Abstract
Coenzyme Q (CoQ) is a component of the electron transport chain that participates in aerobic cellular respiration to produce ATP. In addition, CoQ acts as an electron acceptor in several enzymatic reactions involving oxidation–reduction. Biosynthesis of CoQ has been investigated mainly in Escherichia coli and Saccharomyces cerevisiae, and the findings have been extended to various higher organisms, including plants and humans. Analyses in yeast have contributed greatly to current understanding of human diseases related to CoQ biosynthesis. To date, human genetic disorders related to mutations in eight COQ biosynthetic genes have been reported. In addition, the crystal structures of a number of proteins involved in CoQ synthesis have been solved, including those of IspB, UbiA, UbiD, UbiX, UbiI, Alr8543 (Coq4 homolog), Coq5, ADCK3, and COQ9. Over the last decade, knowledge of CoQ biosynthesis has accumulated, and striking advances in related human genetic disorders and the crystal structure of proteins required for CoQ synthesis have been made. This review focuses on the biosynthesis of CoQ in eukaryotes, with some comparisons to the process in prokaryotes.
Graphical abstract
The enzymes involved in CoQ synthesis form a complex in S. cerevisiae. At least nine peptides are required for the synthesis of CoQ.
Key words:
Introduction
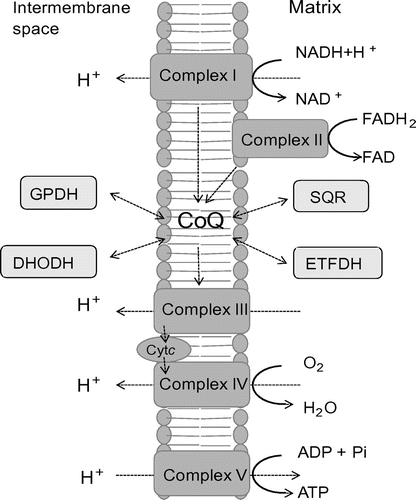
Coenzyme Q (CoQ), also known as ubiquinone or 2,3-dimethoxy-5-methyl-6-polyprenyl-1, 4-benzoquinone, is a well-known component of the electron transport chain that participates in aerobic cellular respiration within the mitochondria of eukaryotes and the plasma membrane of prokaryotes. CoQ exists in both reduced and oxidized forms;Citation1) conversion between these states allows it to transfer electrons to substrates and act as a cofactor of enzymatic reactions (Fig. ). CoQ plays the role of the electron donor during disulfide bond formation in Escherichia coli,Citation2) and its reduction is coupled to sulfide oxidation by sulfide–quinone oxidoreductase in Schizosaccharomyces pombe and mammals.Citation3) CoQ also couples with electron transfer by glycerol-3-phosphate dehydrogenase and electron-transferring flavoprotein dehydrogenase, the latter of which is involved in the beta-oxidation of fatty acids in mammals.Citation4) Moreover, CoQ is required for the de novo synthesis of UMP as a cofactor of dihydroorotate dehydrogenase in many eukaryotes (Fig. ).Citation5,6)
Fig. 1. The electron transfer system and enzymes involved in CoQ biosynthesis. Notes: The position of CoQ in the electron transfer system is shown. Complexes I and II transfer electrons to CoQ from NADH and FADH2, respectively. In yeast, NADH dehydrogenase replaces Complex I in the first reaction. Electrons are transferred to Complex III from CoQH2, a reduced form of CoQ, and then further transferred to Complex IV through cytochrome c (Cytc). Protons are transferred to the intermembrane space, and this proton gradient drives ATP production through Complex V. A number of different enzymes are coupled with CoQ in oxidation–reduction reactions: DHODH, dihydroorotate dehydrogenase; SQR, sulfide quinone reductase; ETFDH, electron transfer flavoprotein dehydrogenase; and GPDH, glycerol-3-phosphate dehydrogenase.
CoQ synthesis is divided into two parts: the synthesis of isoprenoid (Fig. ) and the modification of quinone (Fig. ). The synthesis of isoprenoids has been studied extensively; however, the mechanism by which quinone is synthesized in eukaryotes requires clarification. Living organisms possess a number of species of CoQ with differing isoprenoid side chain lengths. For example, the human and S. pombe CoQ contains ten isoprene units, whereas CoQ from Arabidopsis thaliana, E. coli, and Saccharomyces cerevisiae contains nine, eight, and six units, respectively. The length of the CoQ side chain is defined by the product generated by polyprenyl diphosphate synthases.Citation7,8)
Fig. 2. Biosynthetic pathway of the isoprenoid tail of CoQ. Notes: Isopentenyl diphosphate (IPP) is synthesized via the mevalonate (MVA) pathway in eukaryotes and the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway in prokaryotes and plants. In each organism, trans-polyprenyl diphosphate of a certain length is synthesized by polyprenyl diphosphate synthase. S. cerevisiae Coq1 synthesizes six isoprene units, E. coli IspB synthesizes eight isoprene units, and H. sapiens or S. pombe decaprenyl diphosphate (DPP; a heteromer of PDSS1 and PDSS2 or Dps1 and Dlp1, respectively) synthesize ten isoprene units. S. cerevisiae Coq2, E. coli UbiA, and H. sapiens COQ2 or S. pombe Ppt1(Coq2) condense PHB with trans-polyprenyl diphosphate to form CoQ6, CoQ8, and CoQ10, respectively. DMAPP, dimethylallyl diphosphate; GPP, geranly diphosphate; FPP, farnesyl diphosphate; IPP, isopentenyl diphosphate; HexPP, Hexaprenyl diphosphate; OPP, Octaprenyl diphosphate.
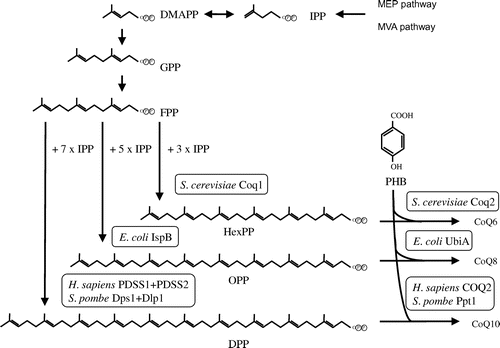
Fig. 3. Overview of the proposed CoQ biosynthetic pathway. Notes: The CoQ biosynthetic pathways of prokaryotes (represented by E. coli) and eukaryotes (represented by S. cerevisiae). In general, the gene names ubi* and COQ(coq)* are used in prokaryotes and eukaryotes, respectively; however, the nomenclature of genes can differ among species. After condensation of PHB with trans-polyprenyl diphosphate, the ring structure is modifed. In E. coli, decarboxylation by UbiD or UbiX is followed by hydroxyation by UbiI, O-methylation by UbiG, hydroxyation by UbiH, C-methylation by UbiE, a final hydroxylation by UbiF, and then O-methylation by UbiG. At least eight genes are responsible for CoQ biosynthesis in E. coli. In S. cerevisiae, pABA is also used as a substrate in addition to PHB. In this species, the first ring is modifed by hydroxyation by Coq6, followed by O-methylation by Coq3; however, the subsequent decarboxylation and hydroxylation steps are unclear. The ring is then modified further by C-methylation by Coq5, a final hydroxylation by Coq7, and O-methylation by Coq3.
