Abstract
The collagenase activity and the fpcol gene were examined in Flavobacterium psychrophilum isolates from cold-water disease (CWD)-affected ayu, Plecoglossus altivelis. Collagenase expression was closely related to the accumulated mortality of CWD-affected ayu. RT-qPCR and bacterial challenge experiments showed that F. psychrophilum ayu isolate WA-1 expressed the fpcol gene more actively and was more virulent than ayu isolate WA-2. The amago (Oncorhynchus masou) isolate WB-1, which possesses a pseudo-fpcol gene, was not harmful to ayu. Hitherto, the well-studied metalloproteases Fpp1 and Fpp2 have been considered virulence factors. However, the most virulent isolate against ayu (WA-1) showed no Fpp activity because of a deletion mutation or an insertion of a transposon in the fpp genes. The less virulent WA-2 isolate showed only Fpp1 activity. Taken together, these results suggest that collagenolytic activity, but not Fpp activity, is related to the virulence of F. psychrophilum isolates in CWD-affected ayu.
Graphical abstract
Comparative study of Flavobacterium psychrophilum isolates from ayu and amago suggests that the collagenase is one of the pathogenic factors for ayu.
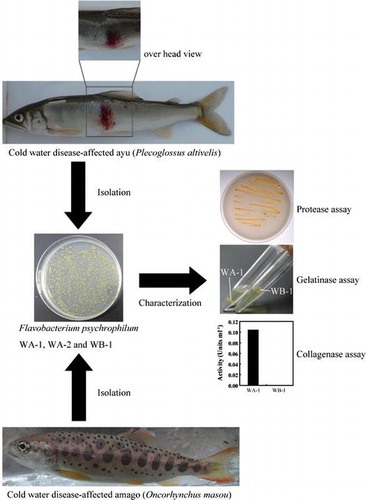
Cold-water disease (CWD) occurs among species of Osmeridae and Salmonidae in fish farms and in aquatic environments including natural rivers and lakes. In Japan, ayu (Plecoglossus altivelis), belonging to the Osmeridae, are actively cultured in fish farms. Ayu are amphidromous fish that typically live for only 1 year. Ayu are stocked in natural rivers with one or more dams for flood-control, irrigation, or electric power generation and are a very popular game fish and fishery species in Japan. A great number of farmed or stocked ayu have been lost to CWD each year over the past 20 years. The gram-negative bacterium Flavobacterium psychrophilum causes CWD, but the serologic and the genetic types of isolates from osmeroids and salmonids differ from each other.Citation1–3) It is also known that the virulence of F. psychrophilum isolates may vary and that some isolates may be specifically virulent for ayu. However, the reasons why some isolates are not harmful to ayu have yet to be determined.
The symptoms of CWD in ayu differ greatly from those in salmonids. CWD-affected ayu primarily show signs such as anemia (pale gills), hemorrhagic lesions or ulcers on the lower jaw, ulcerative lesion on the surface of the body trunk, and shortened trunk in fish that have recovered from CWD.Citation4) CWD can occur in both juvenile and mature ayu when the water temperature ranges between 10 and 20 °C, causing mass mortality in this high-commodity-value fish. In contrast, CWD-affected salmonids primarily show signs such as erosion of fins, tail loss, and dermal ulceration.Citation5) This type of CWD occurs primarily in very young salmonids and rarely occurs in adults when the water temperature ranges between 4 and 10 °C. Taken together, these data suggest that the pathogenic mechanisms of ayu isolates differ from those of salmonid isolates.
A number of putative virulence factors of salmonid isolates have been identified and include extracellular proteases such as Fpp1, Fpp2, and a thermolysin-like protease.Citation6–10) Among these, the metalloproteases Fpp1 and Fpp2 are the most thoroughly studied. Fpp1 and Fpp2 can cleave gelatin, laminin, fibronectin, fibrinogen, and nonfibrillar type IV collagen, but demonstrate minimal activity against fibrillar type I collagen.Citation8,9) Other putative virulence factors have been studied and include lipopolysaccharide, glycocalyx, tlpB, and exbD2.Citation11–15) Although the genome of F. psychrophilum JIP02/86 from rainbow trout has been analyzed,Citation16) the molecular pathogenesis and virulence factors of this isolate have yet to be fully elucidated. Interestingly, the collagenase is not considered a major virulence factor because the collagenase (fpcol) gene of many F. psychrophilum isolates from rainbow trout (Oncorhynchus mykiss) is interrupted by a transposon.Citation16) By contrast, the virulence factors of ayu isolates are believed to be included in the membrane vesicles secreted from the bacteria.Citation17,18) However, the associated proteins and genes have yet to be identified.
In this study, we found that F. psychrophilum ayu isolates WA-1 and WA-2 showed collagenolytic activity, but the amago isolate WB-1 showed no collagenolytic activity. Sequence analysis of the fpcol genes showed that a single mutation disrupts the collagenolytic activity of the WB-1 isolate. The transcription level of the fpcol gene was compared between isolates WA-1 and WA-2 by reverse transcription–quantitative real-time PCR (RT-qPCR). Furthermore, the sequences of two well-known metalloprotease genes (fpp1 and fpp2) were compared in isolates WA-1, WA-2, and WB-1. The virulence of these isolates against ayu was also compared by bacterial challenge. The results of our study are consistent with the hypothesis that collagenase may be an F. psychrophilum virulence factor for infection of ayu.
