± SD. Differences between groups were examined for statistical significance using the Mann–Whitney U-test and the non-repeated measures analysis of variance (ANOVA). A p value < 0.05 denoted the presence of a statistically significant difference.
Results and discussion
Screening of CE accumulation in HMDM and isolation of its inhibitors
We first tested the inhibitory effects of the crude extracts of 22 edible plants on the accumulation of CE in HMDM. The incubation of HMDM for 24 h with 50 μg/mL Ac-LDL increased the accumulation of CE (Fig. ). Under the assay conditions employed, the EtOAc soluble fractions of the MeOH extracts of apple (AP), celery (CE), CC, cucumber (CU), garlic shoots (GS), and avocado (AV) strongly inhibited the accumulation of CE (Fig. ). Since AV contains much fatty acids, probably they inhibited the incorporation of [Citation3H]2-oleic acid into the macrophages.Citation14) CU is known to contain much triterpenes,Citation15,16) and we already reported that the triterpenes in plants inhibited the accumulation of CE and showed the suppressive effect on atherosclerosis in apolipoprotein E (apoE)-deficient mice.Citation9,17) Therefore, the triterpenes in CU possible inhibit the accumulation of CE. AP also contains much triterpenesCitation18) and it is reported that polyphenols and dietary fibers in AP inhibited atherosclerosis in apoE-deficient mice.Citation19) Then, we selected CC, and the bioassay-guided purification of the EtOAc soluble fraction afforded nine compounds 1–9 as the inhibitors of CE accumulation. Their structures were identified on the basis of their 1H NMR and FAB mass spectra (Fig. ).Citation20–22)
Fig. 3. Inhibitory effects of crude extracts of edible plants on CE accumulation in HMDMs induced by Ac-LDL.
Notes: HMDM were incubated with 50 μg/mL Ac-LDL and 0.1 mM [3H]2-oleate conjugated with BSA in the absence or presence of 100 μg/mL crude extracts of edible plants (E: Ethyl acetate soluble fraction, W: H2O soluble fraction, ON, Onion; GO, Green onion; CR, Chinese radish; AP, Apple; OR, Orange; SB, Strawberry; BA, Banana; DA, Darjeeling tea; KI, Kiwi; SD, Shaddock (Citrus grandis); CA, Cabbage; SK, Shekwasha (C. depressa); CE, Celery; CC, Chinese cabbage; KU, Kumquat; CU, Cucumber; CT, Carrot; GS, Garlic shoots; AV, Avocado; TO, Tomato; BB, Blueberry; PP, Pumpkin). After being incubated for 24 h, [3H]2-CE was separated by TLC and its radioactivity was measured with a radioscanner, as described in the Materials and Methods section. Data are presented as the mean ± SD. *, p < 0.01, and **, p < 0.001, vs. control.
![Fig. 3. Inhibitory effects of crude extracts of edible plants on CE accumulation in HMDMs induced by Ac-LDL.Notes: HMDM were incubated with 50 μg/mL Ac-LDL and 0.1 mM [3H]2-oleate conjugated with BSA in the absence or presence of 100 μg/mL crude extracts of edible plants (E: Ethyl acetate soluble fraction, W: H2O soluble fraction, ON, Onion; GO, Green onion; CR, Chinese radish; AP, Apple; OR, Orange; SB, Strawberry; BA, Banana; DA, Darjeeling tea; KI, Kiwi; SD, Shaddock (Citrus grandis); CA, Cabbage; SK, Shekwasha (C. depressa); CE, Celery; CC, Chinese cabbage; KU, Kumquat; CU, Cucumber; CT, Carrot; GS, Garlic shoots; AV, Avocado; TO, Tomato; BB, Blueberry; PP, Pumpkin). After being incubated for 24 h, [3H]2-CE was separated by TLC and its radioactivity was measured with a radioscanner, as described in the Materials and Methods section. Data are presented as the mean ± SD. *, p < 0.01, and **, p < 0.001, vs. control.](/cms/asset/1bf5e46d-1148-41cd-bacd-ed1ff91d4cdb/tbbb_a_1023247_f0003_b.gif)
Inhibitory activity of 1–9 on CE accumulation
Compounds 1–9 isolated from CC and the commercially available chlorophylls a and b (10 and 11) inhibited the accumulation of CE (Fig. ) in HMDM and their inhibitory effects at a concentration of 50 μM were 54, 32, 49, 54, 78, 61, 57, 17, 87, 16, and 19%, respectively. This result indicated that the inhibitors of CE accumulation in CC were chlorophyll derivatives 1–9. Interestingly, the inhibitory activities of 10 and 11 were reduced than those of 7 and 9, respectively, which strongly indicated that the presence of a magnesium ion in 10 and 11 diminished their activities. Furthermore, 9 showed the inhibitory effect on CE accumulation in ACAT-1 CHO cells, whereas 9 showed no effect in ACAT-2 CHO cells (Fig. ), which suggested that chlorophyll derivatives inhibited the foam cell formation by suppressing ACAT-1 activity.