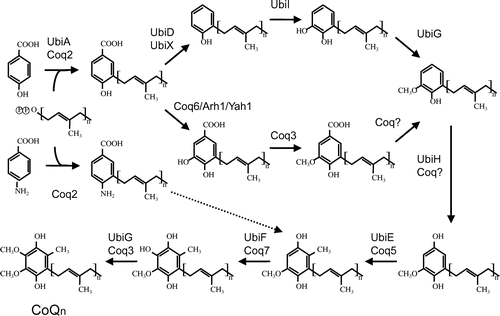
In prokaryotes, the biosynthetic pathway that converts para-hydroxybenzoate (PHB) into CoQ consists of at least eight steps, and the order of reactions is believed to be different in eukaryotes (Fig. ). These steps include the condensation and transfer of the isoprenoid side chain to PHB, followed by hydroxylation, methylation, and decarboxylation. To date, this pathway has been studied most extensively in E. coli and S. cerevisiae using genetic and biochemical analyses. At least 11 genes (ubiA, B, C, D, E, F, G, H, I, J, and X) are involved in CoQ biosynthesis in E. coli, and 11 genes (YAH1, ARH1, and COQ1-COQ9) are required for this process in S. cerevisiae. Involvement of para-amino benzoic acid (pABA) in CoQ biosynthesis is only shown in S. cerevisiae.
Following on from my previous reviews published in 2002Citation9) and 2009,Citation1) the current review provides an overall picture of the biosynthetic pathway of CoQ, with a special focus on recent advances in structurally based insights and the relationship between CoQ and human genetic disorders. Because bacterial biosynthesis of CoQ has been comprehensively reviewed by Aussell et al.Citation10) this review focuses on the process in eukaryotes.
Genes responsible for CoQ biosynthesis
Genes involved in the CoQ biosynthetic pathway have been studied most extensively in two model organisms, namely E. coli and S. cerevisiae, which are representatives of prokaryotes and eukaryotes, respectively.Citation1,9) E. coli ubi (ubiA–J and ubiX) mutants and S. cerevisiae coq (coq1–coq9) mutants, which are unable to synthesize CoQ, were used to define the biosynthetic pathway of CoQ.Citation11–14) Until recently, all corresponding genes for E. coli ubi mutants and S. cerevisiae coq mutants have been identified. While the functions of all E. coli ubi genes except ubiB have been completely assigned, the functions of three S. cerevisiae COQ genes (COQ4, COQ8, and COQ9) remain elusive.Citation1,12) Table shows a comparison of the CoQ biosynthetic genes from seven species, namely Homo sapiens, Mus musculus, Caenorhabditis elegans, A. thaliana, S. cerevisiae, S. pombe, and E. coli. The order of the first three steps of the CoQ biosynthetic pathway differs in E. coli and S. cerevisiae (Fig. ). The decarboxylation stage of the ring modification procedure precedes the hydroxylation and methylation stages in E. coli, but the order of these reactions is thought to be different in S. cerevisiae. The species-specific phenotypes of all coq gene-knockout S. pombeCitation3,15) and C. elegansCitation16) strains have been reported. It is generally considered that the CoQ biosynthesis process is common in eukaryotes (Table ); however, some differences have been reported: (i) polyprenyl diphosphate synthase forms a homodimeric or homotetrameric structure in S. cerevisiae,Citation17) but the homologous enzyme forms a heterotetramer structure composed of two subunits in S. pombe, mouse, and humanCitation18,19); (ii) orthologs of Coq7 are missing in plant genomic databases; and (iii) Coq9 is missing in C. elegans.
Table 1. CoQ biosynthetic genes from various species.
Recent studies of human inherited diseases led to the identification of genetic disorders related to CoQ biosynthesis (Table ). The following sections describe the genes involved in CoQ biosynthesis in S. cerevisiae in detail.
Table 2. Genotype–phenotype correlations in inherited deficiencies of CoQ10 biosynthesis in humans.
Coq1/IspB/PDSS1 + PDSS2/polyprenyl diphosphate synthase
Polyprenyl diphosphate synthase (Coq1 in S. cerevisiae, IspB in E. coli, and a heteromer of PDSS1 and PDSS2 in H. sapiens), the enzyme that synthesizes the isoprenoid chain of CoQ, is classified into two types, namely the homomer and heteromer types. S. cerevisiae Coq1 (hexaprenyl diphosphate synthase) catalyzes the condensation of isopentenyl diphosphate and farnesyl diphosphate or geranylgeranyl diphosphate to produce six units of prenyl diphosphate, which are used to produce CoQ6 (Fig. ).Citation17) Long-chain trans-polyprenyl diphosphate synthases, such as Coq1, have been studied in many species ranging from bacteria to humans. These enzymes possess seven conserved regions, including two DDXXD motifs that involve binding of substrates in association with Mg2+.Citation20) Bacterial polyprenyl diphosphate synthases, S. cerevisiae Coq1, and A. thaliana solanesyl diphosphate synthase are homomeric enzymes.Citation21–24) Meanwhile, decaprenyl diphosphate synthases from S. pombe and human are heterotetramers consisting of dimer of two proteins, namely Dps1/Dlp1 and PDSS1/PDSS2, respectively.Citation18,19,25) PDSS2/Dlp1 lacks the aspartate-rich motifs and is bound to PDSS1/Dps1 to form a heteromeric enzyme.Citation18,19) Recent studies generated functional artificial heteromeric polyprenyl diphosphate synthases comprising S. cerevisiae Coq1 and S. pombe Dps1, or E. coli IspB and S. pombe Dps1, the latter of which (Dps1) is non-functional by itself, suggesting that it may support the activities of polyprenyl diphosphate synthases by partnering with a related protein.Citation17,26) The crystal structures of octaprenyl diphosphate synthase (IspB) from Thermotoga maritime and E. coli have been solved,Citation27,28) and analyses of these enzymes in complex with farnesyl diphosphate and isopentenyl diphosphate clearly defined the substrate-binding sites (Fig. ). Mice carrying mutations in the Pdss2 gene display focal segmental glomerulopathy-like kidney disease that is rescued by CoQ10 supplementation.Citation29) In addition, mutations in the human PDSS1 and PDSS2 genes, such as those associated with Leigh disease and nephropathy, result in poor mitochondrial functionality (Table ).Citation30,31)
Coq2/UbiA/COQ2/PHB-polyprenyl diphosphate transferase
PHB-polyprenyl diphosphate transferase (Coq2 in S. cerevisiae, UbiA in E. coli, and COQ2 in H. sapiens) catalyzes the condensation of PHB with the isoprenoid chain,Citation32,33) and genetic and biochemical analyses have revealed that this enzyme has broad substrate specificity.Citation34) The yeast coq2 mutant and the E. coli ubiA mutant, which display a complete loss of PHB-polyprenyl diphosphate transferase activity, are complemented by the human and S. cerevisiae COQ2 genes, respectively.Citation32) The analysis of a T-DNA insertion mutant revealed that the A. thaliana PPT1 gene (a homolog of S. cerevisiae COQ2) is essential for embryo development.Citation35) Alongside hydroxy benzoic acid, the novel precursor pABA can also be used as a substrate for Coq2 in S. cerevisiae,Citation36,37) but presumably not in humans. Analysis of the crystal structure of UbiA from Aeropyrum pernix (Fig. ) revealed that it comprises nine transmembrane domains and enabled visualization of the potential entry site of the substrate (PHB).Citation38) Mutations in the human COQ2 gene cause encephalomyopathy, cerebellar ataxia, neurological distress, and other disorders (Table ).Citation31,39,40)
Coq3/UbiG/COQ3/O-methyltransferase