Materials and methods
Strains
Flavobacterium psychrophilum WA-1 and WA-2 were isolated from the kidneys of CWD-affected independent ayu (P. altivelis). Isolate WB-1 was from the kidneys of CWD-affected amago (O. masou). These isolates were identified by sequencing the 16S rRNA genes, as described below. The isolates were deposited in the NITE Biological Resource Center (NBRC) under accession numbers NBRC108951, NBRC109952, and NBRC108952 for of WA-1, WA-2, and WB-1, respectively.
Culture conditions
The isolates were cultured in cytophaga (CY) medium (containing 2.0 g of tryptone, 0.5 g of yeast extract, and 0.2 g of sodium acetate per liter) at 18 °C. Growth of the isolates was monitored by determining the optical density of the culture at 620 nm using an NJ-2300 micro-plate reader (Nalge Nunc International, Rochester, NY, USA).
PCR and sequencing
Whole-cell DNA was extracted from isolates WA-1, WA-2, and WB-1 using a Mammalian Genomic DNA Miniprep kit (Sigma-Aldrich, St. Louis, MO, USA). The genomic DNA was used as the PCR template DNA. The 16S rDNA, collagenase (fpcol), and protease (fpp1 and fpp2) genes were amplified using the primer sets 8F-1541R, col1F-col1R (see Fig. (a)), Fpp1F-Fpp1R, and Fpp2F-Fpp2R, respectively. The primers used in this study are listed in Table . The PCR mixtures (20 μL) contained 2 μL of 10× PCR buffer, 2 μL of 2 mM dNTPs, 1 μL of each forward and reverse primer (1 pmol μL−1), 0.5 μL of KOD Dash DNA polymerase (TOYOBO, Osaka City, Osaka, JPN), 1 μL of the template DNA (10 ng μL−1), and 13.5 μL of distilled water. The following thermal cycler program was used: 35 cycles of denaturation (96 °C for 30 s), annealing (55 °C for 15 s), and extension (68 °C for 2 min). The unreacted primers in the PCR products were removed using ExoSAPI (GE Healthcare, Little Chalfont, GBR). The amplified PCR products were resolved on a 1% agarose gel and visualized by staining with 0.1% ethidium bromide. The sequences of the DNA amplicons were analyzed according to the Sanger method (TAKARA BIO INC, Otsu City, Shiga, JPN) using the appropriate primers (see Table ).
Fig. 1. The fpcol gene of F. psychrophilum.
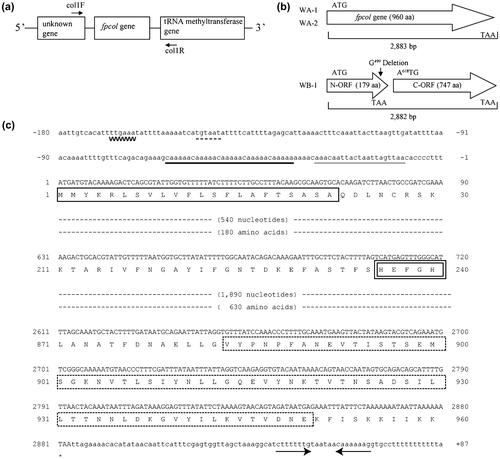
Table 1. Primers used in this study.
Gelatinolytic activity
The isolates were cultured on solid CY medium containing 1.0% gelatin (bovine, Sigma-Aldrich) at 18 °C. The isolates with gelatinolytic activity solated the medium around the colonies within 7 days.
Proteolytic activity
The isolates were cultured on solid CY medium containing 1.5% agarose and 10% horse serum (Invitrogen, Waltham, MA, USA) at 18 °C. Following autoclaving, the serum was added to the medium before the temperature went below 70 °C. When the horse serum was autoclaved with the medium, the proteins within the serum aggregated, causing cloudy patches in the medium. Bacteria exhibiting Fpp activity formed clear zones around streaked colonies within 6 days.
Collagenolytic activity
Supernatants of the isolates were obtained by centrifuging the cultures at 3500 × g for 10 min. The collagenolytic activity of the supernatants was measured using fluorescein isothiocyanate (FITC)-labeled type I collagen, as previously described.Citation19,20) Briefly, the supernatant was mixed with 50 mM Tris–HCl (pH 7.5) containing 0.05% FITC-labeled type I collagen, 5 mM CaCl2 and 200 mM NaCl and incubated at 20 °C for 20 h. After adding EDTA to stop the enzymatic reaction, degraded FITC-labeled collagen fragments were extracted with 50 mM Tris–HCl (pH 9.5) containing 70% ethanol. The fluorescence intensity of the supernatant was measured by fluorescence spectrophotometry (530 nm [emission], 485 nm [excitation]). The results of the collagenase assay are shown as the average of triplicate assays.
