Abstract
Small fish species such as the zebrafish (Danio rerio) and medaka fish (Oryzias latipes) are advantageous animal models and have been used as model organisms in many research areas. However, they have not been utilized for studying the taste system, primarily because of a dearth of molecular biological knowledge. Quantitative methods for analyzing the taste preferences of fish species have also been lacking. Recent progress of the fish genome project has enabled the elucidation of the molecular mechanisms of taste sensation. Taste receptors and a number of signal transduction molecules have been identified. Additionally, the development of quantitative methods of feeding using fluorescently labeled artificial foods has demonstrated taste preferences in small fish species. Comparisons between these results in fish and reports on mammals have proposed a general logic and evolution of vertebrate taste systems. Analysis on the transsynaptic tracer-expressing transgenic medaka fish also suggests the usefulness of small fish in the research of neural circuits for taste.
Graphical Abstract
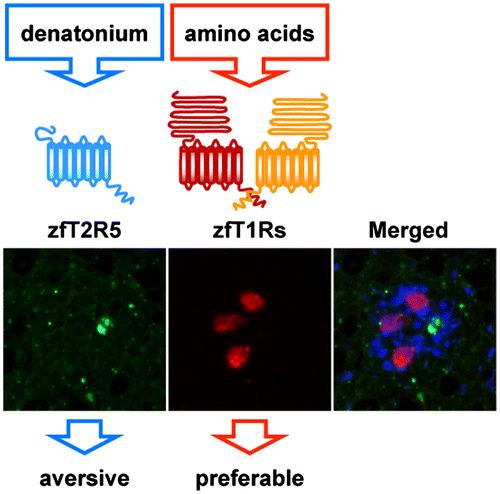
Transgenic labeling of neural circuits linked to a phospholipase C-β2-expressing taste bud cells in medaka fish.
The sense of taste has a vital role in the evaluation and intake of foods. Taste bud cells, located in the epithelia of the oral cavity function as receptor cells for tastants. Taste information is relayed to the brain and its recognition elicits behavioral responses to the food.
Elucidation of the mechanisms of taste sensation in vertebrates has been conducted mainly using rodent models, with very few reports in other vertebrate species. Recently, small fish species such as the zebrafish (Danio rerio) and medaka fish (Oryzias latipes) have been used as model vertebrates in a number of research areas. Several advantages of these small fish species brought breakthrough results. For example, the small number of neurons and small size of their brains have contributed to the research of sensory systems, similar to that in flies and worms. Nonetheless, small fish species have not been utilized for taste research because of insufficient knowledge accumulated regarding their taste sensory systems.
I. Identification and functional analysis of taste signaling molecules in small fish species
The molecular mechanisms of cellular signaling in taste receptor cells in mice, rats, and humans have been elucidated in recent years.Citation1) Taste receptors for sweet, umami, and bitter tastants, belonging to the G protein-coupled receptor family, have been identified. Ion channels have been proposed as candidate taste receptors for sour and salty tastants. The intracellular signaling cascades following these taste receptors and the molecular mechanisms of neurotransmission to the connecting taste neurons have also been elucidated. However, those in teleost fish species have not been well examined and remain unknown.