Dihydroxypolyprenylbenzoate methyltransferase (O-methyltransferase) is encoded by the ubiG gene in E. coli and the COQ3 gene in S. cerevisiae and H. sapiens.Citation41,42) During CoQ biosynthesis, this enzyme catalyzes two O-methylation steps at positions 5 and 6 of the ring structure after hydroxylation by Coq6 and Coq7. The amino acid sequences of the proteins encoded by COQ3 homologs contain four regions that are conserved in a large family of methyl transferase enzymes that utilize S-adenosyl methionine (SAM) as a methyl donor. E. coli UbiG complements the function of S. cerevisiae Coq3, indicating the functional similarity of these proteins.Citation43) S. pombe mutants lacking coq3 display the common phenotypes found in other coq mutants.Citation44) Homozygous C. elegans coq3 mutants that lack methyltransferase activity display delayed development and a sterile phenotype, and these mutants are lethal at the embryonic stage in the next generation.Citation45)
Coq4/COQ4
Coq4 is absolutely required for the biosynthesis of CoQ in S. cerevisiae, S. pombe, and C. elegans. Homologs of Coq4 are also found in cyanobacteria; however, the molecular function of the Coq4 protein has not been elucidated in any organism.Citation15,46) The amino acid sequence of the Coq4 protein does not share any significant homology with protein domains or motifs with known enzymatic activity. The Coq4 protein is peripherally associated with the matrix side of the inner mitochondrial membrane and may play a structural role in the putative polypeptide CoQ biosynthetic complex.Citation46) Lack of COQ4 in S. cerevisiae causes the instability of several Coq proteins, such as Coq7 and Coq3. Haploinsufficiency of COQ4 leads to encephalomyopathy in humans.Citation47)
Coq5/UbiE/COQ5/C-methyltransferase
S. cerevisiae Coq5, E. coli UbiE, and H. sapiens COQ5 catalyze the C-methylation step in the CoQ biosynthetic pathway. These enzymes contain four sequence motifs that are present in a large family of SAM-dependent methyltransferase enzymes. Demonstration of the enzymatic activity of Coq5 methyltransferase using 2-methoxy-6-polypreny-1,4-benzoquinone revealed its exact role in CoQ synthesis.Citation48) E. coli UbiE complements the function of Coq5 in S. cerevisiae,Citation48) and human COQ5 associates with COQ4. Analysis of the crystal structure of S. cerevisiae Coq5 in both the apo and SAM-bound forms revealed that it forms a dimer and contains a typical SAM domain (Fig. ).Citation49)
Coq6/UbiI/COQ6/monooxygenase
S. cerevisiae Coq6, E. coli UbiI, and H. sapiens COQ6 are putative flavin-dependent monooxygenases that are responsible for introducing a hydroxy group to 4-hydroxy-3-polyprenyl benzoic acid. The Coq6 protein contains three conserved regions: an ADP-binding motif, a FAD/NADH-binding motif, and a consensus sequence that binds to the ribityl moiety of FAD.Citation50) A recent analysis demonstrated that Coq6 is involved in C-5 oxidation during the early stage of CoQ synthesis. The involvement of mitochondrial ferredoxin (YAH1) and its reductase (ARH1) in CoQ synthesis has also been proposed.Citation51) Recently, E. coli UbiI was shown to be a counterpart of Coq6.Citation14) Mutations in the human COQ6 gene cause nephrotic syndrome (Table ).Citation52)
Coq7/UbiF/COQ7/monooxygenase
S. cerevisiae Coq7, E. coli UbiF, and H. sapiens COQ7 proteins are putative flavin-dependent enzymes that catalyze monooxygenation in the penultimate step of CoQ biosynthesis. The Coq7 protein belongs to a family of di-iron-binding oxidases that contain a conserved EXXH motif. The clk-1/coq7 mutant of C. elegans, which accumulates the CoQ precursor demethoxyubiquinone (DMQ), shows a prolonged life span, developmental delay, and low egg production.Citation53–55) DMQ is also accumulated in the S. pombe coq7 mutant; however, no apparent role of DMQ has been observed in this species.Citation44) E. coli UbiF also catalyzes the same step of CoQ biosynthesis as Coq7 and C. elegans Clk-1. The Coq7, Clk-1, and UbiF proteins are highly conserved among different kingdoms, but analyses of DNA sequences revealed no apparent ortholog in plants. PKA-mediated phosphorylation and Ptc7-mediated dephosphorylation of Coq7 and other Coq proteins have been reported in S. cerevisiae.Citation56)
Coq8/UbiB/ADCK3 (ADCK4)/protein kinase
S. cerevisiae Coq8 and H. sapiens ADCK3 (or ADCK4) are protein kinases involved in CoQ synthesis. In S. cerevisiae, COQ8 has now been approved as an official gene name because the previously used name (ABC1), which was based on a chaperone of the bc1 complex, has been questioned.Citation57) The S. pombe coq8 gene is also essential for CoQ biosynthesis.Citation58) Coq8 has been classified as a putative protein kinase based on the presence of conserved kinase motifs in its primary structure. A recent study demonstrated that Coq8 is involved in the phosphorylation of Coq3, either directly or indirectly, and now, it is considered to be a regulator of the Coq enzyme complex.Citation59) The structure of human ADCK3 has been solved.Citation60) There are five Coq8-like kinases (ADCK1–5) in humans; ADCK3 is involved in CoQ synthesis, and mutations in the ADCK4 gene are related to human CoQ10 deficiency.Citation61) In fact, mutations in the ADCK3 and ADCK4 genes have been linked with a number of human genetic diseases (Table );Citation62) however, it is unclear whether the other ADCK proteins are also involved in CoQ biosynthesis.
Coq9/COQ9
The COQ9 gene is absolutely required for CoQ biosynthesis in S. cerevisiaeCitation63) and S. pombe;Citation15) however, the function of the encoded protein is still unknown. Although the Coq9 protein is conserved in eukaryotes, it has no primary sequence homology with known proteins. Furthermore, there is no apparent ortholog of COQ9 in E. coli. The Coq9 protein is a component of the multi-subunit CoQ biosynthetic complexCitation64) and is required for the removal of the nitrogen substituent from pABA-derived Q.Citation65) Analysis of the crystal structure of human COQ9 (Fig. )Citation66) revealed that it contains a lipid-binding site and is similar to the bacterial TetR family of transcriptional regulators. Coq9 associates with Coq7 in humans. Notably, the relationship between a human genetic disorder and a mutation in the human COQ9 gene suggests its involvement in the ring modification of CoQ (Table ).Citation67)
Coq10/COQ10A and COQ10B/ CoQ-binding proteins
Although Coq10 is not involved in its biosynthesis directly, it is a unique binding partner of CoQ. The Coq10 protein is localized to the mitochondria but does not belong to the respiration complex in S. cerevisiae.Citation68) The existence of a CoQ-binding protein in mitochondria challenges the current model that CoQ is a free lipid molecule in membranes. Recently, Coq10 from S. pombe was also characterized as a mitochondrial CoQ-binding protein that is required for proper respiration.Citation69) Further characterization of Coq10 suggested that it is essential for proper functioning of the electron transfer system, possibly by assisting in the transfer of CoQ from one site to another in the mitochondrial membranes of eukaryotes.Citation70) A photo-affinity labeling experiment revealed that a FVPFCQK sequence in Coq10 is responsible for binding to CoQ.Citation71)
Coq11
Very recently, Coq11 was identified as a protein associated with the Coq biosynthetic complex.Citation72) The COQ11 gene is not absolutely required for CoQ synthesis, but its deletion reduces the CoQ level in S. cerevisiae.