RNA isolation and cDNA synthesis
Isolates showing collagenolytic activity were cultured to evaluate the transcription of the fpcol gene under various conditions. The ayu isolate WA-1 was cultured in CY medium at 10 and 18 °C. WA-1 was also cultured in CY medium containing 0.1% gelatin or 1 mM CaCl2 at 18 °C. For comparison between WA-1 and the ayu isolate WA-2, WA-2 was grown in CY medium at 18 °C. Aliquots (1 mL) of cultures of the isolates at the different growth stages were collected and centrifuged at 3500 × g for 15 min. After the supernatants were removed, the pellets were stored at −80 °C. Total RNA was extracted from each sample using Isogen (Nippon Gene, Chiyoda-Ku, Tokyo, JPN), following the manufacturer’s instructions. The cDNAs of the fpcol (target) and peptidyl-prolyl cis-trans isomerase C (ppic) (reference) genes were synthesized from the isolated total RNA samples using SuperScriptIII® reverse transcriptase (Invitrogen). The reverse transcription reaction condition was 55 °C for 30 min using the primer sets col2F-col8R and PPIC1388F-PPIC1593R (see Table ). In this study, the ppic gene was used as an internal control for RT-qPCR gene transcription analysis. It is known that the amount of ppic gene is related to the number of F. psychrophilum cells.Citation21) The synthesized cDNAs were used in the RT-qPCR setups.
Standard marker of RT-qPCR experiments
The PCR amplicons of the fpcol and ppic genes were used as standard markers. To amplify them, a preparation of WA-1 genomic DNA and the following primer sets, col2F-col8R or PPIC1388F-PPIC1593R (see Table ), were used. The PCR conditions were the same as those described above. The PCR products were purified by ethanol precipitation, and the concentrations were determined by monitoring the absorbance at 260 nm. A range from 106 to 1010 copies of each gene was used in the RT-qPCR setups.
RT-qPCR experiments
The transcription level of the fpcol and ppic genes was measured by RT-qPCR using an ABI Prism 7900HT (Applied Biosystems, Foster City, CA, USA). PCR mixtures (20 μL each) contained 10 μL of 2× SYBR Premix Ex Taq II (TAKARA BIO INC), 0.4 μL of 50× ROX reference dye, 1 μL of each forward and reverse primer (10 pmol μL−1), 1.6 μL of template DNA, and 6 μL of distilled water. The synthesized cDNAs or PCR amplicons of the fpcol and ppic genes were used as templates. The primer sets col2F-col8R and PPIC1388F-PPIC1593R were used for amplification of the fpcol and ppic genes, respectively. The following thermal cycler program was used: 40 cycles of denaturation (96 °C for 30 s), annealing (55 °C for 15 s), and extension (68 °C for 30 s). The Ct value for each sample was calculated using the program SDS v2.0 (http://www.appliedbiosystems.com/absite/us/en/home/support/tutorials.html). The average Ct value was calculated from a curve prepared from the data from 3 wells. Relative transcription was calculated as the ratio of the average transcription level of the fpcol gene (target) to that of the ppic gene (reference) in each sample.
Bacterial challenge
Ayu (weight, 20–30 g) were held in 176-L tanks that received single-pass well water (16–18 °C) at 500 mL min−1, with supplemental aeration provided by air stones. Prior to infection, the fish were anesthetized with ethyl p-aminobenzoate (0.1 g L−1). Thirty fish per group each received an abdominal cavity injection of 50 μL of isolate WA-1, WA-2, or WB-1 (1.0 × 107 CFU) in PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.76 mM KH2PO4, pH 7.4). The fish were then distributed into 9 experimental tanks containing 10 fish each. The water temperature was controlled to between 16 and 17 °C. As a control, 30 fish received an abdominal cavity injection of 50 μL of PBS; no fish died within 30 days.
DDBJ accession numbers
The sequences of the fpcol gene of isolates WA-1, WA-2, and WB-1 were deposited in the DNA Data Bank of Japan (DDBJ, http://www.ddbj.nig.ac.jp/) under the accession numbers AB921326, AB921327, and AB921328, respectively. Sequence data for the fpp1 and fpp2 genes were also deposited in the DDBJ. The accession numbers for the fpp1 gene of isolates WA-1, WA-2, and WB-1 were AB921320, AB921321, and AB921322, respectively, and the accession numbers for the fpp2 gene from isolates WA-1, WA-2, and WB-1 were AB921323, AB921324, and AB921325, respectively.
