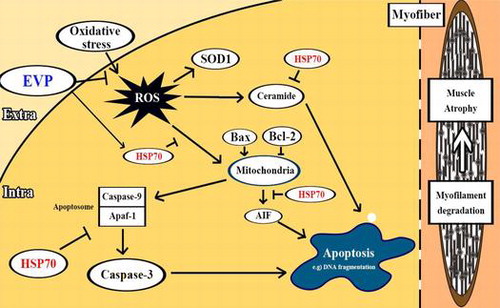
Graphical abstract
Proposed mechanism on anti-skeletal muscle atrophy effect of oenothera odorata root extract via reactive oxygen species-dependent signaling pathways in cellular and mouse models.
Abstract
Skeletal muscle atrophy can be defined as a decrease of muscle volume caused by injury or lack of use. This condition is associated with reactive oxygen species (ROS), resulting in various muscular disorders. We acquired 2D and 3D images using micro-computed tomography in gastrocnemius and soleus muscles of sciatic-denervated mice. We confirmed that sciatic denervation-small animal model reduced muscle volume. However, the intraperitoneal injection of Oenothera odorata root extract (EVP) delayed muscle atrophy compared to a control group. We also investigated the mechanism of muscle atrophy’s relationship with ROS. EVP suppressed expression of SOD1, and increased expression of HSP70, in both H2O2-treated C2C12 myoblasts and sciatic-denervated mice. Moreover, EVP regulated apoptotic signals, including caspase-3, Bax, Bcl-2, and ceramide. These results indicate that EVP has a positive effect on reducing the effect of ROS on muscle atrophy.
Atrophy of skeletal muscle is a condition caused by disuse of muscles via immobilization, bed rest, damage of related muscle, and other factors.Citation1,2) In the case of spaceflight, muscle atrophy is caused by lack of consistent stimulation because gravity is relatively low.Citation3) In the method of regenerating atrophic muscles via myoblast transplantation, transient delivery of dystrophin can improve muscle strength in the injected area.Citation4,5) However, the therapeutic effect of dystrophin is localized in the area of injection, and the injected cells’ survival rate is relatively low.Citation5) Consequently, complete recovery of atrophy was not achieved, necessitating a revised plan. Various muscle atrophy factors, such as loss of muscle mass, are caused by proteolysis and apoptosis of muscle cells.Citation6,7) Other studies have confirmed the increase of proteolysis in the sciatic denervation-small animal model.Citation8,9) In particular, denervation in this model causes loss of myofiber mass and volume. In one study, for example, sciatic-denervated mice had decreased gastrocnemius muscle massCitation10); a reduction by 40% compared to control mice. Thus, to investigate the mechanism of muscle atrophy, we performed sciatic denervation of mice. Muscle volume of sciatic-denervated mice was measured by micro-computed tomography (micro-CT).
There are various factors involved in muscle atrophy. Reactive oxygen species (ROS) are associated with various muscular disorders.Citation11–13) In cases of extreme exercise, the increase in the products of ROS leads to muscle injury.Citation1) Oxygen influx rapidly occurs when muscles contract. An individual then becomes more susceptible to muscle damage.Citation14,15) Heat shock proteins (HSPs) emerge as bioeffector molecules and control various cellular signal cascades in many diseases.Citation16) HSP70 was reported to play a role in protecting the cell from various stress-induced apoptosis, including oxidative stress.Citation17,18) Other studies showed that extreme conditions of skeletal muscle atrophy reduce expression of HSP70.Citation19) Therefore, regulation of HSP70 expression can be expected to have a positive effect by reducing cell death to ROS in skeletal muscle atrophy. Caspases, which are an endoproteases, regulate proteolysis and program apoptosis signaling. The apoptosis intrinsic pathway of caspase-3 activation is complex. It can be initiated by various signals including ROS. Also, caspase-3 regulates pro- and anti-apoptotic proteins, such as Bax and Bcl-2 in the mitochondria.Citation20) Superoxide dismutase-1 (SOD1) is a cytoplasm protein. This enzyme acts as a catalyst with catalase through chemical reaction, which is activated by naturally occurring superoxide radicals such as molecular oxygen and hydrogen peroxide.
Oenothera odorata Jacq. is biennial herbaceous plant commonly known as evening primrose. Evening primrose seed oil has been known to contribute to immune reactions in skin diseases, diabetic complications, and inflammatory disorders.Citation21,22) Moreover, evening primrose seed oil protected against DNA damage and apoptosis induced by palmitic acid.Citation23) Because this plant may be a potential source of antioxidants, this study investigated the effect of O. odorata root extract (EVP) on muscle atrophy induced by oxidative stress.
