Abstract
Three-finger toxins (3FTxs) are one of the major components in snake venoms. In this study, we isolated a cDNA encoding a short-chain 3FTx, Pr-SNTX, from Pseudechis rossignolii. The amino acid sequence of Pr-SNTX is nearly identical to that of its ortholog in Pseudechis australis. Pr-SNTX protein inhibited muscle-type (α2βδε), but not neuronal α7 nicotinic acetylcholine receptor (nAChR) activity.
Mulga snake (or King brown snake), Pseudechis australis, is a venomous snake found throughout most parts of Australia and in few New Guinean localities.Citation1,2) Because of morphological variations, the taxonomy of this species has been complicated. However, based on a recent genetic studyCitation3,4) and on observations of morphological differences between the Australian and New Guinean populations, the New Guinean form was classified as a distinct species, Pseudechis rossignolii, and renamed as Papuan pigmy mulga snake.Citation5)
To compare the venom component of P. rossignolii and P. australis, we randomly selected 148 clones from the venom glands cDNA library of P. rossignolii and identified ten phospholipase A2-like proteinsCitation6) and three Kunitz-type protease inhibitors.Citation7) Among the 148 clones, we found a short-chain three-finger toxin (3FTx) in 8.8% of the selected clones and termed it as Pr-SNTX (Fig. ).
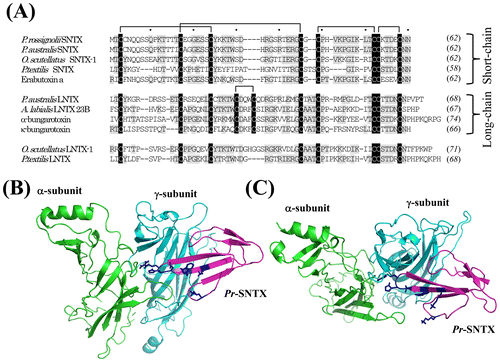
Fig. 1. Multiple alignments of the amino acid sequences of 3FTxs from various species and the structure model of the α/γ subunit interface interacting with Pr-SLTX.
Notes: (A) We isolated the cDNAs of 3FTxs from the venom gland cDNA library of P. rossignolii. The amino acid sequences of three-finger toxins from Elapid snakes were aligned using ClustalW program. The protein accession numbers are the following: P. australis SNTX, ABK63527; Oxyuranus scutellatus SNTX-1, AAZ22673; P. textilis SNTX, ABK63535; Erabutoxin a, BAA75752; P. australis LNTX, ABK 63539; Austrelaps labialis LNTX 23B, ABX58159; α-bungarotoxin, AAL30056; α-bungarotoxin, CAA69971; O. scutellatus LNTX-1, ABK63543; and P. textilis LNTX, ABK63540. Both O. scutellatus LNTX-1 and P. textilis LNTX have four disulfide bonds, and they did not follow the nomenclature of short- and long-type 3FTs. Residues that are identical among the majority of the amino acid sequences are highlighted in gray, and conserved cysteine residues are highlighted in black. The number of amino acid residues is given to the right of each line in italics. Intervals of 10 amino acids are indicated by dots above the alignment. Gaps (-) have been inserted to achieve maximum homology. The nucleotide sequence of Pr-SNTX has been assigned DDBJ/EMBL/GenBank Accession Number AB778565. (B) Overall view of the structure model from the side for the α/γ subunit interface interacting with Pr-SLTX. (C) Overall view of the structure model from the top. The three-dimensional structure of Pr-SNTX was modeled using MODELER v. 9.11 with the structure of cobrotoxin (PDB 1V6P) as a template. Pr-SLTX, α-subunit, and γ-subunit are displayed in magenta, green, and cyan, respectively. Side chains of amino acid residues (Lys27, Trp29, Asp31, His32, Arg33, and Lys47) important for toxin binding to nAChR Citation13) are shown in blue.