Results
Nucleotide and deduced amino acid sequences of the fpcol gene
The sequenced fpcol gene of isolate WA-1 was 2883 bp and encoded a protein with a molecular mass of 102 kDa (Fig. (b) and (c)). The N-terminal amino acid sequence of the collagenase contains a characteristic signal sequence. The N-terminal methionine residue is followed by positively charged amino acids (Lys4-Arg5) and hydrophobic amino acids (Leu6-Phe17). The putative cleavage site (Ala22-Gln23) of the signal peptide was identified using the weight-matrix approach.Citation22) The fpcol gene carries a catalytic amino acid motif (His236-Glu237-x-x-His240) characteristic of a zinc metalloprotease. The C-terminal region of the fpcol gene product possesses a Por secretion tail (Val884-Glu950), which was predicted by a Conserved Domains Search (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
The amino acid sequence of the homologous fpcol gene of ayu isolate WA-2 was nearly identical (99%) to that of WA-1. Although the nucleotide sequence of the pseudo-fpcol gene (2882 bp) of amago isolate WB-1 was nearly identical (>99%) to that of the gene in ayu isolates WA-1 and WA-2, the pseudo-gene lacked a single nucleotide (guanine) at the 499th position. Therefore, the pseudo-gene of WB-1 ends at the 179th residue, meaning that the encoded protein does not exhibit collagenolytic activity in WB-1 cells (Fig. (b)).
In this study, the 5′ leader sequence of the fpcol gene was also sequenced in WA-1 (Fig. (c)) and the other isolates (Fig. ). A putative ribosomal protein S1-binding site (AU-rich sequence)Citation23) was identified upstream of the ATG initiation codon in each isolate. No ribosome-binding site (AG-rich sequence) was identified. A tandem nucleotide repeat (CAAAAA) was identified upstream of the ribosomal protein S1-binding site. A Pribnow Box (TGTAAT) was identified 77-bp upstream of the tandem repeat region. A-35 Box (TTGAAA) was identified upstream of the Pribnow Box, with a gap of 14 bp. Comparison of the 5′ leader sequences between the four isolates, except for the number of CAAAAA tandem repeats, revealed that they are highly similar (Fig. ).
Fig. 2. The 5′ leader sequences and the start region of the fpcol genes.
Collagenolytic and gelatinolytic activity
The culture supernatants of WA-1 and WA-2 digested FITC-labeled type I collagen (Fig. ). The collagenolytic activity of WA-1 was higher than that of WA-2. In contrast, the culture supernatant of isolate WB-1, which possesses a pseudo-fpcol gene, showed no collagenolytic activity (Fig. ). The collagenolytic and gelatinolytic activities of the isolates were similar (Table ). Proliferative WA-1 and WA-2 cells digested gelatin contained in the medium, but WB-1 did not (data not shown).
Fig. 3. Collagenolytic activity.
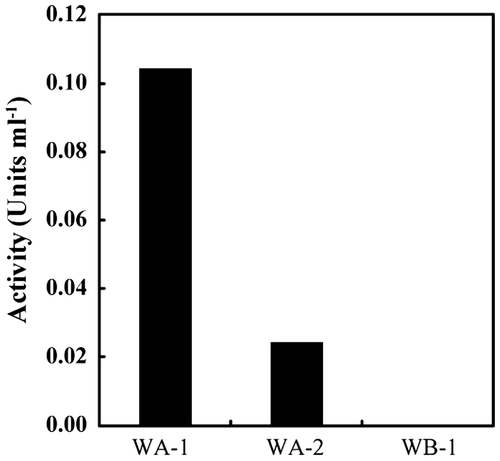
Table 2. Properties of the F. psychrophilum isolates used in this study.
The fpp gene and Fpp activity
The properties of the fpp1 and fpp2 genes are summarized in Table . The fpp1 genes of WA-2 and WB-1 encoded the full-length ORFs, which were nearly identical (>99%) to the known fpp1 gene.Citation8) Although the nucleotide sequence of the fpp1 gene of WA-1 was nearly identical (>99%) to that of the known fpp1 gene,Citation8) it lacked the adenine at nucleotide 43, resulting in a frameshift mutation (Table ).
The fpp2 genes of each of the isolates carried a mutation or a transposon insertion. The guanine at position 466 in the fpp2 gene in WA-2 was mutated to a thymine, resulting in a nonsense mutation. The transposons (1005 and 1019 bp) were inserted at the 1999th and 279th nucleotide of the fpp2 gene in WA-1 and WB-1, respectively (see Supplementary Table 1). Characteristic palindromic structures were also identified at the ends of each transposon. The transposons in WA-1 and WB-1 encoded ORFs of 294 and 297 amino acids, respectively. A BLAST search revealed that both ORFs belong to the transposase IS982 family (data not shown). The transposon identified in the fpp2 gene of WA-1 showed significant identity (E-value=e−130) with the transposon ISFba2 in the genome of Flavobacterium BAL38 (see the IS Database, https://www-is.biotoul.fr//). The transposon identified in the fpp2 gene of WB-1 showed similarity (E-value = 3e−4) with the transposon ISPasp2 in the genome of Parachlamydia sp. UWE25 (see the IS Database).
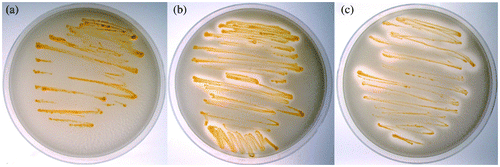
The Fpp activity of each isolate is shown in Fig. . Isolates WA-2 and WB-1 formed clear zones around streaked colonies, indicating that they possess proteolytic activity. In contrast, colonies of isolate WA-1 formed no clear zones.