Three-dimensional structures of proteins involved in CoQ synthesis
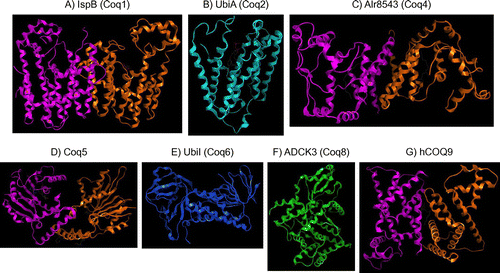
The three-dimensional structures of nine proteins responsible for CoQ synthesis (IspB, UbiA, UbiC, UbiD, UbiX, UbiI, S. cerevisiae Coq5, H. sapiens ADCK3, and H. sapiens COQ9), as well as a protein that is homologous to Coq4, have been determined. The structures of the eukaryotic proteins or their homologs found in other organisms are shown in Figure . E. coli IspB, which is a homolog of Coq1, consists of 14 α-helices.Citation28) The recent cocrystallization of IspB with its substrates (farnesyl diphosphate and isopentenyl diphosphate) revealed that the substrate-binding regions are located around aspartate-rich motifs and identified a pocket that determines the acceptable chain length. Analysis of the crystal structure of A. pernix UbiA, a homolog of Coq2, showed that it comprises nine transmembrane structures and identified the proposed substrate (PHB) binding and entry sites.Citation38) Investigation of a truncated form of E. coli UbiI, which is a homolog of Coq6, revealed that it forms a tetramer that resembles the structure of typical flavin-dependent monooxygenases.Citation14) The crystal structure of Coq5 from S. cerevisiae resembles that of a typical class-I SAM-dependent methyltransferase,Citation49) and the residues involved in the interaction of Coq5 with SAM are located on four loops. Examination of the crystal structure of a truncated form of ADCK3, a member of the UbiB family of protein kinases, revealed an atypical protein kinase with multiple UbiB-specific features positioned to inhibit protein kinase activity.Citation60) These inhibitory regions include an N-terminal domain that occupies the typical substrate-binding pocket, and a unique A-rich loop that limits ATP binding.Citation60) This structure would explain why in vitro kinase activity was not detected for UbiB. H. sapiens COQ9 displays a striking structural homology to members of the TetR family of regulators and contains a lipid-binding pocket. The protein consists of nine α-helices and forms a dimer, and the deduced crystal structure included a phospholipid in its hydrophobic interface.Citation66) In addition to the structures of five proteins that are apparently involved in CoQ synthesis, the crystal structure of the cyanobacteria Alr8543 protein (Nostoc sp. PCC7120), which is homologous to Coq4, has also been solved.Citation73) The direct involvement of the Alr8543 protein in CoQ synthesis has not been proven, but its cocrystallization with geranylgeranyl monophosphate supports a role as a substrate holder during CoQ synthesis.Citation73)
In addition to the three-dimensional structures of Coq proteins and their homologs, the crystal structures of the bacterial decarboxylases UbiD (PDB ID: 4IP2)Citation74) and UbiX (PDB ID: 1SBZ)Citation75) have also been solved. A UbiX homolog named Pad1 exists in yeast, but it is not thought to be involved in CoQ synthesis.Citation76) The crystal structure of the bacterial chorismate lyase UbiC (PDB ID: 1TT8) has also been solved, but its counterpart is not found in eukaryotes.Citation77)
Complex formation
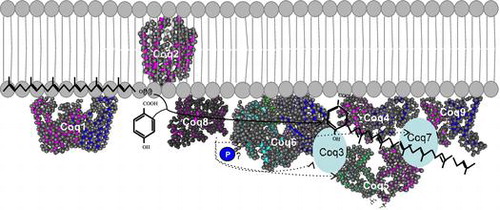
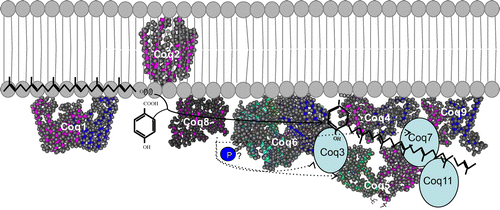
In S. cerevisiae, the enzymes involved in CoQ synthesis are thought to form a large complex termed as CoQ synthome (Fig. ). In this complex, Coq2 spans the inner membrane and the other enzymes are peripherally associated with this membrane on the matrix side. Native polyacrylamide gel electrophoresis analyses identified a 700 kDa band containing Coq3, Coq5, Coq6, Coq9, and Coq4 as well as a larger 1,300 kDa band containing Coq7 also. Coq4 plays a central role in the function of the CoQ synthome and associates with Coq3, Coq5, Coq6, and Coq7. Coq9 associates weakly with the complex, and Coq2 and Coq8 function separately.Citation78) Coq8 is a kinase that is involved in complex formation through the phosphorylation of Coq3, Coq5, and Coq7; however, it is unclear whether Coq8 phosphorylates these proteins directly or indirectly. Several lines of evidence indicate that the human CoQ biosynthetic enzymes also form a complex;Citation66,79) this topic has been comprehensively reviewed by Gonzalez-Mariscal et al.Citation80)
Fig. 5. Structure of the CoQ biosynthetic enzyme complex. Notes: The enzymes involved in CoQ synthesis form a complex in S. cerevisiae.Citation78) There is also some evidence that this complex exists in humans. This figure is modified from the figure reported by Allan et al.Citation72) Proteins in the figure are not proportional to the actual molecular sizes. The structure of S. cerevisiae Coq5 has been solved; for the other enzymes, the structures of homologs from other species are indicated. E. coli IspB is shown as a Coq1 homolog, A. pernix UbiA is shown as a Coq2 homolog, Alr8543 from Nostoc sp. PCC7120 is shown as a Coq4 homolog, E. coli UbiI is shown as a Coq6 homolog, human ADCK3 is shown as a Coq8 homolog, and human COQ9 is shown as a Coq9 homolog. Coq1 is separated from the complex. Coq2 spans the inner membrane. The positions of the other proteins have not been defined experimentally, although Coq4 seems to be in the center, and Coq7 and Coq8 seem to be located at the edge of the complex.