3FTxs are small molecules composed of 60–74 amino acid residues present in elapid and hydrophiid snake venoms that interact with a variety of target molecules, including nicotinic acetylcholine receptors (nAChRs), muscarinic acetylcholine receptors (mAChRs), β-adrenergic receptors, and acetylcholine esterase.Citation8,9) Structurally, 3FTxs have three β-strand loops similar to the fingers that protrude from a small core domain, which is covalently cross-linked by four disulfide bonds. Neurotoxic 3FTxs that target nAChRs are mainly classified into two structural types: short-chain 3FTxs (60–62 amino acid residues, 4 disulfide bonds) preferentially block muscle-type nAChRs, and long-chain 3FTxs (66–75 amino acid residues, 5 disulfide bridges) that in addition to muscle-type nAChRs block neuronal homo-oligomeric types.Citation10)
In P. australis, short-(Pa-SNTX)Citation11) and long-(Pa-LNTX)Citation12) chain 3FTxs have been identified. Pa-SNTX has a lethal activity (LD50) of 76 μg/kg body weight by intravenous injection in mice.Citation11) Pa-LNTX is not toxic to mice at doses smaller than 10 mg/kg and has no affinity to nAChRs purified from the crampfish electric organ.Citation12) However, no data regarding the effects of Pa-SNTX on nAChRs have been reported.
Pr-SNTX and Pa-SNTX are identical in their amino acid sequences except for two amino acid residue substitutions at positions 37 and 39 (97.6% identity in precursor proteins). 3FTxs utilize a common binding-site to interact with key nAChR residues. Mutational analysis of Erabutoxin a, a typical short-chain 3FTx, revealed that in Torpedo nAChR recognition, the common binding-site consisted of the following charged and aromatic residues: Lys27, Trp29, Asp31, Phe32, Arg33, and Lys47 (the numbering refers to Erabutoxin a).Citation13) The amino acid residues of Pr-SNTX binding-site are identical to the corresponding amino acid residues of Erabtoxin a, except for Phe32 (Fig. ).
To predict the possible target molecule of Pr-SNTX, we modeled the structure of Pr-SNTX bound to muscle-type nAChR (α/γ subunit interface). Pr-SNTX structure was constructed with the program MODELER 9v11 (http://www.salilab.org/modeller/about_modeller.html) using cobrotoxin (PDB ID: 1V6P) as a template. The resulting structure was energy-minimized with GROMACS 4.5.Citation14) Model of the extracellular nAChR domain containing α- and γ-subunits was constructed as previously describedCitation15) and used for docking experiments with HEX (version 6.12) program (http://hex.loria.fr/hex.php). The structure obtained was very similar to that of a short-chain 3FTx from Naja oxiana (NTII) with the same extracellular domain.Citation15). The tip of Pr-SNTX loop II enters the ligand-binding pocket between the nAChR subunits, while loops I and III are in contact with nAChR by their tips only (Fig. (B) and (C)). Not only from the primary structure of Pr-SNTX, but also from the binding model of Pr-SNTX to muscle-type nAChR, it was predicted that Pr-SNTX might block the muscle-type nAChR.