RT-qPCR of the collagenase gene
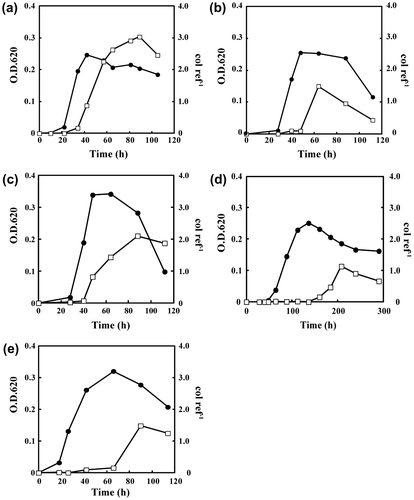
The transcription level of the fpcol gene in WA-1 and WA-2 was analyzed by RT-qPCR (Fig. ). Peak transcription occurred in the death phase for each isolate, irrespective of the culture conditions. The transcription level was highest when cells were cultured in CY medium at 18 °C (Fig. (a)). The addition of 1 mM CaCl2 or 0.1% gelatin to the medium decreased the transcription level by 50 and 75%, respectively (Fig. (b) and (c)). Notably, these additives changed the behavior of WA-1 cells, which formed visible flocs in medium containing 1 mM CaCl2 (data not shown). The cell density increased following addition of gelatin to the medium (Fig. (c)). By culturing at 10 °C, the transcription level was one-third of that at 18 °C (Fig. (d)). Even at 10 °C, the cell density in the late exponential phase was almost the same as that of cultures grown at 18 °C, despite the fact that the growth rate decreased by one-third (Fig. (d)). Although the cell density in the late exponential phase was slightly higher with WA-2 than with WA-1, the level of fpcol gene transcription in WA-2 was 50% less than that in WA-1 (Fig. (e)).
Fig. 5. The growth curves and the transcription levels of the fpcol gene.
Bacterial challenge
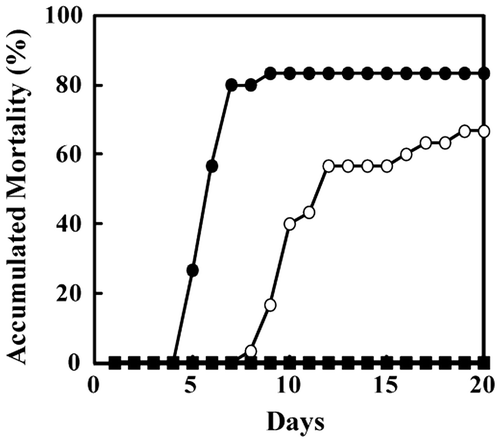
Most of the ayu injected in the abdominal cavity with the isolates WA-1 or WA-2 died within 10 days. The accumulated mortality of ayu injected with WA-1 and WA-2 was 80 and 60%, respectively (Fig. ). In contrast, no fish injected with the amago isolate WB-1 died. Similar results were obtained in experiments in which unaffected ayu were kept with ayu affected by CWD caused by WA-1, WA-2, or WB-1 (data not shown). These results indicate that ayu isolate WA-1 is more harmful than ayu isolate WA-2 or amago isolate WB-1.
Fig. 6. Bacterial challenge.
Discussion
Collagenase genes
A fpcol gene encoding a full-length collagenase possessing an N-terminal signal peptide, a catalytic motif (HExxH), and a C-terminal Por secretion tail was identified in ayu isolates WA-1 and WA-2 (Fig. (c)). In gram-negative bacteria, proteins with a signal peptide and a Por secretion tail are first secreted into the periplasm across the cytoplasmic membrane and then transported from the periplasm across the outer membrane by the Por secretion system (PorSS).Citation24–27) Because the genes constituting the PorSS seem to be widely conserved among the Bacteroidetes, including F. psychrophilum JIP02/86,Citation28), it is believed that F. psychrophilum isolates also secrete the proteins with a signal peptide and a Por secretion tail into the supernatant using the PorSS. More importantly, this study showed that culture supernatants of WA-1 and WA-2 exhibit collagenolytic activity (Fig. ). Taken together, these data suggest that the mature collagenases of F. psychrophilum are secreted from the cytoplasm to the supernatant via the PorSS.
The collagenases from WA-1 and WA-2 share 51% amino acid sequence identity with the homologous collagenase from Cytophaga sp. L43-1,Citation29,30) which is closely related to members of the genus Flavobacterium. These homologous genes belong to the M43 metalloprotease family and have only been identified in F. psychrophilum isolates and Cytophaga sp. L43-1. Despite the fact that the genomes of more than 20 species of the genus Flavobacterium, including bacterial gill disease-causing F. branchiophilum,Citation31) have been completely sequenced (see http://www.ncbi.nlm.nih.gov/), no similar collagenase genes have been found among their genomes.