Progress in the development of genome databases has enabled the exploration of taste receptor genes in fish species. The existence of the orthologous genes for T1Rs, mammalian taste receptors for sweet, and umami, in fish was clarified by comprehensive searches of these databases, based on homologies in amino acid sequences.Citation2) Simultaneously, the orthologous fish genes for T2Rs, mammalian bitter taste receptors, were also identified. T1R and T2R genes were identified as common even in divergent fish species, such as zebrafish, medaka fish, and fugu fish (Takifugu rubripes). This suggests that these gene families already existed in the common ancestor of teleost fishes. The expression of the mRNAs of these orthologous genes was observed in a subset of taste bud cells in zebrafish and medaka fish. This observation strongly suggested that T1R and T2R would be the common receptor families for taste among vertebrate species, ranging at least from teleosts to mammals. Cellular signaling molecules working downstream of these taste receptors, phospholipase-β2 and trpm5 channel, are expressed in the taste bud cells of zebrafish and medaka fish together with other receptors in a similar manner to mammals.Citation2,3)
On the other hand, species-specific aspects of taste-related molecules have also been shown. Tetrapod species commonly have three T1R genes, T1R1, T1R2, and T1R3 in each species. In contrast, teleost fish species have highly conserved orthologs of T1R1 and T1R3, together with weakly conserved multiple T1R orthologs that we named as fish T1R2 genes because of their weak homology to mammalian T1R2 genes as shown by phylogenetic analysis.Citation2) Another phylogenetic analysis, with a different data-set of T1R genes, suggested that the fish T1R2 genes may be closer to the tetrapod T1R1 than T1R2 genes.Citation4) Concerning the G proteins that transduces the signals from T1R and cells are different between teleosts and mammals.Citation5) Co-expression patterns of taste signaling molecules are also different between species, showing the diversification of taste receptor cells in fish. These differences may reflect the differences in the living circumstances and the feeding habitats among species .Citation2,5)
The functional analysis of fish T1Rs and T2Rs was performed using a heterologous expression in cultured cells.Citation6,7) The mammalian T1R1/T1R3 heteromer detects umami substances (amino acids) and the mammalian T1R2/T1R3 heteromer detects sweet substances. Fish T1R1/T1R3 heteromer-expressing cells responded to some L-amino acids, such as L-Arg and L-Ser in a dose-dependent manner. Interestingly, fish T1R2/T1R3 heteromer-expressing cells also responded to several L-amino acids, but not to other tastants, including sugars. Both T1R1/T1R3 and multiple T1R2/T1R3s function as taste receptors for amino acids in fish species. This finding is consistent with reports claiming that fish have multiple amino acid taste receptors. The reason why this might be the case remains unknown. One possibility is that possessing multiple receptors with different sensitivities and response profiles may be advantageous for adaptation to their food circumstances. The responding profiles of T1R2/T1R3s to amino acids were different between zebrafish and medaka fish, suggesting this adaptation in fish T1Rs. Another possibility is that multiple amino acid receptors enable the discrimination of two segregated taste modalities for body-constituting substances and energy resources, as is the case for mammalian T1R1/T1R3 and T1R2/T1R3. Fish T1R1 and T1R2s are expressed in different cell populations, supporting these interpretations. The activation of fish T1Rs for amino acids was not influenced by the addition of inosine monophosphate (IMP), an activator of the response of mammalian T1R1/T1R3, to amino acids. IMP binding and modulation would thus be acquired by T1Rs in the tetrapod lineage. The sole T2R in medaka fish, mfT2R1, and its ortholog in zebrafish, zfT2R5, have been shown to detect denatonium benzoate, which is a bitter tastant for humans. Denatonium benzoate also activates rodent T2Rs. As mentioned below, it has been revealed that amino acids are preferable tastants and denatonium benzoate is an aversive tastant for fish as well as mammals. Thus, T1Rs function as taste receptors for desirable substances, whereas T2Rs function as receptors for aversive substances, commonly among vertebrates. The clear segregation of zfT1R and zfT2R5 expression in zebrafish taste buds strongly suggests a conserved mechanism among vertebrate species, whereby taste modalities are defined by the taste receptor cell types responding to the tastants (Fig. ).
II. Behavioral and physiological methods to quantify the taste preferences of fish species
Behavioral and physiological analyses of taste preferences are useful to evaluate the repertoire, the concentration range of taste substances that an animal can sense, and the animals’ preference or aversion to taste substances. The quantitative methods of behavioral and physiological analysis for terrestrial model animals like rodents have been performed. One major method is the two-bottle preference test. In this test, animals can access two drinking bottles, one containing taste solution and the other containing water, and a preference-aversion score is calculated as the ratio of taste solution intake to total intake. Another major method is the brief access test, also known as the licking test. In this test, the number of licks to the taste solution in a short time is used as the preference-aversion score. However, these methods cannot be adapted to aquatic animals because taste solutions immediately diffuse in water. Behavioral and physiological analyses of fish taste preference have been performed by the observation and scoring of behaviors of food sorting and consumption. Fish species that have a certain body size, and feed while stationary, such as goldfish and fugu fish, can easily have their feeding behavior observed,Citation8,9) while it is difficult to observe the same in small fish species.