Diseases caused by CoQ deficiency
The diseases caused by a deficiency in CoQ have been reviewed previously.Citation81,82) The first mutations related to human CoQ deficiency were identified in COQ2; since then, mutations in seven other CoQ biosynthetic genes (PDSS1, PDSS2, COQ4, COQ6, ADCK3, ADCK4, and COQ9) have been shown to cause various diseases (summarized in Table ). Encephalomyopathy, nephropathy, cerebellar ataxia, and seizures are common features of CoQ deficiency; these symptoms are associated with mitochondrial disorders. The level of CoQ10 differs between patients and depends on the specific mutations, and some disease symptoms are eased by supplementation with CoQ10. There are also reports that secondary CoQ deficiency can be caused by mutations in genes that are not directly involved in CoQ synthesis, such as oncogene B-RAF and aprataxin (APTX). Citation81,82)
Sites of CoQ synthesis
In eukaryotes, CoQ biosynthesis occurs mainly in the mitochondria; indeed, all S. cerevisiae and S. pombe proteins involved in the process localize to this cellular compartment.Citation15) Human COQ proteins also localize to the mitochondria, but the Golgi-localized enzyme UBIAD1 (prenyl transferase), which is homologous to E. coli UbiA, can also participate in CoQ.Citation83) Loss of UBIAD1 apparently reduces the cytosolic pool of CoQ10. As most of other enzymes involves CoQ localization to mitochondria, the mechanism by which UBIAD1 assists with CoQ production in the Golgi is still unclear.
Regulation of the expression of COQ genes and genes regulated by CoQ
Little is currently known about the regulation of COQ gene expression. The expression of S. cerevisiae COQ5 is reportedly regulated by Mig1, Rtg3, and Hap2.Citation84) A microarray analysis revealed that a deficiency of endogenous CoQ in C. elegans clk1 mutants down-regulates a cluster of genes that are important for growth and up-regulates oxidation reactions and protein interactions.Citation85) In addition, a microarray analysis demonstrated the induction of specific genes related to cell survival in CoQ-deficient human cells.Citation86)
Bioproduction of CoQ10 in microorganisms and plants
Because CoQ10 is a commercially sold food supplement, its efficient production by microorganisms has been explored.Citation87) E. coli, Agrobacterium, and photosynthetic bacteria such as Rhodobacter spheroides and Rhodobacter capsulatus have been used to produce CoQ10. E. coli produces CoQ8 naturally, but expressing decaprenyl diphosphate synthase in cells that lack the endogenous ispB gene enables them to produce CoQ10.Citation21) A previous study generated an engineered E. coli strain that expressed the Agrobacterium tumefaciens decaprenyl diphosphate synthase gene (ddsA) and had a strengthened mevalonate pathway; this strain was capable of producing substantial amounts of CoQ10.Citation88) By optimizing the growth medium and conditions, photosynthetic Rhodobacter capable of producing CoQ10 at concentrations up to 8.70 mg/mg dry cell weight was generated.Citation89) In addition, Agrobacterium can produce CoQ10 at concentrations up to 11.84 mg/mg dry cell weight.Citation90) The fission yeast S. pombe is a good candidate for CoQ10 production, and the yield can be doubled by genetic engineering, although it is not as high as that obtained from other microorganisms.Citation91) In addition to microbial production, researchers have attempted to produce CoQ10 using other systems; the attempts to produce CoQ10 in rice are worth noting.Citation92,93)
Concluding remarks
Recent studies of the mechanisms of CoQ synthesis have been very fruitful. In particular, current understanding of the correlation between defective genes involved in CoQ synthesis and human diseases is surprisingly advanced. The three-dimensional structures of proteins involved in CoQ synthesis have also been determined recently. Despite this accumulating knowledge of CoQ biosynthesis, we still do not have a clear picture of the whole biosynthetic pathway in eukaryotes. For example, Coq4 and Coq9 are absolutely required for CoQ biosynthesis in eukaryotes, but their specific functions are unclear. Furthermore, additional factors, such as Coq10 and Coq11, also appear to be involved in CoQ biosynthesis. Besides the electron transport system, reactions involving sulfide-quinone oxidoreductase, glycerol-3-phosphate dehydrogenase, dihydroorotate dehydrogenase, and electron-transferring flavoprotein dehydrogenase require CoQ as a cofactor. Overall, additional work is required to understand the complete pathway of CoQ biosynthesis.
Disclosure statement
No potential conflict of interest was reported by the author.
Acknowledgment
The author would like to thank Dr. T. Kaino for critical reading of the manuscript.
Additional information
Funding
Notes
Abbreviations: CoQ, coenzyme Q; DMQ, demethoxyubiquinone; pABA, para-amino benzoic acid; PHB, para-hydroxybenzoate; SAM, S-adenosyl methionine.
References
- Kawamukai M. Biosynthesis and bioproduction of coenzyme Q 10 by yeasts and other organisms. Biotechnol. Appl. Biochem. 2009;53:217–226.10.1042/BA20090035
- Inaba K. Disulfide bond formation system in Escherichia coli. J. Biochem. (Tokyo). 2009;146:591–597.10.1093/jb/mvp102
- Zhang M, Wakitani S, Hayashi K, Miki R, Kawamukai M. High production of sulfide in coenzyme Q deficient fission yeast. BioFactors. 2008;32:91–98.10.1002/biof.v32:1/4
- Šimkovič M, Frerman FE. Alternative quinone substrates and inhibitors of human electron-transfer flavoprotein-ubiquinone oxidoreductase. Biochem. J. 2004;378:633–640.10.1042/BJ20031272
- Lopez-Martin JM, Salviati L, Trevisson E, et al. Missense mutation of the COQ2 gene causes defects of bioenergetics and de novo pyrimidine synthesis. Hum. Mol. Genet. 2007;16:1091–1097.10.1093/hmg/ddm058
- Matsuo Y, Nishino K, Mizuno K, et al. Polypeptone induces Dramatic cell lysis in ura4 deletion mutants of fission yeast. PLoS ONE. 2013;8:e59887.10.1371/journal.pone.0059887
- Okada K, Suzuki K, Kamiya Y, et al. Polyprenyl diphosphate synthase essentially defines the length of the side chain of ubiquinone. Biochim. Biophys. Acta. 1996;1302:217–223.10.1016/0005-2760(96)00064-1
- Okada K, Kainou T, Matsuda H, Kawamukai M. Biological significance of the side chain length of ubiquinone in Saccharomyces cerevisiae. FEBS Lett. 1998;431:241–244.10.1016/S0014-5793(98)00753-4
- Kawamukai M. Biosynthesis, bioproduction and novel roles of ubiquinone. J. Biosci. Bioeng. 2002;94:511–517.10.1016/S1389-1723(02)80188-8
- Aussel L, Pierrel F, Loiseau L, Lombard M, Fontecave M, Barras F. Biosynthesis and physiology of coenzyme Q in bacteria. Biochim. Biophys. Acta. 2014;1837:1004–1011.10.1016/j.bbabio.2014.01.015
- Meganathan R. Ubiquinone biosynthesis in microorganisms. FEMS Microbiol. Lett. 2001;203:131–139.
- Tran UC, Clarke CF. Endogenous synthesis of coenzyme Q in eukaryotes. Mitochondrion. 2007;7:S62–S71.10.1016/j.mito.2007.03.007
- Tzagoloff A, Dieckmann CL. PET genes of Saccharomyces cerevisiae. Microbiol. Rev. 1990;54:211–225.