Materials and methods
Materials
Penicillin–streptomycin was obtained from Lonza (Walkersville, MD, USA). An EZ-Cytox cell viability kit was purchased from Daeil Lab (Seoul, Korea). Antibody against HSP70 was purchased from Enzo Life Sciences, AG (Lausen, Switzerland). Antibodies against Bcl-2, Bax, procaspase-3, cleaved caspase-3, SOD1, and β-actin antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). N-acetyl-l-cysteine (NAC), 2′,7′-dichlorofluorescein diacetate (DCF-DA) and ortho-phthaldialdehyde were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sphingomyelin, dihydrosphingomyelin, ceramide, and Sphingolipid ceramide N-deacylase were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). High-performance thin layer chromatography (HPTLC) silica-gel plate, chloroform, and high-performance liquid chromatography (HPLC) grade methanol were purchased from Merck (Darmstadt, Germany). Vectashield mounting medium with DAPI was purchased from Vector Laboratories (Burlingame, CA, USA). All other chemicals used were the highest analytical grade that is commercially available.
Oenothera odorata root extract
The Oenothera odorata plants were cultivated according to the good agricultural practices method of the Korea Rural Development Administration and were harvested in 2009 in Eumseong-gun (GPS: E 128°62´ N 36°56´). For sample preparation, the roots were extracted with 99% MeOH three times at 25 °C for three days. The extracts were filtrated through Whatman No. 1 filter paper (GE Healthcare, Buckinghamshire, UK) and combined followed by concentration using a rotary evaporator (EYELA N-1000, Japan) at 40 °C. The obtained dried extracts were lyophilized and then powdered. The EVP was dissolved in dimethylsulfoxide (DMSO) and added to Dulbecco’s modified Eagle’s medium (DMEM) or phosphate-buffered saline (PBS) with a maximum final DMSO concentration 0.05%.
Animal experiments
Male C57/BL6 mice (4 week old) were purchased from Orient Bio (Gangneung, Korea) and were housed in wired cages with a temperature of 20–22 °C and humidity of 40–50%. The Institutional Animal Care and Use Committee (IACUC, YWC-130204-1) at Yonsei University (Wonju, Korea) approved the protocol for this study. An attempt was made to minimize the pain of the animals. The sciatic nerve in the right leg of each mouse was surgically removed fragmentally to induce immobilization. These mice had induced muscle atrophy by sciatic denervation for 7 days. Then, EVP (1–2 mg/kg) was injected by intraperitoneal injection 10 times during a 2-week period. The mice were sacrificed 21 days after sciatic denervation.
Micro-computed tomography (Micro-CT)
Images of the muscle in the tibia of each mouse (n = 5) were acquired at day 21 after the induced muscle atrophy, using micro-CT (SkyScan 1076, Bruker AXS, Germany). Scan parameters were set to 18 µm resolutions, 100 kV voltages, 100 µA current, 2065 ms exposure time, 1 mm thickness of aluminum filter, and 0.7° rotation step. The mice were under anesthesia during the scanning. The beam-hardening errors were corrected to improve the quality of the micro-CT images by flat-field correction before scanning and beam-hardening correction during reconstruction. For the evaluation of muscle volume, three-dimensional models were reconstructed using CT-Analyzer 1.11 (CT-An 1.11, Bruker, Germany). The raw data acquired by micro-CT were translated to two-dimensional (2D) cross-sectional gray scale image slices using NRecon (Bruker AXS). From the acquired 2D images, the volumes of interest for the measurement of muscle volumes (MV, mm3) of the right lower legs were calculated using CT-Analyzer 1.11.
Cell culture
C2C12 myoblasts were cultured in DMEM (Sigma-Aldrich) supplemented with 10% (v/v) fetal bovine serum (FBS; Lonza, Walkersville, MD, USA), 100 μg/mL penicillin-streptomycin, 8 mM N-(2-hydroxyelthyl)piperazine-N′-2-ethanesulfonic acid, and 2 mM l-glutamine. C2C12 myoblasts were maintained at 37 °C in a humidified 5% CO2 incubator.
Cell viability
Cell viability was determined using the EZ-Cytox cell viability kit following the manufacturer’s instructions. Initially, C2C12 myoblasts were seeded into 96-well culture plates at 2 × 104 cells/mL and it is cultured in DMEM containing 10% (v/v) FBS at 37 °C. When C2C12 myoblasts reached 70% confluence, the medium was replaced with serum-free DMEM containing various concentrations of EVP (0–50 µg/mL) for 23 h. Phosphate-buffered saline (PBS, pH 7.2) washing, the medium was replaced with serum-free DMEM containing 1 mM H2O2 for 1 h. EZ-Cytox kit reagents were added to the medium, the C2C12 myoblasts were incubated for 1 h, and then the optical density was determined at 450 nm using a microplate reader (BioTek Instruments Inc., Winooski, VT, USA).
Detection of apoptotic cells
C2C12 myoblasts were cultured on a cover glass for 23 h with DMSO or EVP (25 or 50 µg/mL) in serum-free DMEM. After PBS washing, the serum-free medium was treated with 1 mM H2O2 (by addition to the medium) for 1 h. Then, these C2C12 myoblasts were fixed in 4% para-formaldehyde and membrane-permeabilized by 30 min exposure to 0.1% Triton X-100 in PBS at room temperature. After C2C12 myoblasts were washed with PBS, samples were mounted with DAPI-mounting media. Apoptotic cells were identified by the morphological changes in the stained cells by fluorescence microscopy. For each determination, three separate counts of 100 cells were scored. Apoptosis (i.e. the number of cells with apoptotic nuclear morphology) was expressed as a percentage of the total number of C2C12 myoblasts counted.