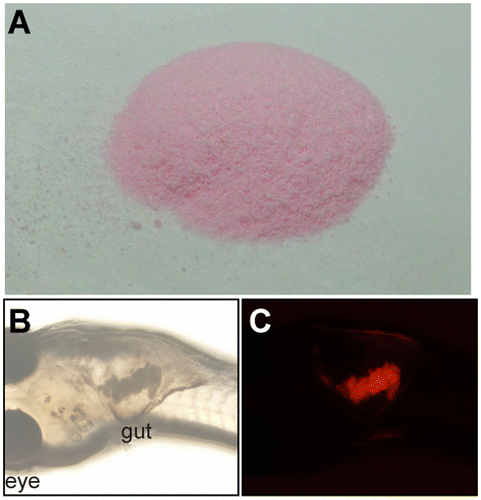
Our group recently developed quantitative methods for doing so by taking advantage of the transparency of small fish species.Citation6,10) Artificial foods containing a taste substance and a fluorescent dye are fed to medaka fish larvae in a short time and the fluorescence intensities of the ingested foods in the gut are measured by fluorescence microscopic observation to determine the preference-aversion score (Fig. ). The preference for amino acids, and the aversion to denatonium benzoate, a bitter compound, were both shown in medaka fish by this method. The inhibition of G protein signaling by the expression of the dominant-negative Gα mutant in both T1R and T2R-expressing taste bud cells caused the loss of the preference and the aversion, indicating the usefulness of this method to evaluate the preference-aversion behavior originated from the taste sense.Citation10) The fluorescently labeled artificial foods can also be used for the analysis of adult fish. As before, foods containing amino acids or denatonium benzoate were fed to adult zebrafish and the remaining food was collected. The fluorescence of the remaining foods was measured. The taste preferences of zebrafish were thus revealed to be similar to those of medaka fish.Citation6)
Fig. 2. Fluorescently labeled artificial foods for small fish. A. Observation of the foods. Artificial foods containing a taste substance and a fluorescent dye (DiI) are grinded to a fine powder. B and C. Images of a 20-dpf fish fed the fluorescently labeled food. Bright-field images (B) and fluorescence images (C) are shown. The red fluorescence derived from DiI was detected in the gut.
III. Utilization of small fish species to the research on the neural circuits for taste signals
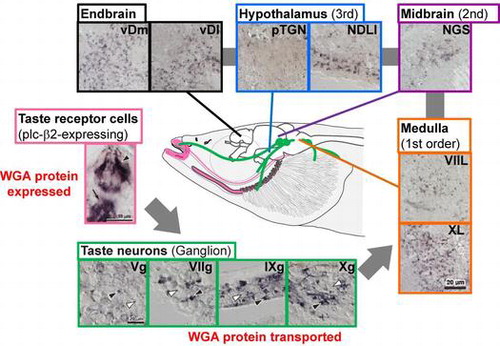
Wheat germ agglutinin (WGA) is a plant lectin that is able to be transported transneuronally and is used as a tracer for visualizing neural circuits by immunohistochemical staining. The recent innovative technique of inducing cell-specific expression of WGA cDNA as a transgene by genetic engineering has enabled the visualization of neural circuits originating in specific cells.Citation11) This technique has already been used for the analysis of neural circuits from some types of taste receptor cells in mice.Citation12–15) WGA labeling has been observed in a subset of neurons limited to the primary gustatory nuclei in these reports. A reason for this would be that only a small portion of the WGA protein in the WGA-expressing or WGA-transported cells is transported transneuronally to their connecting neurons. The utilization of a model which has more target cells, and a strong promoter, to induce the expression of more WGA transcripts in target cells was expected to better visualize higher-order neural circuits. To do this, we used medaka fish as a model and medaka fish plc-β2 (mfplcb2) promoter for the expression of WGA transcripts. Our observation confirmed that the medaka fish has a small-volume brain and many taste buds. Thus, its taste neural circuits would converge from many taste receptor cells to a small number of central nerves. mfplcb2 is expressed strongly in a large subset, about half, of taste bud cells, including T1R and T2R-expressing cells as described above. For these techniques, small fishes have an advantage in whole-brain analysis because of their small brains.