In order to characterize the physiological functions of Pr-SNTX, Pr-SNTX mature protein was expressed in E. coli as a histidine hexamer fusion protein. Pr-SNTX cDNA fragment was amplified by PCR using a forward primer SNeuTX-5′ [5′-GTGGATCCCTGGAAGTTCTGTTCCAGGGGCCCATGACATGTTGCAAC-3′: BamHI site is underlined; the PreScission recognition amino acid sequence (Leu-Glu-Val-Leu-Phe-Gln-Gly-Pro) is double underlined] and a reverse primer SNeuTX-3′ (5′-ATGAATTCTAATTGTTGCATTCGTC-3′: EcoRI site is underlined). To eliminate the histidine hexamer tag from the recombinant protein, a DNA sequence that code PreScission (GE Healthcare UK Ltd., Buckinghamshire, UK) recognition amino acid sequence was inserted in the boundary between the tag and Pr-SNTX protein coding regions. After the amplified DNA was digested with BamHI and EcoRI, the products were subcloned into the expression vector pCold II (Takara, Otsu, Japan). E. coli strain Origami B (Novagen, Madison, WI, USA) was transformed with the plasmids, and the soluble proteins were prepared by using BugBuster (Novagen). After purification of the histidine hexamer fusion proteins by chromatography on TALON His-Tag Purification Resin (Clontech, Mountain View, CA, USA), the histidine hexamer tag was cleaved by PreScission protease by incubation at 4 °C overnight in the cleavage buffer (150 mM NaCl, 1.0 mM EDTA, 1.0 mM DTT, and 50 mM Tris HCl, pH 7.0). The recombinant protein was further purified by reverse-phase HPLC on an Intrada WP-RP column (Imtakt, Kyoto, Japan). The molecular weight of recombinant Pr-SNTX was verified by SDS-PAGE (Fig. (A)) and matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS) (Fig. (B)) using AXIMA performance (Shimadzu Techno-Research, Kyoto, Japan). The observed average molecular mass [M + H]+ of the recombinant Pr-SNTX was 6949.66. It was nearly identical to the theoretical average molecular mass of the Pr-SNTX S-S oxidized form including Gly-Pro residues, remaining after the PreScission digestion at the N-terminus (6949.78).
Fig. 2. SDS-PAGE and MALDI-TOF-MS spectrometry analyses of recombinant Pr-SNTX protein.
Notes: (A) Recombinant Pr-SLTX protein was expressed in E. coli and purified by TALON affinity column and a reverse-phase HPLC column, Intrada WP-RP. Purified protein (2 μg) was loaded on 12% NuPAGE (Life Technologies, Carlsbad, CA) and stained with Coomassie Brilliant Blue R-250. (B) The molecular weight of recombinant Pr-SNTX was verified by MALDI-TOF-MS using AXIMA Performance. The observed average molecular mass [M + H]+ of recombinant Pr-SNTX protein was 6949.66. The mass peaks at m/z 3476.14 correspond to [M + 2H]2+ for Pr-SNTX.
![Fig. 2. SDS-PAGE and MALDI-TOF-MS spectrometry analyses of recombinant Pr-SNTX protein.Notes: (A) Recombinant Pr-SLTX protein was expressed in E. coli and purified by TALON affinity column and a reverse-phase HPLC column, Intrada WP-RP. Purified protein (2 μg) was loaded on 12% NuPAGE (Life Technologies, Carlsbad, CA) and stained with Coomassie Brilliant Blue R-250. (B) The molecular weight of recombinant Pr-SNTX was verified by MALDI-TOF-MS using AXIMA Performance. The observed average molecular mass [M + H]+ of recombinant Pr-SNTX protein was 6949.66. The mass peaks at m/z 3476.14 correspond to [M + 2H]2+ for Pr-SNTX.](/cms/asset/e862a90a-673f-4665-94fd-5eb699621968/tbbb_a_1065169_f0002_b.gif)
Using the two-electrode voltage clamp technique on Xenopus laevis oocytes, we investigated the effects of recombinant Pr-SNTX on mouse muscle-type nAChR (α2βδε) and rat neuronal α7 nAChRs. The electrophysiology measurements using X. laevis oocytes were described in a previous study.Citation16)
Mouse α-, β-, δ-, and ε-subunits as well as rat α7 subunit of AChR cDNAs were isolated by RT-PCR (Suppl. Table 1). The amplified DNA products were subcloned into the X. laevis oocytes expression vector pSD64TRER. Oocytes were injected with approximately 27 nl rat nicotinic AChR α7 (1 μg/μl) or with a combination mixture of mouse α-, β-, δ-, and ε- subunits (0.4, 0.2, 0.2, and 0.2 μg/μl, respectively) of nAChR cRNAs and incubated in modified Barth solution (MBS) at 18 °C for 1 to 2–4 days prior to electrophysiological analysis. Current traces were evoked using 10 and 100 μM acetylcholine (ACh) in oocytes for muscle and neuronal nAChRs, respectively.