Among F. psychrophilum isolates, fpcol genes encoding the full-length collagenase have only been identified in ayu isolates. In addition to WA-1 and WA-2, the other two ayu isolates have a homologous fpcol gene encoding the full-length collagenase (data not shown). By contrast, salmonid isolates carry the pseudo-fpcol gene with a transposonCitation16) or a single nucleotide mutation, similar to the amago isolate WB-1 described in this study (Fig. (b)). As discussed in a previous study, it is believed that the collagenase is not involved in the pathogenesis of CWD in salmonids.Citation16) However, notably, pseudo-genes lacking the transposon or the single nucleotide mutation encode a collagenase nearly identical (99% amino acid sequence identity) to that of the ayu isolates. These data strongly suggest that an ancestor of the salmonid isolates lost the collagenolytic activity due to a mutation or the insertion of a transposon because the collagenase is not essential for survival. As a result, some salmonid isolates may have lost pathogenicity for ayu. By contrast, the question whether ayu isolates with collagenolytic activity are pathogenic for salmonids has yet to be answered.
The mechanism of fpcol gene regulation
The results of RT-qPCR and bacterial challenge experiments suggest that isolates that actively express the collagenase may be highly virulent for ayu (Figs. , (a), (e), and ). The results of RT-qPCR experiments also indicate that transcription of the fpcol gene is influenced by bacterial growth stage, temperature, and the presence of calcium or gelatin. These factors may affect the development of CWD in ayu.
Peak transcription (Fig. ) seems to occur later for the fpcol gene compared with the collagenase gene (colA) derived from the gram-positive bacterium Clostridium perfringens. The colA gene is strongly transcribed in the period from the late exponential to the early stationary phase, and its translation is regulated by the temperature-dependent formation of a characteristic secondary structure in the mRNA encoding the 5′ leader sequence of the ORF.Citation32) This regulatory mechanism appears to differ from that of the fpcol gene because no such secondary structure is predicted from the 5′ leader sequence of the fpcol gene.
The expression level of the collagenase is believed to differ among the ayu isolates (Fig. (a) and (e)). Interestingly, comparison of the 5′ leader sequences of the fpcol genes between WA-1 and WA-2 shows that they are nearly identical, with the exception of the number of 6-nt repeats (Fig. ). Notably, the repeat region is next to the ribosomal protein S1-binding region located upstream of the initiation codon (Fig. ). Ribosomal protein S1 is part of the translational apparatus and is known to stimulate transcription in vitro by binding to the S1-binding region.Citation33) These data suggest that the difference in the number of 6-nt repeats adjacent to the ribosomal protein S1-binding region may affect transcription of the fpcol gene. The mechanism by which expression of the fpcol gene is regulated remains unclear.
Occurrence of CWD outbreaks at low water temperature
Water temperature is also a critical factor in the development of CWD in ayu, which commonly occurs at around 18 °C. The low bacterial growth rate and decreased fpcol gene transcription observed at 10 °C (Fig. (a) and (d)) suggest that CWD outbreaks in ayu are repressed at low water temperature. However, CWD outbreaks can also occur at around 10 °C. It is known that humoral immunity in fish is suppressed at low temperature.Citation34) Therefore, at low temperature, CWD outbreaks can occur when bacterial virulence, which is influenced by bacterial growth rate and the collagenase expression, exceeds the capacity of the ayu immune response.
On the other hand, it is known that CWD in salmonids tends to occur at rather low temperatures, from 4 to 10 °C. In contrast to the fpcol gene, the level of transcription of some genes, including the two-component histidine sensor kinase (FP1516), ATP-dependent RNA helicase (FP0666), outer membrane protein (FP2096), hypothetical protein (FP0029), ATP-binding cassette transporter (FP0834), and M43 metalloprotease (FP1619), is known to be higher at 10 °C than 18 °C in F. psychrophilum isolated from rainbow trout.Citation15) These data also suggest that the pathogenic mechanism and virulence factors associated with CWD in ayu and salmonids differ.
Virulence factors in ayu-CWD
The results of this study suggest that the collagenase produced by F. psychrophilum is a virulence factor in ayu-CWD, because only collagenase can digest the collagen component of the skin, which is the preliminary barrier protecting the host fish from bacterial infection. To date, no other enzymes that digest collagen have been identified in F. psychrophilum. Regarding the well-studied Fpp1 and Fpp2 enzymes, the results of our study strongly suggest that the Fpps do not play a critical role in the pathogenesis of CWD in ayu because the most pathogenic isolate, WA-1, showed no Fpp activity following nucleotide deletion in the fpp1 gene and the insertion of the transposon in the fpp2 gene (Fig. and Table ). It is noteworthy that the pathogenicity of WA-1, exhibiting only collagenolytic activity, was higher than that of WA-2, which exhibited both collagenolytic and Fpp activity (Fig. and Table ). However, the possibility that Fpp activity exacerbates the lesions associated with CWD has not yet been fully ruled out.
When F. psychrophilum invades a host fish, the bacteria are surrounded by an abundance of calcium and gelatin. In this study, transcription of the fpcol gene was shown to be partially repressed in the presence of calcium or gelatin (Fig. (a)–(c)). These results suggest that the collagenase may play an important role in the period spanning bacterial adherence to initiation of the invasion process. After invasion, other proteases encoded in the F. psychrophilum genome, such as serine proteases and metalloproteases,Citation16) may facilitate bacterial dispersion and function as lethal toxic factors in the host. Most of these proteases have not been studied. In order to develop approaches to rapidly resolve CWD outbreaks in ayu, it is very important that the biological functions of these proteases be elucidated.
