Abstract
Collagen hydrolysate (CH) was orally administered to UVB-irradiated hairless mice at doses of 20, 200–2000 mg/kg BW/day. The low dose of CH increased the skin hydration and reduced the transepidermal water loss on damaged skin. These results suggested the optimal dose of collagen to improve the UV-damaged skin condition.
Photoaging is a major cause of skin aging. Irradiation of UV results in aged skin with a decrease in moisture content and viscoelasticity. Oral supplementation with some forms of polyphenol has been shown to improve the skin moisture content of UVB-irradiated hairless mice.Citation1) Collagen hydrolysate (CH) also improves the skin moisture contentCitation2–5) and skin elasticity.Citation6) In these experiments, the dosage of CH was 50–2000 mg/kg body weight (BW). There is no study on dosage of CH on UVB-irradiated hairless mice, except the low dose (50 mg/kg BW) and high dose (200 mg/kg BW). In this paper, we determine the most suitable dose of dietary CH providing pharmacological effects on hairless mouse skin after repeated UVB irradiation.
All animal experiments were approved by the Ethics Committee of Tokyo University of Agriculture and Technology. Six-week-old male Hos: HR-1 hairless mice were purchased from SLC Japan. The mice were first housed in collective cages at 20 ± 2 °C on a 12-h light/12-h dark cycle with free access to water and Labo MR Stock diet (Nosan Corp., Japan) throughout the experimental period. After 5 days of acclimation, mice were divided into five groups (seven mice per group) such that the BW and hydration of the stratum corneum did not differ significantly between UV( − ) control, UV(+) control, UV(+) 20, UV(+) 200, and UV(+) 2000 groups. CH (from Jellice Corp., Japan, Mw = 3000) was dissolved in water (2, 20, and 200 mg/ml) and administered orally at 20, 200, and 2000 mg/kg BW daily, respectively.
Mice were then housed in individual stainless steel cages (5 × 9 × 4 cm) and subjected to UVB irradiation (0.3 mW/cm2) emitted by a UV lamp (GL20SE; Sanyo Denki, Japan). UVB energy was measured with a digital ultraviolet meter (AS ONE, Japan). UV(+) groups were exposed to UVB irradiation three times per week for 1 min each in the first week. Exposure time was then increased to 3 × 2 min per week, 3 × 3 min and 2 × 4 min in the following weeks, and was finally maintained at 7 × 3 min so that the skin did not become red. Test samples were taken daily.
Mice were maintained at 20 ± 2 °C and 50 ± 5% humidity for 2 h before measuring skin hydration. Hydration of the stratum corneum of the lumbar skin was measured once a week with a Corneometer CM 825 (Courage+Khazaka Electronic GMBH, Germany). Transepidermal water loss (TEWL) of UVB-irradiated hairless mouse skin was measured using a Tewameter TM300 (Courage+Khazaka Electronic GMBH, Germany).
Hyaluronic acid (HA) from UVB-irradiated hairless mouse skin was prepared following the protocol given in a previous paper.Citation7) The skin samples were treated with 0.5 M sodium hydroxide at 4 °C overnight, and then neutralized with 1 M hydrochloric acid. The samples were digested with Actinase E (Kaken Pharmaceutical, Japan) at 50 °C for 48 h. The distribution of HA was analyzed using the Lee and CowmanCitation8) method. HA was electrophoresed with 1% agarose gel at 200 mA, 50 V for 4 h. Agarose gels were stained with Stains-All (Sigma, USA), and washed with water. HA standards were supplied from Kewpie Co.
Metabolome analysis was carried out by Human Metabolome Technology Inc., Tsuruoka, Japan. Capillary electrophoresis–time-of-flight mass spectrometry (CE–TOFMS) was carried out using an Agilent CE system equipped with an Agilent 6210 TOFMS (Agilent Technologies, Waldbronn, Germany). Approximately 50 mg of frozen skin was used. The metabolites were analyzed as described in a previous paper.Citation9–11) Hierarchical cluster analysis and principal component analysis were performed by proprietary software, PeakStat and SampleStat, respectively. Detected metabolites were plotted on metabolic pathway maps using VANTED (Visualization and Analysis of Networks containing Experimental Data) software. All data of amino acid composition were shown relative to a value of 100% for the amino acid composition of UV(+) control. Data are shown as mean ± standard error.
All data are presented as mean ± standard error. Differences in the mean for each group were analyzed by a one-way ANOVA, with post hoc analyses being carried out using the Tukey–Kramer multiple comparisons test with equal dispersion (Statcel 3, Japan).