Measurement of intracellular ROS
The levels of intracellular ROS were quantified by fluorescence with DCF-DA as described by Bass et al. with some modifications.Citation24) C2C12 myoblasts were collected and incubated with 10 μM of DCF-DA (dissolved in DMSO) for 30 min at 37 °C. Then, C2C12 myoblasts were washed with PBS, and the relative levels of fluorescence were quantified with FLx800 microplate fluorescence reader (BioTek Instruments Inc.) at the excitation and emission wavelengths of 485 and 535 nm, respectively. In addition, ROS production images were acquired with an LSM710 confocal microscope (Zeiss, Jena, Germany). During confocal microscopic observation, all the images were taken using the same settings.
Immunoblot analysis
C2C12 myoblasts were cultured for 23 h with DMSO or EVP (50 µg/mL) in serum-free DMEM. After PBS washing, the medium was replaced with serum-free DMEM containing 1 mM H2O2 for 1 h. Atrophy of GM and SM was induced by sciatic denervation with PBS (including 5% DMSO) or EVP (1–2 mg/kg) injection. Protein lysate was extracted using the PRO-PREP protein extraction kit (iNtRON, Sungnam-Si, Korea) following the manufacturer’s instructions. The whole lysates were analyzed using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) on 10–15% polyacrylamide gels. The proteins were transferred to PVDF membrane (Bio-Rad, Hercules, CA, USA). The membranes were blocked overnight at 4 °C in Tris-buffered saline containing 0.1% Tween-20 (TBS/T) and 5% skimmed milk powder and then incubated with each primary antibody. Blots were washed with TBS/T and incubated with each secondary antibody of horseradish peroxidase conjugated. Proteins were detected using an enhanced chemiluminescence detection reagent for immunoblot analysis (GE Healthcare, Buckinghamshire, UK).
High-performance liquid chromatography (HPLC)
Samples for lipid extraction provide C2C12 myoblasts and tissues from mice. Ceramide quantification was performed according to previously described procedures with some modifications.Citation25) In brief, total lipids were extracted using chloroform/methanol/1 M NaCl solution (8:4:2.5, v/v/v) with an addition of dihydrosphingomyelin as an internal standard and dried. Then, it was centrifuged at 13,000 rpm (4 °C) for 10 min, and the supernatant was transferred to a clean 1.5 mL tube. The dried samples were dissolved in 30 μL of chloroform/methanol (1:2, v/v) and spotted on an HPTLC silica-gel plate for ceramide separation. Then ceramide was converted simultaneously to sphingosine by ceramidase in a reaction buffer containing 25 mM Tris–HCl buffer, pH 7.5, containing 1% sodium chlorate, and 15% fatty acid-free bovine serum albumin. The released sphingosine from ceramide was analyzed using HPLC following ortho-phthalaldehyde derivatization.
Immunocytochemistry
C2C12 myoblasts were cultured on a cover glass for 23 h with DMSO or EVP (50 µg/mL) in serum-free DMEM. After PBS washing, the medium was treated with 1 mM H2O2 (by addition to the medium) for 1 h. C2C12 myoblasts fixed in 4% para-formaldehyde. 0.1% Triton-X treated for 25 min and 3% BSA blocking for 30 min in shaking rocker. C2C12 myoblasts were incubated with antibodies against SOD1 (1:1000 dilution) overnight at 4 °C. After PBS washing, C2C12 myoblasts were incubated with secondary antibodies, Alexa Fluor 488 anti-rabbit IgG (Invitrogen, Seoul, Korea), for 3 h at 4 °C. After being washed with PBS, C2C12 myoblasts were stained and mounted with DAPI-mounting media (Vector, Burlingame, CA, USA). Images were acquired with an LSM710 confocal microscope (Zeiss). During confocal microscopic observation, all the images were taken using the same settings.
Statistical analysis
Experimental results are expressed as the mean ± SD. One-way analysis of variance was followed by Tukey’s Multiple Comparison test using GraphPad Prism 5.0 software from GraphPad Software, Inc. (San Diego, CA, USA). Values of p < 0.01 were considered to indicate statistically significant differences.
Results
Effects of EVP on disuse muscle atrophy by sciatic denervation in mice
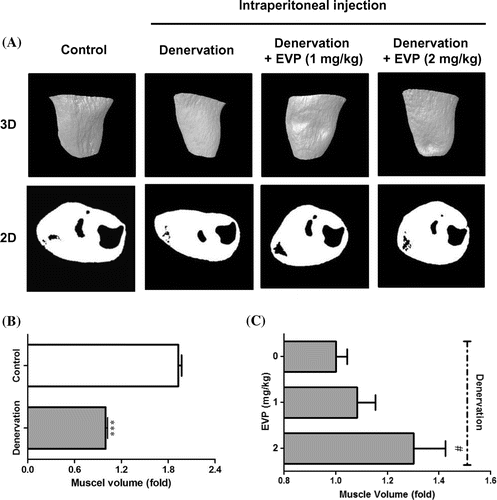
Muscle atrophy by sciatic denervation rapidly occurred during the first 14 days in Sprague Dawley rats.Citation26) First experiment was designed to investigate whether the sciatic-denervated mice model is relation with loss of muscle volume. Muscle volume was measured in vivo micro-CT imaging (Fig. (A)). As expected, the sciatic-denervated group had a decrease of muscle volume compared to the control group (Fig. (B)). This result indicates that skeletal muscles of sciatic-denervated mice undergo atrophy leading to muscle loss. In contrast, muscle volume of the EVP-treated group increased to compared with non-treated group (only sciatic denervation) (Fig. (C)).