Fig. 4. Inhibitory effects of 1–11 on CE accumulation in HMDM induced by Ac-LDL.
Notes: HMDM was incubated with 50 μg/mL Ac-LDL and 0.1 mM [3H]2-oleate conjugated with BSA in the absence or presence of 1–11 (50 μM). After being incubated for 24 h, [3H]2-CE was separated by TLC and its radioactivity was measured with a radioscanner, as described in the Materials and Methods section. Data are presented as the mean ± SD. *, p < 0.05, **, p < 0.01 and ***, p < 0.001, vs. control.
![Fig. 4. Inhibitory effects of 1–11 on CE accumulation in HMDM induced by Ac-LDL.Notes: HMDM was incubated with 50 μg/mL Ac-LDL and 0.1 mM [3H]2-oleate conjugated with BSA in the absence or presence of 1–11 (50 μM). After being incubated for 24 h, [3H]2-CE was separated by TLC and its radioactivity was measured with a radioscanner, as described in the Materials and Methods section. Data are presented as the mean ± SD. *, p < 0.05, **, p < 0.01 and ***, p < 0.001, vs. control.](/cms/asset/5c07d551-11a9-4342-a44f-9d2679a73ae5/tbbb_a_1023247_f0004_b.gif)
Fig. 5. Inhibitory effects of 9 on CE accumulation in CHO cells overexpressing human ACAT-1 or ACAT-2.
Notes: hACAT-1 CHO (A) and hACAT-2 CHO (B) cells were incubated with 50 μg/mL Ac-LDL and 0.1 mM [3H]2-oleate conjugated with BSA in the absence or presence of indicated concentration with 9. After being incubated for 24 h, [3H]2-CE was separated by TLC and its radioactivity was measured with a radioscanner, as described in the Materials and Methods section. Data are presented as the mean ± SD. *, p < 0.01, vs. control.
![Fig. 5. Inhibitory effects of 9 on CE accumulation in CHO cells overexpressing human ACAT-1 or ACAT-2.Notes: hACAT-1 CHO (A) and hACAT-2 CHO (B) cells were incubated with 50 μg/mL Ac-LDL and 0.1 mM [3H]2-oleate conjugated with BSA in the absence or presence of indicated concentration with 9. After being incubated for 24 h, [3H]2-CE was separated by TLC and its radioactivity was measured with a radioscanner, as described in the Materials and Methods section. Data are presented as the mean ± SD. *, p < 0.01, vs. control.](/cms/asset/2d94e285-7ab1-4462-80d8-6248ba0740ad/tbbb_a_1023247_f0005_b.gif)
Previous studies reported the inhibitory effects of pheophorbide a (1), pheophytin a (7), and chlorophyllin on the LPS-induced production of nitric oxide and IL-1β in macrophages, and suggested that chlorophyll derivatives may possess anti-inflammatory activities in macrophages.Citation23–25) However, the other activities of chlorophyll derivatives in macrophages remained unknown. This is the first study to examine the inhibitory effects of chlorophyll derivatives on the accumulation of CE.
Conclusion
We isolated nine chlorophyll derivatives from CC as potent inhibitors of CE accumulation in macrophages. Our results indicated that the chlorophyll derivatives contained in edible plants are useful for the prevention and treatment of atherosclerosis.
Additional information
Funding
References
- Ross R. Atherosclerosis is an inflammatory disease. Am. Heart J. 1999;138:419–420.10.1016/S0002-8703(99)70266-8
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989;320:915–924.
- Kodama T, Freeman M, Rohrer L, Zabrecky J, Matsudaira P, Krieger M. Type I macrophage scavenger receptor contains α-helical and collagen-like coiled coils. Nature. 1990;343:531–535.10.1038/343531a0
- Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J. Biol. Chem. 1993;268:11811–11816.
- Acton SL, Scherer PE, Lodish HF, Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J. Biol. Chem. 1994;269:21003–21009.
- Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77.10.1038/386073a0
- Chang TY, Chang CC, Lin S, Yu C, Li BL, Miyazaki A. Roles of acyl-coenzyme A:cholesterol acyltransferase-1 and -2. Curr. Opin. Lipidol. 2001;12:289–296.10.1097/00041433-200106000-00008
- Eguchi K, Fujiwara Y, Hayashida A, Horlad H, Kato H, Rotinsulu H, Losung F, Mangindaan RE, de Voogd NJ, Takeya M, Tsukamoto S. Manzamine A, a marine-derived alkaloid, inhibits accumulation of cholesterol ester in macrophages and suppresses hyperlipidemia and atherosclerosis in vivo. Bioorg. Med. Chem. 2013;21:3831–3838.10.1016/j.bmc.2013.04.025
- Fujiwara Y, Kiyota N, Hori M, Matsushita S, Iijima Y, Aoki K, Shibata D, Takeya M, Ikeda T, Nohara T, Nagai R. Esculeogenin A, a new tomato sapogenol, ameliorates hyperlipidemia and atherosclerosis in ApoE-deficient mice by inhibiting ACAT. Arterioscler. Thromb. Vasc. Biol. 2007;27:2400–2406.10.1161/ATVBAHA.107.147405
- Fujiwara Y, Kiyota N, Tsurushima K, Yoshitomi M, Horlad H, Ikeda T, Nohara T, Takeya M, Nagai R. Tomatidine, a tomato sapogenol, ameliorates hyperlipidemia and atherosclerosis in ApoE-deficient mice by inhibiting acyl-CoA:cholesterol acyl-transferase (ACAT). J. Agric. Food Chem. 2012;60:2472–2479.10.1021/jf204197r
- Connor RI, Shen L, Fanger MW. Evaluation of the antibody-dependent cytotoxic capabilities of individual human monocytes. Role of Fc gamma RI and Fc gamma RII and the effects of cytokines at the single cell level. J. Immunol. 1990;145:1483–1489.