- Hajj Chehade M, Loiseau L, Lombard M, et al. ubil, a new gene in Escherichia coli coenzyme Q biosynthesis, is involved in aerobic C5-hydroxylation. J. Biol. Chem. 2013;288:20085–20092.10.1074/jbc.M113.480368
- Hayashi K, Ogiyama Y, Yokomi K, Nakagawa T, Kaino T, Kawamukai M. Functional conservation of coenzyme Q biosynthetic genes among yeasts, plants, and humans. PLoS ONE. 2014;9:e99038.10.1371/journal.pone.0099038
- Gavilán ÁNgela, Asencio C, Cabello J, Rodríguez-Aguilera JC, Schnabel R, Navas P. C. elegans knockouts in ubiquinone biosynthesis genes result in different phenotypes during larval development. BioFactors. 2005;25:21–29.10.1002/biof.v25:1/4
- Zhang M, Luo J, Ogiyama Y, Saiki R, Kawamukai M. Heteromer formation of a long-chain prenyl diphosphate synthase from fission yeast Dps1 and budding yeast Coq1. FEBS J. 2008;275:3653–3668.10.1111/j.1742-4658.2008.06510.x
- Saiki R, Nagata A, Uchida N, Kainou T, Matsuda H, Kawamukai M. Fission yeast decaprenyl diphosphate synthase consists of Dps1 and the newly characterized Dlp1 protein in a novel heterotetrameric structure. Eur. J. Biochem. 2003;270:4113–4121.10.1046/j.1432-1033.2003.03804.x
- Saiki R, Nagata A, Kainou T, Matsuda H, Kawamukai M. Characterization of solanesyl and decaprenyl diphosphate synthases in mice and humans. FEBS J. 2005;272:5606–5622.10.1111/ejb.2005.272.issue-21
- Kainou T, Okada K, Suzuki K, Nakagawa T, Matsuda H, Kawamukai M. Dimer formation of octaprenyl-diphosphate synthase (IspB) is essential for chain length determination of ubiquinone. J. Biol. Chem. 2001;276:7876–7883.10.1074/jbc.M007472200
- Okada K, Kainou T, Tanaka K, Nakagawa T, Matsuda H, Kawamukai M. Molecular cloning and mutational analysis of the ddsA gene encoding decaprenyl diphosphate synthase from Gluconobacter suboxydans. Eur. J. Biochem. 1998;255:52–59.10.1046/j.1432-1327.1998.2550052.x
- Okada K, Kamiya Y, Zhu X, et al. Cloning of the sdsA gene encoding solanesyl diphosphate synthase from Rhodobacter capsulatus and its functional expression in Escherichia coli and Saccharomyces cerevisiae. J. Bacteriol. 1997;179:5992–5998.
- Jun L, Saiki R, Tatsumi K, Nakagawa T, Kawamukai M. Identification and subcellular localization of two solanesyl diphosphate synthases from Arabidopsis thaliana. Plant Cell Physiol. 2004;45:1882–1888.10.1093/pcp/pch211
- Ducluzeau AL, Wamboldt Y, Elowsky CG, Mackenzie SA, Schuurink RC, Basset GJ. Gene network reconstruction identifies the authentic trans-prenyl diphosphate synthase that makes the solanesyl moiety of ubiquinone-9 in Arabidopsis. Plant J. 2012;69:366–375.10.1111/tpj.2011.69.issue-2
- Suzuki K, Okada K, Kamiya Y, et al. Analysis of the decaprenyl diphosphate synthase (dps) gene in fission yeast suggests a role of ubiquinone as an antioxidant. J. Biochem. (Tokyo). 1997;121:496–505.10.1093/oxfordjournals.jbchem.a021614
- Cui TZ, Kaino T, Kawamukai M. A subunit of decaprenyl diphosphate synthase stabilizes octaprenyl diphosphate synthase in Escherichia coli by forming a high-molecular weight complex. FEBS Lett. 2010;584:652–656.10.1016/j.febslet.2009.12.029
- Guo RT, Kuo CJ, Chou CC, et al. Crystal structure of octaprenyl pyrophosphate synthase from hyperthermophilic Thermotoga maritima and mechanism of product chain length determination. J. Biol. Chem. 2004;279:4903–4912.
- Han X, Chen CC, Kuo CJ, et al. Crystal structures of ligand-bound octaprenyl pyrophosphate synthase from Escherichia coli reveal the catalytic and chain-length determining mechanisms. Proteins. 2015;83:37–45.10.1002/prot.v83.1
- Saiki R, Lunceford AL, Shi Y, et al. Coenzyme Q10 supplementation rescues renal disease in Pdss2kd/kd mice with mutations in prenyl diphosphate synthase subunit 2. Am. J. Physiol. Renal Physiol. 2008;295:F1535–F1544.10.1152/ajprenal.90445.2008
- López LC, Schuelke M, Quinzii CM, et al. Leigh syndrome with nephropathy and CoQ10 Deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am. J. Hum. Genet. 2006;79:1125–1129.10.1086/510023
- Mollet J, Giurgea I, Schlemmer D, et al. Prenyldiphosphate synthase, subunit 1 (PDSS1) and OH-benzoate polyprenyltransferase (COQ2) mutations in ubiquinone deficiency and oxidative phosphorylation disorders. J. Clin. Invest. 2007;117:765–772.10.1172/JCI29089
- Uchida N, Suzuki K, Saiki R, et al. Phenotypes of fission yeast defective in ubiquinone production due to disruption of the gene for p-hydroxybenzoate polyprenyl diphosphate transferase. J. Bacteriol. 2000;182:6933–6939.10.1128/JB.182.24.6933-6939.2000
- Ashby MN, Kutsunai SY, Ackerman S, Tzagoloff A, Edwards PA. COQ2 is a candidate for the structural gene encoding para-hydroxybenzoate:polyprenyltransferase. J. Biol. Chem. 1992;267:4128–4136.
- Suzuki K, Ueda M, Yuasa M, Nakagawa T, Kawamukai M, Matsuda H. Evidence that Escherichia coli ubiA product is a functional homolog of yeast COQ2, and the regulation of ubiA gene expression. Biosci. Biotech. Biochem. 1994;58:1814–1819.10.1271/bbb.58.1814
- Okada K, Ohara K, Yazaki K, et al. The AtPPT1 gene encoding 4-hydroxybenzoate polyprenyl diphosphate transferase in ubiquinone biosynthesis is required for embryo development in Arabidopsis thaliana. Plant Mol. Biol. 2004;55:567–577.