Fig. 1. Effects of intraperitoneal injection of EVP on disuse muscle atrophy by sciatic denervation in mice.
Effects of EVP on substances and proteins relating to the pathway between ROS and apoptosis in muscle of sciatic-denervated mice
Several studies identified the relationship of ceramide with various muscular diseases.Citation27,28) Ceramide plays a key role which promotes protein synthesis and expression of muscle-specific transcription factors. HSP expression increases in response to stressors. Significant reduction of HSP70 by muscle atrophy indicates that HSP70 activity may protect muscle via several cellular responses.Citation19) SOD1 is known to be a major underlying factor for familial amyotrophic lateral sclerosis, muscular degeneration, and muscle atrophy.Citation29–32)
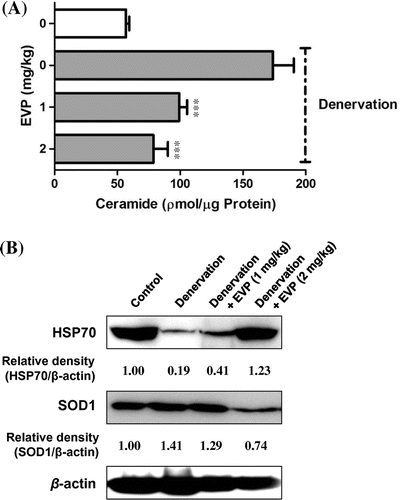
We investigated the changes of ceramide levels in sciatic-denervated mice through HPLC analysis. The amount of ceramide increased in sciatic-denervated mice. The GM and SM of sciatic-denervated mice exhibited 3.06-fold increase in ceramide levels. However, ceramide levels of the EVP group were reduced approximately 42.95 and 54.74% in the EVP group (1–2 mg/kg injection) compared with non-EVP-treated group of sciatic-denervated mice (Fig. (A)). Also, we confirmed that HSP70 protein decreased significantly in sciatic-denervated mice (Fig. (B)). In contrast, HSP70 expression was higher in the EVP-treated group than the non-treated group of sciatic-denervated mice. In addition, EVP reduced the expression of SOD1 in sciatic-denervated mice. This result suggests HSP70 is a regulator of skeletal muscle system and HSP70 expression is related to muscle atrophy.
Fig. 2. Effects of EVP on substances and proteins relating to the pathway between ROS and apoptosis in muscle of sciatic-denervated mice.
Effect of EVP on H2O2-induced oxidative stress in C2C12 myoblasts
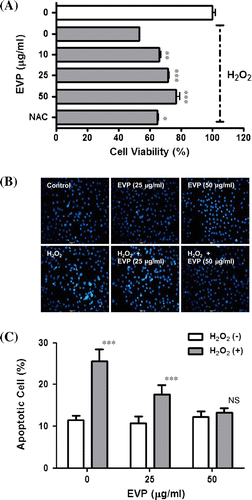
Muscle cell injury by ROS is closely associated with various muscular disorders and their pathogenic conditions.Citation1,11–13) In order to identify how EVP affects oxidative stress status, C2C12 myoblasts were treated with 1 mM H2O2 for 1 h, which reduced cell viability via extreme ROS. Cell viability was reduced approximately up to 48%; however, EVP (0–50 µg/mL) recovered cell viability (Fig. (A)) compared to the antioxidant NAC positive control. In addition, to investigate the effect of EVP on apoptotic cell death, we evaluated the change of chromatin morphology on oxidative stress by H2O2 in C2C12 myoblasts using DAPI staining. During apoptosis or necrosis, membrane permeability is increased. Therefore, strong fluorescence appears via an increase of DAPI influx. Cell death is characterized by chromatin condensation, apoptotic bodies, cytoplasmic condensation, and cell shrinkage. Apoptosis of C2C12 myoblasts was induced by 1 mM H2O2 for 1 h (Fig. (A)). Apoptosis by H2O2 increased to 25.53% compared to the un-treated control (11.45%). Pre-treatment for 23 h with EVP (25, 50 µg/mL) reduced apoptosis to 17.58 and 13.36%, respectively (Fig. (B)).
Fig. 3. Effect of EVP on H2O2-induced oxidative stress in C2C12 myoblasts.