Author contribution
All of the authors made substantial contributions to conception, study design, and interpretation of data. Nakayama H., K. Tanaka, and N. Teramura contributed to acquisition of data and analysis. Nakayama H. drafted the manuscript and K. Tanaka, N. Teramura, and S. Hattori revised it critically for important intellectual content. All of the authors gave final approval of the version to be submitted and any revised version.
Funding
This work was supported by the Japan Science and Technology Agency [AS242Z02004N]; Adaptable and Seamless Technology Transfer Program grant through Target-driven R&D (A-STEP) [AS2531213N].
Supplemental material
Supplemental material for this article can be accessed at http://dx.doi.org/10.1080/09168451.2015.1079477.
Supp150701.doc
Download MS Word (60 KB)Acknowledgments
RT-qPCR data were collected at Wakayama Prefectural Research Center of Environment and Public Health, technically supported by F. Terasoma and H. Naka. We thank Drs C. Nakayasu, Y. Yoshiura, and M. Ototake for helpful discussions. We also thank Dr K. Yoshimoto for proofreading the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Izumi S, Wakabayashi H. Further study on serotyping of Flavobacterium psychrophilum. Fish Pathol. 1999;34:89–90.10.3147/jsfp.34.89
- Izumi S, Aranishi F, Wakabayashi H. Genotyping of Flavobacterium psychrophilum using PCR-RFLP analysis. Dis. Aquat. Organ. 2003;56:207–214.10.3354/dao056207
- Tabata K. Relationships of the infectivity of Flavobacterium psychrophilum between native fishes and released ayu Plecoglossus altivelis in a river. Jap. Soc. Fish. Sci. 2004;70:318–323. Japanese.
- Miwa S, Nakayasu C. Pathogenesis of experimentally induced bacterial cold water disease in ayu Plecoglossus altivelis. Dis. Aquat. Organ. 2005;67:93–104.10.3354/dao067093
- Dalsgaard I. Virulence mechanisms in Cytophaga psychrophila and other Cytophaga-like bacteria pathogenic for fish. Annu. Rev. Fish Dis. 1993;3:127–144.10.1016/0959-8030(93)90032-7
- Bertolini JM, Wakabayashi H, Watral VG, Whipple MJ, Rohovec JS. Electrophoretic detection of proteases from selected strains of flexibacter psychrophilus and assessment of their variability. J. Aquat. Anim. Health. 1994;6:224–233.10.1577/1548-8667(1994)006<0224:EDOPFS>2.3.CO;2
- Ostland VE, Byrne PJ, Hoover G, Ferguson HW. Necrotic myositis of rainbow trout, Oncorhynchus mykiss (Walbaum): proteolytic characteristics of a crude extracellular preparation from Flavobacterium psychrophilum. J. Fish Dis. 2000;23:329–336.10.1046/j.1365-2761.2000.00251.x
- Secades P, Alvarez B, Guijarro JA. Purification and characterization of a psychrophilic, calcium-induced, growth-phase-dependent metalloprotease from the fish pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 2001;67:2436–2444.10.1128/AEM.67.6.2436-2444.2001
- Secades P, Alvarez B, Guijarro JA. Purification and properties of a new psychrophilic metalloprotease (Fpp2) in the fish pathogen Flavobacterium psychrophilum. FEMS Microbiol. Lett. 2003;226:273–279.10.1016/S0378-1097(03)00599-8
- Sudheesh PS, LaFrentz BR, Call DR, et al. Identification of potential vaccine target antigens by immunoproteomic analysis of a virulent and a non-virulent strain of the fish pathogen Flavobacterium psychrophilum. Dis. Aquat. Organ. 2007;74:37–47.10.3354/dao074037
- Dalsgaard I, Madsen L. Bacterial pathogens in rainbow trout, Oncorhynchus mykiss (Walbaum), reared at Danish freshwater farms. J. Fish Dis. 2000;23:199–209.10.1046/j.1365-2761.2000.00242.x
- MacLean LL, Vinogradov E, Crump EM, Perry MB, Kay WW. The structure of the lipopolysaccharide O-antigen produced by Flavobacterium psychrophilum. Eur. J. Biochem. 2001;268:2710–2716.10.1046/j.1432-1327.2001.02163.x
- Alvarez B, Secades P, Prieto M, McBride MJ, Guijarro JA. A mutation in Flavobacterium psychrophilum tlpB inhibits gliding motility and induces biofilm formation. Appl. Environ. Microbiol. 2006;72:4044–4053.10.1128/AEM.00128-06
- LaFrentz BR, Lindstrom NM, LaPatra SE, Call DR, Cain KD. Electrophoretic and Western blot analyses of the lipopolysaccharide and glycocalyx of Flavobacterium psychrophilum. Fish Shellfish Immunol. 2007;23:770–780.10.1016/j.fsi.2007.02.005
- Hesami S, Metcalf DS, Lumsden JS, MacInnes JI. Identification of cold-temperature-regulated genes in Flavobacterium psychrophilum. Appl. Environ. Microbiol. 2011;77:1593–1600.10.1128/AEM.01717-10
- Duchaud E, Boussaha M, Loux V, et al. Complete genome sequence of the fish pathogen Flavobacterium psychrophilum. Nat. Biotechnol. 2007;25:763–769.10.1038/nbt1313
- Kondo M, Kawai K, Yagyu K, Nakayama K, Kurohara K, Oshima S. Changes in the cell structure of Flavobacterium psychrophilum with length of culture. Microbiol. Immunol. 2001;45:813–818.10.1111/mim.2001.45.issue-12
- Aoki M, Kondo M, Nakatsuka Y, Kawai K, Oshima S. Stationary phase culture supernatant containing membrane vesicles induced immunity to rainbow trout Oncorhynchus mykiss fry syndrome. Vaccine. 2007;25:561–569.10.1016/j.vaccine.2006.07.047
- Nagai Y, Hori H, Hattori S, et al. A micro-assay method for collagenase activity and its application in the study of collagen metabolism in pathological tissues. Japn. J. Inflammation. 1984;4:121–130.