- Marbois B, Xie LX, Choi S, Hirano K, Hyman K, Clarke CF. para-aminobenzoic acid is a precursor in coenzyme Q6 biosynthesis in Saccharomyces cerevisiae. J. Biol. Chem. 2010;285:27827–27838.10.1074/jbc.M110.151894
- Pierrel F, Hamelin O, Douki T, et al. Involvement of mitochondrial ferredoxin and para-aminobenzoic acid in yeast coenzyme Q biosynthesis. Chem. Biol. 2010;17:449–459.10.1016/j.chembiol.2010.03.014
- Cheng W, Li W. Structural insights into ubiquinone biosynthesis in membranes. Science. 2014;343:878–881.10.1126/science.1246774
- Quinzii C, Naini A, Salviati L, et al. A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am. J. Hum. Genet. 2006;78:345–349.10.1086/500092
- Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, et al. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J. Am. Soc. Nephrol. 2007;18:2773–2780.10.1681/ASN.2006080833
- Poon WW, Barkovich RJ, Hsu AY, et al. Yeast and rat Coq3 and Escherichia coli UbiG polypeptides catalyze both O-methyltransferase steps in coenzyme Q biosynthesis. J. Biol. Chem. 1999;274:21665–21672.10.1074/jbc.274.31.21665
- Jonassen T, Clarke CF. Isolation and functional expression of human COQ3, a gene encoding a methyltransferase required for ubiquinone biosynthesis. J. Biol. Chem. 2000;275:12381–12387.10.1074/jbc.275.17.12381
- Hsu AY, Poon WW, Shepherd JA, Myles DC, Clarke CF. Complementation of coq3 mutant yeast by mitochondrial targeting of the Escherichia coli UbiG polypeptide: evidence that UbiG catalyzes both O-methylation steps in ubiquinone biosynthesis. Biochemistry. 1996;35:9797–9806.10.1021/bi9602932
- Miki R, Saiki R, Ozoe Y, Kawamukai M. Comparison of a coq7 deletion mutant with other respiration-defective mutants in fission yeast. FEBS J. 2008;275:5309–5324.10.1111/j.1742-4658.2008.06661.x
- Hihi AK, Gao Y, Hekimi S. Ubiquinone is necessary for Caenorhabditis elegans development at mitochondrial and non-mitochondrial sites. J. Biol. Chem. 2002;277:2202–2206.10.1074/jbc.M109034200
- Marbois B, Gin P, Gulmezian M, Clarke CF. The yeast Coq4 polypeptide organizes a mitochondrial protein complex essential for coenzyme Q biosynthesis. Biochim. Biophys. Acta. 2009;1791:69–75.10.1016/j.bbalip.2008.10.006
- Salviati L, et al. Haploinsufficiency of COQ4 causes coenzyme Q10 deficiency. J. Med. Genet. 2012;49:187–191.10.1136/jmedgenet-2011-100394
- Barkovich RJ, Shtanko A, Shepherd JA, et al. Characterization of the COQ5 gene from Saccharomyces cerevisiae. Evidence for a C-methlytransferase in ubiquinone biosynthesis. J. Biol. Chem. 1997;272:9182–9188.
- Dai YN, Zhou K, Cao DD, et al. Crystal structures and catalytic mechanism of the C-methyltransferase Coq5 provide insights into a key step of the yeast coenzyme Q synthesis pathway. Acta Crystallogr. D. Biol. Crystallogr. 2014;70:2085–2092.10.1107/S1399004714011559
- Gin P, Hsu AY, Rothman SC, et al. The Saccharomyces cerevisiae COQ6 gene encodes a mitochondrial flavin-dependent monooxygenase required for coenzyme Q biosynthesis. J. Biol. Chem. 2003;278:25308–25316.10.1074/jbc.M303234200
- Ozeir M, Mühlenhoff U, Webert H, Lill R, Fontecave M, Pierrel F. Coenzyme Q biosynthesis: Coq6 is required for the C5-Hydroxylation reaction and substrate analogs rescue Coq6 deficiency. Chem. Biol. 2011;18:1134–1142.10.1016/j.chembiol.2011.07.008
- Heeringa SF, et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J. Clin. Invest. 2011;121:2013–2024.10.1172/JCI45693
- Ewbank JJ, Barnes TM, Lakowski B, Lussier M, Bussey H, Hekimi S. Structural and functional conservation of the caenorhabditis elegans timing gene clk-1. Science. 1997;275:980–983.10.1126/science.275.5302.980
- Nakai D, Shimizu T, Nojiri H, et al. coq7/clk-1 regulates mitochondrial respiration and the generation of reactive oxygen species via coenzyme Q. Aging Cell. 2004;3:273–281.10.1111/ace.2004.3.issue-5
- Larsen PL, Clarke CF. Extension of life-span in caenorhabditis elegans by a diet lacking coenzyme Q. Science. 2002;295:120–123.10.1126/science.1064653
- Martín‑Montalvo A, González‑Mariscal I, Padilla S, et al. Respiratory-induced coenzyme Q biosynthesis is regulated by a phosphorylation cycle of Cat5p/Coq7p. Biochem. J. 2011;440:107–114.10.1042/BJ20101422
- Hsieh EJ, Dinoso JB, Clarke CF. A tRNATRP gene mediates the suppression of cbs2-223 previously attributed to ABC1/COQ8. Biochem. Biophys. Res. Commun. 2004;317:648–653.10.1016/j.bbrc.2004.03.096
- Saiki R, Ogiyama Y, Kainou T, Nishi T, Matsuda H, Kawamukai M. Pleiotropic phenotypes of fission yeast defective in ubiquinone-10 production. A study from the abc1Sp (coq8Sp) mutant. Biofactors. 2003;18:229–235.10.1002/biof.v18:1/4
- Tauche A, Krause-Buchholz U, Rodel G. Ubiquinone biosynthesis in Saccharomyces cerevisiae: the molecular organization of O-methylase Coq3p depends on Abc1p/Coq8p. FEMS Yeast Res. 2008;8:1263–1275.10.1111/fyr.2008.8.issue-8
- Stefely JA, Reidenbach AG, Ulbrich A, et al. Mitochondrial ADCK3 employs an atypical protein kinase-like fold to enable coenzyme q biosynthesis. Mol. Cell. 2015;57:83–94.10.1016/j.molcel.2014.11.002
- Ashraf S, Gee HY, Woerner S, et al. ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J. Clin. Invest. 2013;123:5179–5189.10.1172/JCI69000
- Lagier-Tourenne C, Tazir M, López LC, et al. ADCK3, an Ancestral kinase, is mutated in a form of recessive ataxia associated with coenzyme Q10 deficiency. Am. J. Hum. Genet. 2008;82:661–672.10.1016/j.ajhg.2007.12.024
- Johnson A, Gin P, Marbois BN, et al. COQ9, a new gene required for the biosynthesis of coenzyme Q in Saccharomyces cerevisiae. J. Biol. Chem. 2005;280:31397–31404.10.1074/jbc.M503277200
- Hsieh EJ, Gin P, Gulmezian M, et al. Saccharomyces cerevisiae Coq9 polypeptide is a subunit of the mitochondrial coenzyme Q biosynthetic complex. Arch. Biochem. Biophys. 2007;463:19–26.10.1016/j.abb.2007.02.016
- He CH, Black DS, Nguyen TP, Wang C, Srinivasan C, Clarke CF. Yeast Coq9 controls deamination of coenzyme Q intermediates that derive from para-aminobenzoic acid, Biochim. Biophys. Acta. 2015;1851:1227–1239.