Throughout the experimental period, BW did not differ significantly between UV(−) control, UV(+) control, UV(+) CH20, UV(+) CH200, and UV(+) 2000 groups (data not shown).
The epidermis of hairless mice increased in thickness following UVB irradiation (data not shown). The epidermal thickness in the UV(+) control , UV(+) CH20, UV(+) CH200, and UV(+) CH2000 groups was found to be about 34.5 ± 2.8, 34.7 ± 2.7, 31.1 ± 1.5, and 33.2 ± 2.2 μm, respectively. The administration of CH did not affect the thickness of epidermis with UVB irradiation.
After 7 weeks, the water content of the stratum corneum in the UV(+) control group was significantly lower than that in the UV(−) control group (Fig. (A)). The water contents of the UV(+) CH groups were significantly higher than those of the UV(+) control group, (UV(+) CH groups vs. UV(+) control, p < 0.01, Tukey–Kramer’s test). These results suggest that the administration of CH counteracts the decrease in stratum corneum hydration induced by repeated UVB irradiation without the addition of CH. These data were very similar to our previous paper.Citation2)
Fig. 1. Water content and TEWL of dorsal skin of hairless mouse after repeated UVB irradiation and administration of collagen hydrolysate (CH).
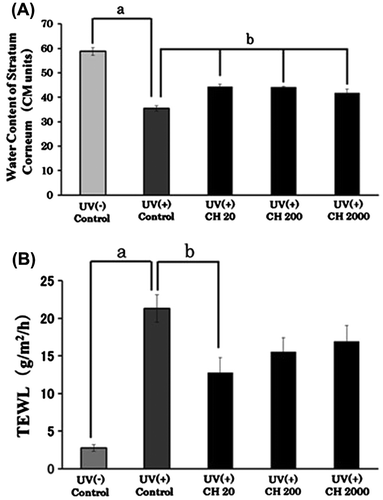
TEWL of dorsal skin of UVB-irradiated hairless mice after the administration of CH is shown in Fig. (B). TEWL increased with UVB irradiation of the skin. It is worth noting that TEWL of UV(+) CH20 compared to the UV(+) control decreased significantly in statistical terms (p < 0.01, Tukey–Kramer’s test). The administration of CH decreased TEWL, the value for UV(+) CH20 being the lowest. There was no statistical significant difference between the value for UV(+) CH groups. It is considered that this optimum dose of 20 mg/kg BW of CH had alleviated UV-induced skin inflammation. Oba et al. showed that the administration of the dosage of CH at about 2000–3300 mg/kg BW (2% CH containing diet) improves the loss of epidermal barrier function and skin elasticity induced by repeated UVB irradiation in hairless mice.Citation6) Our result suggested the possibility that the administration of low dose of CH improved the skin damage on UVB-irradiated hairless mice. Furthermore, it is necessary to try the examination at the low dose.
The electrophoretic pattern of HA from dorsal skin is shown in Fig. . The molecular weight (Mw) distribution was similar to that of HA standard of Mw 800 k. Mw distribution of HA in the dorsal skin of the UV(+) control group was extended to the small values compared to that of the UV(−) control group. The lowest Mw points of UV(−) control and UV(+) control were 800 and 500 k, respectively. When compared to the Mw distribution of HA in the UV(+) control, that in the UV(+) CH groups was small. The administration of CH inhibited the production of low Mw of HA produced without CH. That tendency became strong at higher dosage of CH.
Fig. 2. Agarose gel electrophoretic pattern of hyaluronic acid (HA) from hairless mouse dorsal skin after repeated UV irradiation.
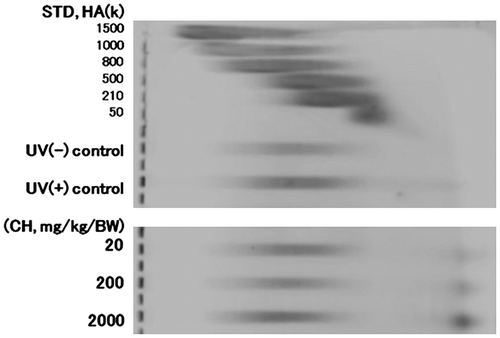
Table shows the difference of free amino acid on skin from UVB-irradiated hairless mice following the administration of CH at different dosages. Values for 4-Hyp and 5-Hyp of UV(+) CH vs. UV(+) control increased in a dose-dependent manner. 4-Hyp is the main amino acid of collagen and 5-Hyp levels were low. Data for 5-Hyp showed a relative increase. This suggests that Hyp absorbed from CH was detected in the skin. Iwai et al. showed that Pro-Hyp was detected in blood after the administration of CH.Citation12) Pro-Hyp was detected in skin and cartilage after 30 min of CH administration.Citation13) The absorbed peptide and amino acid from CH was transferred to skin, and affected the synthesis and degradation of the extracellular matrix. About 55 metabolites with statistical significance were detected, but it was difficult to understand the relationship between administrated CH and that metabolites.