Effect of EVP on ceramide level on H2O2-induced oxidative stress in C2C12 myoblasts
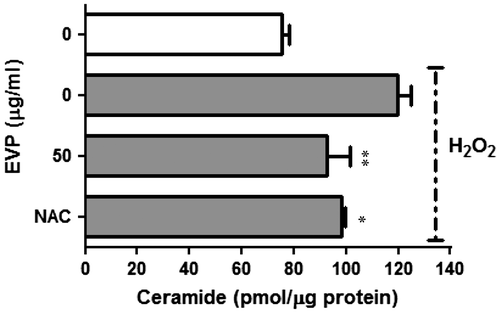
During differentiation of myoblasts, ceramide reduces expression of the myogenic transcription factor. Also, ceramide inhibits synthesis of myogenin and myotube formation.Citation33) We performed quantitative analysis of endogenous ceramide on oxidative stress by H2O2 in C2C12 myoblasts. HPTLC analysis method was used to separate ceramide from whole lipid extract. HPLC was used to quantify ceramide in C2C12 myoblasts. Ceramide increased up to 1.59-fold by H2O2 in C2C12 myoblasts. On the other hand, ceramide decreased by 20.83% in the presence of EVP (50 µg/mL) (Fig. ). This result suggests that the de novo synthesis of ceramide induced by ROS in vitro.
Fig. 4. Effect of EVP on ceramide level in H2O2-induced oxidative stress in C2C12 myoblasts.
Effect of EVP on oxidative stress induced by ROS in C2C12 myoblasts
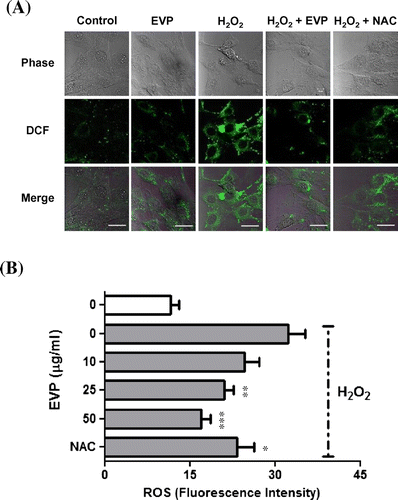
The generation of intracellular ROS was measured with DCF-DA. The expression of high fluorescence increases proportional to the level of intracellular ROS.Citation34) Our results showed high expression of fluorescence on oxidative stress by H2O2 in C2C12 myoblasts (Fig. (A)). However, the EVP group (50 µg/mL) fluorescence expression was similar to that of the control group. Additionally, we confirmed fluorescence by intracellular ROS in the presence or absence of EVP (0–50 µg/mL) in C2C12 myoblast via microplate fluorescence reader (Fig. (B)). Intracellular ROS by H2O2 (32.3 ± 3.1) had an increase in fluorescence density compared to control (11.7 ± 1.5). In contrast, pre-treatment of EVP (17 ± 1.7) had a reduced fluorescence density in C2C12 myoblasts.
Fig. 5. Effect of EVP on oxidative stress induced by ROS in C2C12 myoblasts.
Effect of EVP on SOD1 expression on H2O2-induce oxidative stress in C2C12 myoblasts
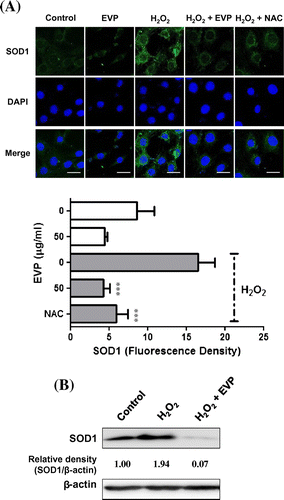
SOD1 is Cu–Zn superoxide dismutase located in the cytosol. Catalysis action of SOD1 has known as decomposition of superoxide or hydrogen peroxide.Citation35) So, we investigated the expression of SOD1 on oxidative stress by H2O2 in C2C12 myoblasts. In this study, we confirmed anti-oxidant capacity of EVP through SOD1 expression in sciatic-denervated mice (Fig. (B)). In addition, we performed immunocytochemistry using a confocal laser microscope to confirm the expression of SOD1 on oxidative stress by H2O2. C2C12 myoblasts had a high expression of SOD1 with exposure to H2O2. However, SOD1 measured by fluorescence decreased in the EVP group (50 µg/mL) (Fig. (A)). Following H2O2-induced oxidative stress without EVP, expression of the SOD1 protein increased significantly (Fig. (B)). Apparently, treatment of EVP downregulated SOD1 protein expression. This result suggests that EVP can decrease intracellular ROS-induced oxidative stress. These effects of EVP can support cell viability via suppressed of ROS production.
Fig. 6. Effect of EVP on SOD1 expression in H2O2-induced oxidative stress in C2C12 myoblasts.