- Lohman DC, Forouhar F, Beebe ET, et al. Mitochondrial COQ9 is a lipid-binding protein that associates with COQ7 to enable coenzyme Q biosynthesis. Proc. Natl. Acad. Sci. U S A. 2014;111:E4697–E4705.10.1073/pnas.1413128111
- Duncan AJ, Bitner-Glindzicz M, Meunier B, et al. A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset Primary coenzyme Q10 deficiency: a potentially treatable form of mitochondrial disease. Am. J. Hum. Genet. 2009;84:558–566.10.1016/j.ajhg.2009.03.018
- Barros MH, Johnson A, Gin P, Marbois BN, Clarke CF, Tzagoloff A. The Saccharomyces cerevisiae COQ10 gene encodes a START domain protein required for function of coenzyme Q in respiration. J. Biol. Chem. 2005;280:42627–42635.10.1074/jbc.M510768200
- Cui T-Z, Kawamukai M. Coq10, a mitochondrial coenzyme Q binding protein, is required for proper respiration in Schizosaccharomyces pombe. FEBS J. 2009;276:748–759.10.1111/j.1742-4658.2008.06821.x
- Busso C, Tahara EB, Ogusucu R, et al. Saccharomyces cerevisiae coq10 null mutants are responsive to antimycin A. FEBS J. 2010;277:4530–4538.10.1111/j.1742-4658.2010.07862.x
- Murai M, Matsunobu K, Kudo S, Ifuku K, Kawamukai M, Miyoshi H. Identification of the binding site of the quinone-head group in mitochondrial Coq10 by photoaffinity labeling. Biochemistry. 2014;53:3995–4003.10.1021/bi500347s
- Allan CM, Awad AM, Johnson JS, et al. Identification of Coq11, a new coenzyme Q biosynthetic protein in the CoQ-synthome in Saccharomyces cerevisiae. J. Biol. Chem. 2015;290:7517–7534.10.1074/jbc.M114.633131
- Rea SL, Graham BH, Nakamaru-Ogiso E, Kar A, Falk MJ. Bacteria, yeast, worms, and flies: exploiting simple model organisms to investigate human mitochondrial diseases. Dev. Disabil. Res. Rev. 2010;16:200–218.10.1002/ddrr.v16:2
- Jacewicz A, Izumi A, Brunner K, Schnell R, Schneider G. Structural insights into the UbiD Protein family from the crystal structure of PA0254 from Pseudomonas aeruginosa. PLoS ONE. 2013;8:e63161.10.1371/journal.pone.0063161
- Rangarajan ES, Li Y, Iannuzzi P, et al. Crystal structure of a dodecameric FMN-dependent UbiX-like decarboxylase (Pad1) from Escherichia coli O157: H7. Protein Sci. 2004;13:3006–3016.
- Mukai N, Masaki K, Fujii T, Kawamukai M, Iefuji H. PAD1 and FDC1 are essential for the decarboxylation of phenylacrylic acids in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2010;109:564–569.10.1016/j.jbiosc.2009.11.011
- Smith N, Roitberg AE, Rivera E, et al. Structural analysis of ligand binding and catalysis in chorismate lyase. Arch. Biochem. Biophys. 2006;445:72–80.10.1016/j.abb.2005.10.026
- He CH, Xie LX, Allan CM, Tran UC, Clarke CF. Coenzyme Q supplementation or over-expression of the yeast Coq8 putative kinase stabilizes multi-subunit Coq polypeptide complexes in yeast coq null mutants. Biochim. Biophys. Acta. 2014;1841:630–644.10.1016/j.bbalip.2013.12.017
- Nguyen TP, Casarin A, Desbats MA, et al. Molecular characterization of the human COQ5 C-methyltransferase in coenzyme Q10 biosynthesis. Biochim. Biophys. Acta. 2014;1841:1628–1638.10.1016/j.bbalip.2014.08.007
- González-Mariscal I, García-Testón E, Padilla S, et al. Regulation of coenzyme Q biosynthesis in yeast: a new complex in the block. IUBMB Life. 2014;66:63–70.10.1002/iub.1243
- Wang Y, Hekimi S. Molecular genetics of ubiquinone biosynthesis in animals. Critical reviews in biochemistry and molecular biology. 2013;48:69–88.10.3109/10409238.2012.741564
- Doimo M, Desbats MA, Cerqua C, Cassina M, Trevisson E, Salviati L. Genetics of coenzyme Q10 deficiency. Mol. Syndromol. 2014;5:156–162.
- Mugoni V, Postel R, Catanzaro V, et al. Ubiad1 is an antioxidant enzyme that regulates eNOS activity by CoQ10 synthesis. Cell. 2013;152:504–518.10.1016/j.cell.2013.01.013
- Hagerman RA, Willis RA. The yeast gene COQ5 is differentially regulated by Mig1p, Rtg3p and Hap2p. Biochim. Biophys. Acta. 2002;1578:51–58.10.1016/S0167-4781(02)00496-7
- Fischer A, Niklowitz P, Menke T, Döring F. Promotion of growth by Coenzyme Q10 is linked to gene expression in C. elegans. Biochem. Biophys. Res. Commun. 2014;452:920–927.10.1016/j.bbrc.2014.09.016
- Fernandez-Ayala DJ, Guerra I, Jimenez-Gancedo S, et al. Survival transcriptome in the coenzyme Q10 deficiency syndrome is acquired by epigenetic modifications: a modelling study for human coenzyme Q10 deficiencies, BMJ Open, 2013;3:e002524.
- Cluis CP, Burja AM, Martin VJ. Current prospects for the production of coenzyme Q10 in microbes. Trends Biotechnol. 2007;25:514–521.10.1016/j.tibtech.2007.08.008
- Zahiri HS, Yoon SH, Keasling JD, et al. Coenzyme Q10 production in recombinant Escherichia coli strains engineered with a heterologous decaprenyl diphosphate synthase gene and foreign mevalonate pathway. Metab. Eng. 2006;8:406–416.10.1016/j.ymben.2006.05.002
- Yoshida H, Kotani Y, Ochiai K, Araki K. Production of ubiquinone-10 using bacteria. J. Gen. Appl. Microbiol. 1998;44:19–26.10.2323/jgam.44.19
- Ha SJ, Kim SY, Seo JH, et al. Ca2+ increases the specific coenzyme Q10 content in Agrobacterium tumefaciens. Bioprocess. Biosyst. Eng. 2009;32:697–700.
- Moriyama D, Hosono K, Fujii M, et al. Production of CoQ10 in fission yeast by expression of genes responsible for CoQ10 biosynthesis. Biosci. Biotechnol. Biochem.. 2015;79:1026–1033.
- Takahashi S, Ogiyama Y, Kusano H, Shimada H, Kawamukai M, Kadowaki K. Metabolic engineering of coenzyme Q by modification of isoprenoid side chain in plant. FEBS Lett. 2006;580:955–959.10.1016/j.febslet.2006.01.023
- Takahashi S, Ohtani T, Satoh H, Nakamura Y, Kawamukai M, Kadowaki K. Development of coenzyme Q10-enriched rice using sugary and shrunken mutants. Biosci. Biotechnol. Biochem. 2010;74:182–184.10.1271/bbb.90562
- Mitsui J, Matsukawa T, Ishiura H, et al.. Mutations in COQ2 in familial and sporadic multiple-system atrophy. N. Engl. J. Med. 2013;369:233–244.
- Mollet J, Delahodde A, Serre V, et al. CABC1 gene mutations cause ubiquinone deficiency with cerebellar ataxia and seizures. Am. J. Hum. Genet. 2008;82:623–630.10.1016/j.ajhg.2007.12.022
- Gerards M, van den Bosch B, Calis C, et al. Nonsense mutations in CABC1/ADCK3 cause progressive cerebellar ataxia and atrophy. Mitochondrion. 2010;10:510–515.10.1016/j.mito.2010.05.008