- Chan CC, Sakashita N, Ornvold K, Lee O, Chang ET, Dong R, Lin S, Lee CY, Strom SC, Kashyap R, Fung JJ, Farese RV Jr, Patoiseau JF, Delhon A, Chang TY. Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J. Biol. Chem. 2000;275:28083–28092.
- Miyazaki A, Rahim AT, Araki S, Morino Y, Horiuchi S. Chemical cross-linking alters high-density lipoprotein to be recognized by a scavenger receptor in rat peritoneal macrophages. Biochim. Biophys. Acta. 1991;1082:143–151.10.1016/0005-2760(91)90188-N
- Kritchevsky D, Tepper SA, Wright S, Czarnecki SK, Wilson TA, Nicolosi RJ. Cholesterol vehicle in experimental atherosclerosis 24: avocado oil. J. Am. Coll. Nutr. 2003;22:52–55.10.1080/07315724.2003.10719275
- Akiyama K, Hayashi H. Arbuscular mycorrhizal fungus-promoted accumulation of two new triterpenoids in cucumber roots. Biosci. Biotechnol. Biochem. 2002;66:762–769.10.1271/bbb.66.762
- Kai H, Baba M, Okuyama T. Two new megastigmanes from the leaves of Cucumis sativus. Chem. Pharm. Bull. 2007;55:133–136.10.1248/cpb.55.133
- Fujiwara Y, Hayashida A, Tsurushima K, Nagai R, Yoshitomi M, Daiguji N, Sakashita N, Takeya M, Tsukamoto S, Ikeda T. Triterpenoids isolated from Zizyphus jujuba inhibit foam cell formation in macrophages. J. Agric. Food Chem. 2011;59:4544–4552.10.1021/jf200193r
- Xiangjiu H, Liu RH. Triterpenoids isolated from apple peels have potent antiproliferative activity and may be partially responsible for apple’s anticancer activity. J. Agric. Food Chem. 2007;55:4366–4370.
- Auclair S, Silberberg M, Gueux E, Morand C, Mazur A, Milenkovic D, Scalbert A. Apple polyphenols and fibers attenuate atherosclerosis in apolipoprotein E-deficient mice. J. Agric. Food Chem. 2008;56:5558–5563.10.1021/jf800419s
- Ohshima T, Hirata M, Oda T, Sasaki A, Shiratsuchi M. Pheophorbide a, a potent endothelin receptor antagonist for both ETA and ETB subtypes. Chem. Pharm. Bull. 1994;42:2174–2176.10.1248/cpb.42.2174
- Ma L, Dolphin D. Nucleophilic reaction of 1,8-diazabicyclo[5.4.0]undec-7-ene and 1,5-diazabicyclo[4.3.0]non-5-ene with methyl pheophorbide a. Unexpected products. Tetrahedron. 1996;52:849–860.10.1016/0040-4020(95)00944-2
- Nakatani Y, Ourisson G, Beck JP. Chemistry and biochemistry of Chinese drugs. VII. Cytostatic pheophytins from silkworm excreta, and derived photocytotoxic pheophorbides. Chem. Pharm. Bull. 1981;29:2261–2269.10.1248/cpb.29.2261
- Cho KJ, Han SH, Kim BY, Hwang SG, Park KK, Yang KH, Chung AS. Chlorophyllin suppression of lipopolysaccharide-induced nitric oxide production in RAW 264.7 cells. Toxicol. Appl. Pharmacol. 2000;166:120–127.10.1006/taap.2000.8958
- Yun CH, Jeon YJ, Yang Y, Ju HR, Han SH. Chlorophyllin suppresses interleukin-1 beta expression in lipopolysaccharide-activated RAW 264.7 cells. Int. Immunopharmacol. 2006;6:252–259.10.1016/j.intimp.2005.08.012
- Islam MN, Ishita IJ, Jin SE, Choi RJ, Lee CM, Kim YS, Jung HA, Choi JS. Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents pheophorbide a and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem. Toxicol. 2013;55:541–548.10.1016/j.fct.2013.01.054