Thus, a transgenic medaka fish in which WGA transcripts were expressed specifically in mfplcb2-expressing cells was produced (Fig. ).Citation16) Transported WGA proteins were observed clearly in a subset of the neurons in the cranial sensory ganglia and the primary gustatory nuclei, and even in the larvae of fish 12 days post fertilization. WGA-positive neurons were also observed in the second and third gustatory nuclei in adult fish, whereas such neurons have not been observed in mice. Furthermore, a significant number of the positive neurons were observed in the nuclei of the telencephalon. The structure of the telencephalon in fish has not been well-defined because their brains develop with contrasting cell movements to those of mammals. However, these data are the first to demonstrate the tracing of higher order gustatory neuronal circuitry associated with a specific subpopulation of taste bud cells. Thus, although WGA protein transport experiments in transgenic medaka fish in which WGA transcripts are expressed by another promoter should be conducted, the fish itself would constitute a good model organism for research on the neural circuits of taste.
Fig. 3. Schematic of WGA transport in 9 month-old transgenic fish. Transgene-expressed WGA proteins in plc-β2-positive taste bud cells (pink box) were transported to the taste nerves (green boxes) and the brain. WGA-positive (black arrowheads) and WGA-negative (white arrowheads) neurons were detected in the facial (VIIg), glossopharyngeal (IXg), and vagal (Xg) ganglia of taste neurons (green box). WGA-positive neurons were also detected in the facial lobe (VIIL) and the vagal lobe (XL) in the medulla (orange boxes), the secondary gustatory nucleus (NGS) in the midbrain (purple box), the preglomerular tertiary gustatory nucleus (pTGN) and the diffuse nucleus of the inferior lobe (NDLI) in the hypothalamus (blue boxes), the ventral region of the Dm (vDm), and the ventral region of the Dl (lateral part of the dorsal telencephalic area [vDl]) in the endbrain (black boxes). The likely pathways of WGA transport are indicated by gray arrows and lines.Citation16)
![Fig. 3. Schematic of WGA transport in 9 month-old transgenic fish. Transgene-expressed WGA proteins in plc-β2-positive taste bud cells (pink box) were transported to the taste nerves (green boxes) and the brain. WGA-positive (black arrowheads) and WGA-negative (white arrowheads) neurons were detected in the facial (VIIg), glossopharyngeal (IXg), and vagal (Xg) ganglia of taste neurons (green box). WGA-positive neurons were also detected in the facial lobe (VIIL) and the vagal lobe (XL) in the medulla (orange boxes), the secondary gustatory nucleus (NGS) in the midbrain (purple box), the preglomerular tertiary gustatory nucleus (pTGN) and the diffuse nucleus of the inferior lobe (NDLI) in the hypothalamus (blue boxes), the ventral region of the Dm (vDm), and the ventral region of the Dl (lateral part of the dorsal telencephalic area [vDl]) in the endbrain (black boxes). The likely pathways of WGA transport are indicated by gray arrows and lines.Citation16)](/cms/asset/32b3b706-7c7f-44d3-b20b-3013c07a5c44/tbbb_a_1023251_f0003_oc.gif)
IV. Future perspectives
The further elucidation of the molecular mechanisms of taste sensation in fish is required. Many genetic techniques used in other model species can also be used in small fish species, such as Gal4/UAS, Cre/loxP, and others. The relative ease of obtaining fertilized eggs and the high efficiency of transgenesis are the advantages of small fish compared with rodents. Recently, targeted genome editing using a CRISPR/Cas system has been shown to work in small fish.Citation17) Thus, research using gene knockout and knock-in in fish would be possible. Advances using these techniques constitute good reason to utilize fish as model organisms for taste research. Furthermore, comparative molecular biology would lead to understanding a general logic of taste systems in vertebrates.