- Teramura N, Tanaka K, Iijima K, et al. Cloning of a novel collagenase gene from the gram-negative bacterium Grimontia (Vibrio) hollisae 1706B and its efficient expression in Brevibacillus choshinensis. J. Bacteriol. 2011;193:3049–3056.10.1128/JB.01528-10
- Ohara K, Kageyama T, Kuwada T, Umino T, Furusawa S, Yoshiura Y. Quantitative estimation of Flavobacterium psychrophilum infected ayu Plecoglossus altivelis altivelis by real-time PCR. Jap. Soc. Fish. Sci. 2009;75:258–260. Japanese.
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic. Acids Res. 1986;14:4683–4690.10.1093/nar/14.11.4683
- Boni IV, lsaeva DM, Musychenko ML, Tzareva NV. Ribosome-messenger recognition: mRNA target sites for ribosomal protein S1. Nucleic Acids Res. 1991;19:155–162.10.1093/nar/19.1.155
- Rhodes RG, Samarasam MN, Shrivastava A, et al. Flavobacterium johnsoniae gldN and gldO are partially redundant genes required for gliding motility and surface localization of sprb. J. Bacteriol. 2010;192:1201–1211.10.1128/JB.01495-09
- Rhodes RG, Samarasam MN, Van Groll EJ, McBride MJ. Mutations in Flavobacterium johnsoniae sprE result in defects in gliding motility and protein secretion. J. Bacteriol. 2011;193:5322–5327.10.1128/JB.05480-11
- Sato K, Naito M, Yukitake H, et al. A protein secretion system linked to bacteroidete gliding motility and pathogenesis. Proc. Natl. Acad. Sci. USA. 2010;107:276–281.10.1073/pnas.0912010107
- Shrivastava A, Rhodes RG, Pochiraju S, Nakane D, McBride MJ. Flavobacterium johnsoniae RemA is a mobile cell surface lectin involved in gliding. J. Bacteriol. 2012;194:3678–3688.10.1128/JB.00588-12
- McBride MJ, Zhu Y. Gliding motility and Por secretion system genes are widespread among members of the phylum bacteroidetes. J. Bacteriol. 2013;195:70–78.
- Sasagawa Y, Kamio Y, Matsubara Y, et al. Purification and properties of collagenase from Cytophaga sp. L43-1 strain. Biosci. Biotechnol. Biochem. 1993;57:1894–1898.10.1271/bbb.57.1894
- Sasagawa Y, Izaki K, Matsubara Y, Suzuki K, Kojima H, Kamio Y. Molecular cloning and sequence analysis of the gene encoding the collagenase from Cytophaga sp. L43-1 strain. Biosci. Biotechnol. Biochem. 1995;59:2068–2073.10.1271/bbb.59.2068
- Touchon M, Barbier P, Bernardet JF, et al. Complete genome sequence of the fish pathogen Flavobacterium branchiophilum. Appl. Environ. Microbiol. 2011;77:7656–7662.10.1128/AEM.05625-11
- Obana N, Nomura N, Nakamura K. Structural requirement in Clostridium perfringens Collagenase mRNA 5′ leader sequence for translational induction through small RNA-mRNA base pairing. J. Bacteriol. 2013;195:2937–2946.10.1128/JB.00148-13
- Sukhodolets MV, Garges S, Adhya S. Ribosomal protein S1 promotes transcriptional cycling. RNA. 2006;12:1505–1513.10.1261/rna.2321606
- Chen WH, Sun LT, Tsai CL, Song YL, Chang CF. Cold-stress induced the modulation of catecholamines, cortisol, immunoglobulin M, and leukocyte phagocytosis in Tilapia. Gen. Comp. Endocrinol. 2002;126:90–100.10.1006/gcen.2001.7772
- Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948.10.1093/bioinformatics/btm404