Fetal and adult muscle-type nAChRs are known, which are composed of α2βγδ and α2βδε subunits, respectively. The ligand-binding-sites of both nAChR types are located at the α/γ, α /δ, and α/ε subunit interfaces. A short-chain 3FTx of Naja mossambica mossambica (NmmI) bound with high affinity to the α/γ and α/δ subunit interfaces (Kd = 0.14 nM). Binding affinity of NmmI to the α/ε subunit (Kd = 130 nM) was 1000 times lower than the binding affinity to the α/γ and α /δ subunit interfaces.Citation17) Despite the different potential affinities of neurotoxic 3FTxs to muscle-type nAChRs, adult-type nAChR (α2βδε) were used to assess the activity of 3FTxs in this study.
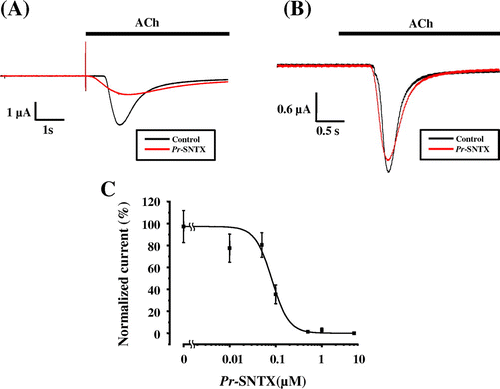
Pr-SNTX showed fairly high inhibitory activity against muscle-type nAChR (α2βδε) with a half inhibitory concentration (IC50) of 82.9 ± 12.3 nM (Fig. (A) and (C)) and had no effects on neuronal α7 nAChR (Fig. (B)) at concentrations up to 10 μM. The inhibitory activity of Pr-SNTX against muscle-type nAChR was less effective than α-bungarotoxin (IC50 = 52.9 ± 10.1 nM, Suppl. Fig. 1).
Fig. 3. Pr-SNTX inhibits the acetylcholine-evoked current in oocyte expressed muscle-type nAChR (α2βδε), but not neuronal α7 nAChR.
Notes: (A) Acetylcholine-evoked currents of muscle-type nAChRs (α2βδε) were recorded in the absence (black) or presence (red) of 100 nM Pr-SNTX protein; bar indicates acetylcholine (10 μM) application. (B) Acetylcholine-evoked currents of neuronal α7 nAChRs were recorded in the absence (black) or presence (red) of 10 μM Pr-SNTX protein; bar indicates acetylcholine (100 μM) application. (C) Dose-dependent inhibitory effects of Pr-SNTX. Data were analyzed and plotted using Origin 8 (OriginLab., Northampton, MA, USA). The 100% activity value was determined by measurements in the absence of Pr-SNTX protein. Each data point represents the mean from a single experiment performed in triplicate. Mean values obtained from five oocytes. Error bars show the standard error of the mean.
Both short- and long-chain 3FTxs have been identified from many snake species. In P. rossignolii, we have also identified a long-chain 3FTx (Pr-LNTX) which is quite different from its P. australis ortholog (data not shown) in amino acid sequence. In future studies, we will characterize the physiological functions of Pr-LNTX and elucidate the synergistic neurotoxicity of Pr-SNTX and Pr-LNTX.
Author contributions
YY isolated Pr-SNTX cDNA. YY, HK, and HI expressed and purified the recombinant protein. XY and TK isolated the nAChR subunit cDNAs. YY and HI performed electrophysiological recordings. SF and YU modeled the structure of Pr-SNTX bound to muscle-type nAChR. HI wrote the manuscript.
Supplemental material
The supplemental material for this article is available at http://dx.doi.org/10.1080/09168451.2015.1065169.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Ministry of Education, Science, Sports, and Culture, Grant-in-Aid for Scientific Research (C), [2010, 21600021]. YU and SF were partially supported by the Russian Foundation for Basic Research [project No. 15-04-01843a].
Suppl._DATA.pptx
Download MS Power Point (71.7 KB)Acknowledgments
We thank Mami Takeda for the isolation of muscle-type nAChR cDNAs. We also thank the deceased Dr. Michihisa Toriba in the Japan Snake Institute for providing and classifying P. rossignolii with valuable advice of snake taxonomy.