The transparency of their bodies is one of the excellent advantageous properties of small fish species for taste research, as well as for a wide variety of research areas. Cells in the body can be easily observed with fluorescent protein-labeling or other histochemical techniques. With respect to taste systems, their numerous taste buds, which are not assembled into papillae, are suitable for microscopic observation. Combined with another advantageous property—that their embryonic developments occur rapidly in clear-shelled eggs—small fish have been proven immensely useful for the developmental studies. Indeed, they have recently been used for developmental study of the taste system.Citation18) Many central and peripheral neurons can be observed in vivo even in the larval stages of these animals. Therefore, in vivo imaging of neuronal activities responding to various stimulation, such as odorants and visual stimuli, have been performed using transgenic fish expressing a genetically encoding calcium indicator, or other indicator, in their neurons.Citation19,20) There have been no reports about in vivo neuronal imaging of taste sensation in fish. Although in vivo neuronal imaging of taste cortex and cranial ganglia has been reported in mice, Citation21,22) studies using fish would bring appreciable results because of their ease and comprehensiveness. Laser ablation of specific neurons or brain areas can be performed in small fish. This is also helpful in elucidating the neuronal circuits for taste sensation. These various advantageous properties of small fish species will undoubtedly bring breakthrough achievements in the study of taste systems.
Disclosure statement
No potential conflict of interest was reported by the author.
Funding
This work was supported in part by the Grant-in-Aid for Young Scientists (A) [23688016] and Scientific Research (B) [26292064], the Nissin Seifun Foundation, the Iijima Foundation for the Promotion of Food Science and Technology, the Salt Science Research Foundation [0844], and the LOTTE Foundation.
Acknowledgments
This research was performed at the Laboratory of Biological function and the ILSI-Endowed Chair of Functional Food Science and Nutrigenomics, Department of Applied Biological Chemistry, Graduate School of Agricultural and Life Sciences at the University of Tokyo. I would like to express my sincere gratitude to Dr. Keiko Abe and Dr. Soichi Arai for their guidance and continuous encouragement. I also thank all my collaborators and the members of the laboratories for their pertinent advices and experimental supports.
Notes
This review was written in response to the author’s receipt of the JSBBA Award for Young Scientists in 2013.
References
- Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244.10.1016/j.cell.2009.10.001
- Ishimaru Y, Okada S, Naito H, Nagai T, Yasuoka A, Matsumoto I, Abe K. Two families of candidate taste receptors in fishes. Mech. Dev. 2005;122:1310–1321.10.1016/j.mod.2005.07.005
- Yoshida Y, Saitoh K, Aihara Y, Okada S, Misaka T, Abe K. Transient receptor potential channel M5 and phospholipaseC-β2 colocalizing in zebrafish taste receptor cells. NeuroReport. 2007;18:1517–1520.10.1097/WNR.0b013e3282ec6874
- Picone B, Hesse U, Panji S, Van Heusden P, Jonas M, Christoffels A. Taste and odorant receptors of the coelacanth-A gene repertoire in transition. J. Exp. Zool. B. Mol. Dev. Evol. 2014;322:403–414.