Table 1. Amino acid variations in the skin of UVB-irradiated hairless mice by collagen hydrolysate treatment at different dosages.
The administration of CH improves skin moisture contents and the loss of epidermal barrier on UVB-irradiated hairless mice. The Mw of HA in UV(+) control mouse back skin was distributed more in the lower Mw compared to that of UV(−) control mouse back skin. However, the administration of CH decreased the HA of low Mw. These results suggest that dietary CH improves the skin damage of UVB-induced aging by inhibiting the digestion of HA. In this experiment, the high dose of CH (2000 mg/kg BW) showed improvement in skin damage on UVB-irradiated hairless mice, and the low dose of CH (20 mg/kg BW) showed the highest efficacy. It is considered that the overdosage of CH put a burden on kidney. That dosage is equivalent to 1.2 g of human weight 60 kg. In human study, the dosage of CH was 5 or 10 g per day.Citation14–17) It is possible to take collagen by a normal diet in the case of humans. It is considered the most suitable dose to produce the pharmacological effects of dietary CH on human skin following repeated UVB irradiation.
Author contributions
Analysis and interpretation of data was done by N. Jinbo and C. Kawada. Y. Nomura carried out the critical revision of the article for important intellectual content.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Mukherjee PK, Maity N, Nema NK, et al. Bioactive compounds from natural resources against skin aging. Phytomedicine. 2011;19:64–73.
- Tanaka M, Koyama Y, Nomura Y. Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci. Biotechnol. Biochem. 2009;73:930–932.
- Zhuang Y, Hou H, Zhao X, et al. Effects of collagen and collagen hydrolysate from jellyfish (Rhopilema esculentum) on mice skin photoaging induced by UV irradiation. J. Food Sci. 2009;74:H183–H188.
- Pyun HB, Kim M, Park J, et al. Effect of collagen tripeptide supplement on photoaging and epidermal skin barrier in UVB-exposed hairless mice. Prev. Nutr. Food Sci. 2012;17:245–253.
- Fan J, Zhuang Y, Li B. Effects of collagen and collagen hydrolysate from jellyfish umbrella on histological and immunity changes of mice photoaging. Nutrients. 2013;5:223–233.
- Oba C, Ohara H, Morifuji M, et al. Collagen hydrolysate intake improves the loss of epidermal barrier function and skin elasticity induced by UVB irradiation in hairless mice. Photodermatol. Photoimmunol. Photomed. 2013;29:204–211.
- Nomura Y, Takahashi K, Shirai K, et al. Features of collagen matrix reconstructed with proteodermatan sulfate from pigskin insoluble collagen. Agric. Biol. Chem. 1989;53:939–948.
- Lee HG, Cowman MK. An agarose gel electrophoretic method for analysis of hyaluronan molecular weight distribution. Anal. Biochem. 1994;219:278–287.
- Soga T, Heiger DN. Amino acid analysis by capillary electrophoresis electrospray ionization mass spectrometry. Anal. Chem. 2000;72:1236–1241.
- Soga T, Ueno Y, Naraoka H, et al. Simultaneous determination of anionic intermediates for Bacillus subtilis metabolic pathways by capillary electrophoresis electrospray ionization mass spectrometry. Anal. Chem. 2002;74:2233–2239.
- Soga T, Ohashi Y, Ueno Y, et al. Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J. Proteome Res. 2003;2:488–494.
- Iwai K, Hasegawa T, Taguchi Y, et al. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem. 2005;53:6531–6536.
- Kawaguchi T, Nanbu PN, Kurokawa M. Distribution of prolylhydroxyproline and its metabolites after oral administration in rats. Biol. Pharm. Bull. 2012;35:422–427.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology. Skin Pharmacol. Physiol. 2013;27:47–55.
- Proksch E, Schunck M, Zague V, et al. Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin Pharmacol. Physiol. 2014;27:113–119.
- Koyama Y, Kuwaba K, Kondo S, et al. Supplemental ingestion of collagen peptide suppresses ultraviolet-induced erythema. Jpn. Pharmacol. Ther. 2014;42:781–790.
- Kuwaba K, Koyama Y, Koikeda T, et al. Effect of collagen peptide ingestion on skin properties. Jpn. Pharmacol. Ther. 2014;42:995–1004.