References
- Cogger HG. Reptiles and amphibians of Australia. Sydney: Reed New Holland; 2000.
- O’M.Shea A guide to the snakes of Papua New Guinea. Port Moresby: Independent Publishing; 1996.
- Wüster W, Dumbrell AJ, Hay C, Pook CE, Williams DJ, Fry BG. Snakes across the Strait:trans-Torresian phylogeographic relationships in three genera of Australasian snakes (Serpentes:Elapidae:Acanthophis, Oxyuranus, and Pseudechis). Mol. Phylogenet. Evol. 2005;34:1–14.10.1016/j.ympev.2004.08.018
- Williams D, Wüster W. Venomous Bites and Stings in Papua New Guinea. In: Williams D, Jensen S, Nimorakiotakis B, Winkel KD, editors. Snakes of Papua New Guinea. Melbourne.: Australia Venom Research Unit; 2005. p. 33–64.
- Hoser R. A new species of snake (Serpentes:Elapidae) from Irian Jaya. Litt. Serpentium. 2000;20:178–186.
- Inagaki H, Yamauchi Y, Toriba M, Kubo T. Regional divergence of phospholipase A(2)-like protein cDNAs between New Guinean and Australian Pseudechis australis. Toxicon. 2010;56:637–639.10.1016/j.toxicon.2010.04.018
- Inagaki H, Kimoto H, Yamauchi Y, Toriba M, Kubo T. Functional characterization of Kunitz-type protease inhibitor Pr-mulgins identified from New Guinean Pseudechis australis. Toxicon. 2012;59:74–8010.1016/j.toxicon.2011.10.005
- Kini RM, Doley R. Structure, function and evolution of three-finger toxins: mini proteins with multiple targets. Toxicon. 2010;56:855–867.10.1016/j.toxicon.2010.07.010
- Tsetlin V, Utkin Y, Kasheverov I. Polypeptide and peptide toxins, magnifying lenses for binding sites in nicotinic acetylcholine receptors. Biochem. Pharmacol. 2009;78:720–731.10.1016/j.bcp.2009.05.032
- Tsetlin VI. Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors:pharmacological tools and endogenous modulators. Trends Pharmacol. Sci. 2015;36:109–123.10.1016/j.tips.2014.11.003
- Takasaki C, Tamiya N. Isolation and amino acid sequence of a short-chain neurotoxin from an Australian elapid snake, Pseudechis australis. Biochem. J. 1985;232:367–371.
- Takasaki C. Amino acid sequence of a long-chain neurotoxin homologue, Pa ID, from the venom of an Australian elapid snake, Pseudechis australis. J. Biochem. 1989;106:11–16.
- Trémeau O, Lemaire C, Drevet P, et al. Genetic engineering of snake toxins. The functional site of Erabutoxin a, as delineated by site-directed mutagenesis, includes variant residues. J. Biol. Chem. 1995;270:9362–9369.
- Pronk S, Páll S, Schulz R, et al. GROMACS 4.5:a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics. 2013;29:845–854.10.1093/bioinformatics/btt055
- Mordvintsev DY, Polyak YL, Levtsova OV, et al. A model for short α-neurotoxin bound to nicotinic acetylcholine receptor from Torpedo californica: comparison with long-chain α-neurotoxins and α-conotoxins. Comput. Biol. Chem. 2005;29:398–411.10.1016/j.compbiolchem.2005.08.007
- Yassaka RT, Inagaki H, Fujino T, Nakatani K, Kubo T. Enhanced activation of the transient receptor potential channel TRPA1 by ajoene, an allicin derivative. Neurosci. Res. 2010;66:99–105.10.1016/j.neures.2009.09.1712
- Osaka H, Malany S, Kanter JR, Sine SM, Taylor P. Subunit interface selectivity of the α-neurotoxins for the nicotinic acetylcholine receptor. J. Biol. Chem. 1999;274:9581–9586.10.1074/jbc.274.14.9581