Effect of EVP on HSP70 expression on H2O2-induced oxidative stress in C2C12 myoblasts
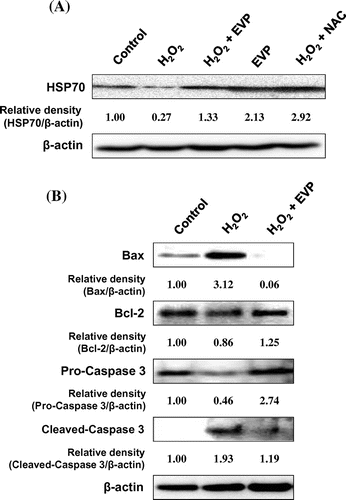
HSP protects cells from various stressors such as heat shock, tumor necrosis factor, oxidative stress, radiation, and ceramide.Citation36,37) We hypothesized that EVP could protect C2C12 myoblasts through modulation of HSP70 signaling in the cellular response to stress. Therefore, we performed immunoblot analysis to investigate the effect of EVP on HSP70 expression in C2C12 myoblasts. In this result, HSP70 expression was decreased by oxidative stress in C2C12 myoblasts (Fig. (A)). On the other hand, EVP (50 µg/mL) significantly recovered HSP70 protein expression. Also, we performed immunoblot analysis to investigate oxidative stress apoptotic signals in C2C12 myoblasts. The treatment of H2O2 increased intracellular ROS production in C2C12 myoblasts, and thereby induce cell apoptosis through apoptotic signals. We confirmed increased expression of Bax and cleavage caspase 3 as pro-apoptotic factors and decreased expression of anti-apoptotic factors such as Bcl-2 (Fig. (B)). In contrast, EVP suppressed these pro-apoptotic factors and increased anti-apoptotic factors. These results indicate that the apoptosis signals induced by oxidative stress can be regulated by the anti-oxidant capacity of EVP.
Fig. 7. Effect of EVP on HSP70 expression in H2O2-induced oxidative stress in C2C12 myoblasts.
Discussion
In this study, sciatic-denervated mice showed that the volume of muscle was altered by disuse muscle atrophy. In our previous study,Citation38) EVP has been efficient in sciatic denervation mice model via intramuscular injection. Therefore, we wanted to determine the effects of intraperitoneal injection. So, EVP was injected by intraperitoneal injection. Sciatic-denervated mice had significantly reduced volume of muscle; however, the intraperitoneal injection of EVP (1–2 mg/kg) delayed loss of muscle volume. EVP protected C2C12 myoblasts from oxidative stress, recovering cell viability under conditions of H2O2-induced oxidative stress. In addition, DAPI staining showed that EVP pre-treatment inhibits cell death in C2C12 myoblasts undergoing H2O2-induced oxidative stress. Apoptosis of myoblasts via oxidative stress is a key cause in muscle atrophy. Activation of caspase3 can be initiated by various signals such as ROS and pro- to anti-apoptotic proteins in mitochondria.Citation39) Apoptosis, a process of programmed cell death, is a tightly regulated system resulting in cellular self-destruction without inflammation or damage to the surrounding tissue.Citation40) We confirmed that EVP protects myoblasts from oxidative stress-induced cell death. EVP regulated apoptotic factors, including Bax, Bcl-2, and caspase 3, in H2O2-treated C2C12 myoblasts. We also found the anti-apoptotic effect of EVP via induction of HSP70. HSP70 is expected to increase under various stresses including heat shock, infection, inflammation, exercises, exposure to toxins, starvation, and hypoxia.Citation41,42,43) The protective effects of HSP70 are closely linked to apoptosis inhibition. Particularly, HSP70 is significantly down-regulated in various models of skeletal muscle atrophy.Citation44) We identified significant decreases in the HSP70 protein in both in vivo muscular atrophy conditions and in vitro oxidative stress conditions. The sciatic denervation-animal model has been used in rodents as a model of disuse to induce muscle atrophy. Muscle-specific ubiquitin ligases such as atrogin-1 and MuRF1 are induced by sciatic denervation.Citation45) Muscular diseases such as muscle atrophy are closely related to oxidative stress.Citation11–13) Sciatic denervation state is a destroyed arrangement of myosin filament by increase of ROS and degradation of muscle-specific protein. Other studies have shown that flavonoids, secondary metabolites of plants, are an effective countermeasure to reduce the muscle mass loss induced by sciatic denervation.Citation46) Many studies reported that EVP is composed of 65–75% linoleic acid; 7–10% γ-linolenic acid (GLA); oleic, palmitic, and stearic acids; and the steroids campesterol and β-sitosterol.Citation47,48) The GLA was effect of antioxidant among the components, which protected against DNA damage and apoptosis induced by palmitic acid.Citation23) In many studies, various fatty acids, including linoleic acid, GLA, oleic acid, palmitic acid, were known long ago as modulation materials in proliferation and differentiation of myoblast.Citation49,50) So, we checked the effect of linoleic acid, oleic acid, stearic acid, and campesterol as components of EVP in myoblast. The linoleic acid and oleic acid increased cell viability of C2C12 myoblasts on H2O2-induced stress. But, stearic acid and campesterol did not have the effect on cell viability of C2C12 myoblasts (data not shown). Therefore, we think that linoleic acid and oleic acid of main component of EVP would improve skeletal muscle atrophy. Now, we are trying to oral administration of a single compound of EVP. We are expecting that the component of EVP has a positive effect via oral administration in muscular disease. Due to this reason, anti-oxidative protection may be a useful strategy to prevent oxidative injury or to delay the progress of related diseases.Citation14)
In summary, muscle atrophy was regulated by the redox system of proteolysis. Many studies suggest that antioxidants can be therapeutic agents in muscle atrophy.Citation39) Therefore, these results can be of use in the rehabilitation of physically handicapped patients with muscular atrophy.