- Ohmoto M, Okada S, Nakamura S, Abe K, Matsumoto I. Mutually exclusive expression of Gαia and Gα14 reveals diversification of taste receptor cells in zebrafish. J. Comp. Neurol. 2011;519:1616–1629.10.1002/cne.v519.8
- Oike H, Nagai T, Furuyama A, Okada S, Aihara Y, Ishimaru Y, Marui T, Matsumoto I, Misaka T, Abe K. Characterization of ligands for fish taste receptors. J. Neurosci. 2007;27:5584–5592.10.1523/JNEUROSCI.0651-07.2007
- Misaka T. Development of a cultured cell-based human-taste evaluation system. Biosci. Biotechnol. Biochem. 2013;77:1613–1616.10.1271/bbb.130288
- Hidaka I, Ohsugi T, Kubomatsu T. Taste receptor stimulation and feeding behaviour in the puffer, Fugu pardalis. I. Effect of single chemicals. Chem. Senses. 1978;3:341–354.10.1093/chemse/3.4.341
- Lamb CF, Finger TE. Gustatory control of feeding behavior in goldfish. Physiol. Behav. 1995;57:483–488.10.1016/0031-9384(94)00287-F
- Aihara Y, Yasuoka A, Iwamoto S, Yoshida Y, Misaka T, Abe K. Construction of a taste-blind medaka fish and quantitative assay of its preference-aversion behavior. Genes Brain Behav. 2008;7:924–932.10.1111/gbb.2008.7.issue-8
- Yoshihara Y, Mizuno T, Nakahira M, Kawasaki M, Watanabe Y, Kagamiyama H, Jishage K, Ueda O, Suzuki H, Tabuchi K, Sawamoto K, Okano H, Noda T, Mori K. A genetic approach to visualization of multisynaptic neural pathways using plant lectin transgene. Neuron. 1999;22:33–41.10.1016/S0896-6273(00)80676-5
- Damak S, Mosinger B, Margolskee RF. Transsynaptic transport of wheat germ agglutinin expressed in a subset of type II taste cells of transgenic mice. BMC Neurosci. 2008;9:96.10.1186/1471-2202-9-96
- Ohmoto M, Matsumoto I, Yasuoka A, Yoshihara Y, Abe K. Genetic tracing of the gustatory and trigeminal neural pathways originating from T1R3-expressing taste receptor cells and solitary chemoreceptor cells. Mol. Cell. Neurosci. 2008;38:505–517.10.1016/j.mcn.2008.04.011
- Ohmoto M, Maeda N, Abe K, Yoshihara Y, Matsumoto I. Genetic tracing of the neural pathway for bitter taste in t2r5-WGA transgenic mice. Biochem. Biophys. Res. Commun. 2010;400:734–738.10.1016/j.bbrc.2010.08.139
- Yamamoto K, Ishimaru Y, Ohmoto M, Matsumoto I, Asakura T, Abe K. Genetic tracing of the gustatory neural pathway originating from Pkd1l3-expressing type III taste cells in circumvallate and foliate papillae. J. Neurochem. 2011;119:497–506.10.1111/jnc.2011.119.issue-3
- Ieki T, Okada S, Aihara Y, Ohmoto M, Abe K, Yasuoka A, Misaka T. Transgenic labeling of higher order neuronal circuits linked to phospholipase C-β2-expressing taste bud cells in medaka fish. J. Comp. Neurol. 2013;521:1781–1802.10.1002/cne.23256
- Ansai S, Kinoshita M. Targeted mutagenesis using CRISPR/Cas system in medaka. Biol. Open. 2014;3:362–371.10.1242/bio.20148177
- Kapsimali M, Kaushik AL, Gibon G, Dirian L, Ernest S, Rosa FM. Fgf signaling controls pharyngeal taste bud formation through miR-200 and Delta-Notch activity. Development. 2011;138:3473–3484.10.1242/dev.058669
- Muto A, Ohkura M, Abe G, Nakai J, Kawakami K. Real-time visualization of neuronal activity during perception. Curr. Biol. 2013;23:307–311.10.1016/j.cub.2012.12.040
- Friedrich RW, Wiechert MT. Neuronal circuits and computations: pattern decorrelation in the olfactory bulb. FEBS Lett. 2014;588:2504–2513.10.1016/j.febslet.2014.05.055
- Chen X, Gabitto M, Peng Y, Ryba NJ, Zuker CS. A gustotopic map of taste qualities in the mammalian brain. Science. 2011;333:1262–1266.
- Barretto RP, Gillis-Smith S, Chandrashekar J, Yarmolinsky DA, Schnitzer MJ, Ryba NJ, Zuker CS. The neural representation of taste quality at the periphery. Nature. 2015;517:373–376.