Author contributions
YH Lee and WJ Kim designed the experiments. DH Seo and HS Kim did micro-computed tomography analysis for sciatic denervation. MH Lee and SY Kim performed the revised experiments. M Gelinsky and TJ Kim formulated the hypothesis and wrote the article. HS Kim and TJ Kim received financial support for this study. All authors reviewed and approved the final draft.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning [grant number 2010-00757]; Leading Space Core Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning [grant number 2011-0030888].
Notes
Abbreviations: CT, computed tomography; DCF-DA, 2′,7′-dichlorofluorescein diacetate; EVP, Oenothera odorata root extract; FBS, fetal bovine serum; GM, gastrocnemius muscle; HPLC, high-performance liquid chromatography; HSP, heat shock protein; NAC, N-acetyl cysteine; OPA, ortho-phthaldialdehyde; ROS, reactive oxygen species; SCDase, Sphingolipid ceramide N-deacylase; SOD1, superoxide dismutase-1; SM, soleus muscle; GLA, γ-linolenic acid.
References
- Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982;107:1198–1205.10.1016/S0006-291X(82)80124-1
- Gwag T, Lee K, Ju H, Shin H, Lee JW, Choi I. Stress and signaling responses of rat skeletal muscle to brief endurance exercise during hindlimb unloading: a catch-up process for atrophied muscle. Cell. Physiol. Biochem. 2009;24:537–546.10.1159/000257510
- Chowdhury P, Soulsby M, Kim K. L-carnitine influence on oxidative stress induced by hind limb unloading in adult rats. Aviat. Space Environ. Med. 2007;78:554–556.
- Qu-Petersen Z, Deasy B, Jankowski R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J. Cell Biol. 2002;157:851–864.10.1083/jcb.200108150
- Huard J, Cao B, Qu-Petersen Z. Muscle-derived stem cells: potential for muscle regeneration. Birth Defects Res. C. Embryo Today. 2003;69:230–237.10.1002/(ISSN)1542-9768
- Leeuwenburgh C. Role of apoptosis in sarcopenia. J. Gerontol. A. Biol. Sci. Med. Sci. 2003;58:999–1001.
- Nader GA. Molecular determinants of skeletal muscle mass: getting the “AKT” together. Int. J. Biochem. Cell Biol. 2005;37:1985–1996.10.1016/j.biocel.2005.02.026
- Furuno K, Goodman MN, Goldberg AL. Role of different roteolytic systems in the degradation of muscle proteins during denervation atrophy. J. Biol. Chem. 1990;265:8550–8557.
- Goldberg AL. Protein turnover in skeletal muscle. II. Effects of denervation and cortisone on protein catabolism in skeletal muscle. J. Biol. Chem. 1969;244:3223–3229.
- Muller FL, Song W, Jang YC, et al. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1159–R1168.10.1152/ajpregu.00767.2006
- Rodriguez MC, Tarnopolsky MA. Patients with dystrophinopathy show evidence of increased oxidative stress. Free Radic. Biol. Med. 2003;34:1217–1220.10.1016/S0891-5849(03)00141-2
- Haycock JW, MacNeil S, Jones P, Harris JB, Mantle D. Oxidative damage to muscle protein in Duchenne muscular dystrophy. NeuroReport. 1996;8:357–361.10.1097/00001756-199612200-00070
- Haycock JW. Differential protein oxidation in Duchenne and Becker muscular dystrophy. NeuroReport. 1998;9:2201–2207.10.1097/00001756-199807130-00010
- Chan KM, Decker EA. Endogenous skeletal muscle antioxidants. Crit. Rev. Food Sci. Nutr. 1994;34:403–426.10.1080/10408399409527669
- Nishida H, Ichikawa H, Konishi T. Shengmai-san enhances antioxidant potential in C2C12 myoblasts through the induction of intracellular glutathione peroxidase. J. Pharmacol. Sci. 2007;105:342–352.10.1254/jphs.FP0071371
- Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Annu. Rev. Cell Biol. 1993;9:601–634.10.1146/annurev.cb.09.110193.003125
- Mailhos C, Howard MK, Latchman DS. Heat shock protects neuronal cells from programmed cell death by apoptosis. Neuroscience. 1993;55:621–627.10.1016/0306-4522(93)90428-I
- Ahn JH, Ko YG, Park WY, Kang YS, Chung HY, Seo JS. Suppression of ceramide-mediated apoptosis by HSP70. Mol. Cells. 1999;9:200–206.
- Senf SM, Dodd SL, McClung JM, Judge AR. Hsp70 overexpression inhibits NF-κB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J. 2008;22:3836–3845.10.1096/fj.08-110163
- Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:337–344.
- Horrobin DF. Nutritional and medical importance of gamma-linolenic acid. Prog. Lipid Res. 1992;31:163–194.10.1016/0163-7827(92)90008-7
- Jack AM, Keegan A, Cotter MA, Cameron NE. Effects of diabetes and evening primrose oil treatment on responses of aorta, corpus cavernosum and mesenteric vasculature in rats. Life Sci. 2002;71:1863–1877.10.1016/S0024-3205(02)01912-4
- Beeharry N, Lowe JE, Hernandez AR, et al. Linoleic acid and antioxidants protect against DNA damage and apoptosis induced by palmitic acid. Mutat. Res. 2003;530:27–33.10.1016/S0027-5107(03)00134-9
- Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J. Immunol. 1983;130:1910–1917.
- Lee S, Lee YS, Choi KM, et al. Quantitative analysis of sphingomyelin by high-performance liquid chromatography after enzymatic hydrolysis. Evid. Based Complement. Alternat. Med. 2012;2012: 396218.
- Sacheck JM, Hyatt JP, Raffaello A, et al. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007;21:140–155.
- Meadows KA, Holly JM, Stewart CE. Tumor necrosis factor-α-induced apoptosis is associated with suppression of insulin-like growth factor binding protein-5 secretion in differentiating murine skeletal myoblasts. J. Cell. Physiol. 2000;183:330–337.10.1002/(ISSN)1097-4652
- Strle K, Broussard SR, McCusker RH, et al. Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology. 2004;145:4592–4602.10.1210/en.2003-1749
- Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 2004;27:723–749.10.1146/annurev.neuro.27.070203.144244
- Valentine JS, Doucette PA, Zittin Potter S. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu. Rev. Biochem. 2005;74:563–593.10.1146/annurev.biochem.72.121801.161647
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007;43:477–503.10.1016/j.freeradbiomed.2007.03.034
- Elchuri S, Oberley TD, Qi W, et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380.10.1038/sj.onc.1208207
- Mebarek S, Komati H, Naro F, et al. Inhibition of de novo ceramide synthesis upregulates phospholipase D and enhances myogenic differentiation. J. Cell Sci. 2007;120:407–416.10.1242/jcs.03331
- Azzawi M, Hasleton PS, Turner DM, et al. Tumor necrosis factor-alpha gene polymorphism and death due to acute cellular rejection in a subgroup of heart transplant recipients. Hum. Immunol. 2001;62:140–142.10.1016/S0198-8859(00)00235-4
- Tsang CK, Liu Y, Thomas J, Zhang Y, Zheng XF. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat. Commun. 2014;5:3346–3356.
- Simon MM, Reikerstorfer A, Schwarz A, et al. Heat shock protein 70 overexpression affects the response to ultraviolet light in murine fibroblasts. Evidence for increased cell viability and suppression of cytokine release. J. Clin. Invest. 1995;95:926–933.10.1172/JCI117800
- Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp. Cell. Res. 1996;223:163–170.10.1006/excr.1996.0070
- Lee YH, Seo DH, Park JH, et al. Effect of Oenothera odorata root extract on microgravity and disuse-induced muscle atrophy. Evid. Based Complement. Alternat. Med. 2015;2015:130513–130521.
- Scott KP, Andreas NK, Keith CD. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:337–344.
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257.10.1038/bjc.1972.33
- Wong HR. Heat shock proteins. Facts, thoughts, and dreams. Shock. 1999;12:323–325.10.1097/00024382-199910000-00012
- De Maio A. Heat shock proteins. Facts, thoughts, and dreams. Shock. 1999;11:1–12.
- Santoro MG. Heat shock factors and the control of the stress response. Biochem. Pharmacol. 2000;59:55–63.10.1016/S0006-2952(99)00299-3
- Kondo H, Miura M, Itokawa Y. Antioxidant enzyme systems in skeletal muscle atrophied by immobilization. Pflugers Arch. 1993;422:404–406.10.1007/BF00374299
- Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. USA. 2001;98:14440–14445.10.1073/pnas.251541198
- Mukai R, Horikawa H, Fujikura Y, et al. Prevention of disuse muscle atrophy by dietary ingestion of 8-prenylnaringenin in denervated mice. PLoS One. 2012;7:e45048.10.1371/journal.pone.0045048
- Blumenthal M. The American Botanical Council Guide to Herbs. NY: Thieme Medical Publishers; 2003.
- Kim HY, Oh H, Li X, Cho KW, Kang DG, Lee HS. Ethanol extract of seeds of Oenothera odorata induces vasorelaxation via endothelium-dependent NO-cGMP signaling through activation of Akt-eNOS-sGC pathway. J. Ethnopharmacol. 2011;133:315–323.10.1016/j.jep.2010.09.024
- Zhang G, Chen X, Lin L, Wen C, Rao S. Effects of fatty acids on proliferation and differentiation of myoblast. Wei. Sheng. Yan. Jiu. 2012;41:883–888.
- Lee JH, Tachibana H, Morinaga Y, Fujimura Y, Yamada K. Modulation of proliferation and differentiation of C2C12 skeletal muscle cells by fatty acids. Life Sci. 2009;84:415–420.10.1016/j.lfs.2009.01.004
