Abstract
Tomato (Solanum lycopersicum) is rich in anthocyanins, which are polyphenolic pigments. This study aimed to analyze and characterize the anthocyanin composition in cultivated blue tomato in Japan. The extracts of peel, seed, and pulp of tomatoes were purified following which anthocyanins and lycopene contents were analyzed using high-performance liquid chromatography and electrospray ionization mass spectrometry. Eleven types of anthocyanins were identified, including delphinidin, petunidin, and malvidin. Further, the antioxidant activity of anthocyanins was evaluated using 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt radical quenching assays and electron spin resonance. “Blue tomato” extracts exert antioxidant activity. Thus, we showed that petunidin was present in the “blue tomato” peel while lycopene was present in the peel and pulp. Additionally, the blue tomato peel extract was found to significantly inhibit H2O2-induced cell death in vitro. This is the first study on cell protective effects of Japanese blue tomato extract and petunidin in murine photoreceptor cells.
Graphical abstract
This is the first study that shows qualitative and quantitative chemical characterization of the individual components responsible for the bioactivity of blue tomato extracts.
Tomato (Solanum lycopersicum) is a highly useful vegetable that helps preventing arteriosclerosis, diabetes, and cancer because it is rich in carotenoids, such as lycopene and β-carotene.Citation1–3) Experimental evidences show that carotenoids possess antioxidative effects, promote blood flow, and inhibit LDL cholesterol oxidation.Citation4–6) Some of the related wild species of tomatoes contain high levels of anthocyanins in the fruit peel for protection against the ultraviolet rays present in sunlight. Anthocyanins are naturally occurring pigments that impart red, blue, and purple colors to plants. They are found in the peel of most fruits and vegetables, such as grape, apple, and eggplant. Anthocyanins are polyphenols that have high antioxidative properties and have long been used as health foods and dietary supplements for various health benefits. For example, blueberry and bilberry, which are one of the richest sources of anthocyanins, are used for improving eye health. We have previously reported that bilberry extract promotes visual acuity through protection of the retinal ganglion cell, prevents photoreceptor cell damage caused by oxidative stress, and inhibits angiogenesis in mammalian cell.Citation7–11)
The anthocyanin in plants is synthesized when exposed to the ultraviolet rays present in sunlight. The fruits and vegetables accumulate large amount of anthocyanins in order to protect themselves from damage caused by ultraviolet light. It has been observed that a large amount of ultraviolet light exposure increases the synthesis of anthocyanin in plants.
In this study, we performed both quantitative and qualitative analysis of the anthocyanin compounds present in Japanese blue tomato by using high-performance liquid chromatography with diode-array detection (HPLC–DAD) and high-performance liquid chromatography/electrospray ionization tandem mass spectrometry (HPLC–ESI–MS/MS). In this study, we use a cultivar of “Indigo Rose” and call it “blue tomato.” This cultivar was bred from purple tomatoes and shown blue skin. In addition, antioxidant activities of anthocyanins were studied by electron spin resonance (ESR). Protective effects of the blue tomato extract and its constituents against oxidative stress were investigated using a photoreceptor cell line. This is the first report that functional effect of blue tomatoes anthocyanin.
Materials and methods
Chemicals
For this work, standard anthocyanins, delphinidin-3-glucoside, petunidin-3-glucoside, and malvidin-3-glucoside, the standard carotenoids, lycopene, and β-carotene were purchased from Extrasynthese (Genay, France). The standard anthocyanidin, petunidin, was purchased from Tokiwa Phytochemical Co., Ltd. (Chiba, Japan). Trifluoroacetic acid (TFA), triethylamine (TEA), hypoxanthine, xanthine oxidase, H2O2, and FeSO4 were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). ABTS [2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt] and 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) were purchased from Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan). 2,2-Diphenyl-1-picryl-hydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and N-acetyl-l-cysteine (NAC) were purchased from Sigma-Aldrich (St Louis, MO, USA). Hoechst 33342, propidium iodide (PI),was purchased from Life Technologies (Carlsbad, USA). The 661W cells, a mouse photoreceptor-derived cell line, were obtained as a kind gift from Dr. Muayyad R. Al-Ubaidi of the University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA.
Blue tomato
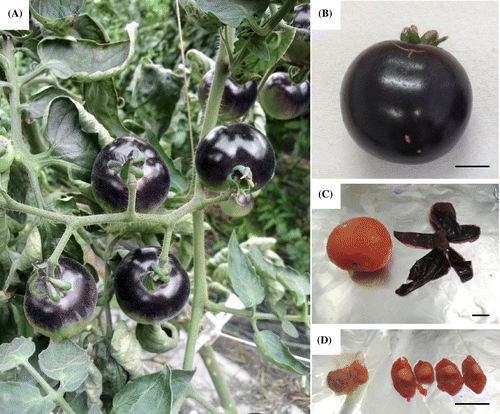
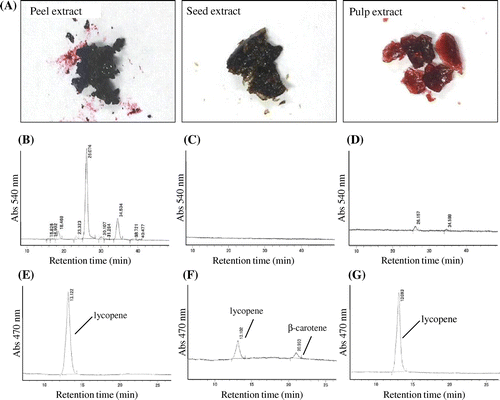
The fresh blue tomatoes were kindly donated from British Seed Ltd. (Gifu, Japan). Blue tomatoes, used in this study, are a cultivar of “Indigo Rose” that has been cultivated in Ogaki, Gifu in Japan. These tomatoes cultivated in the greenhouse from May 2013, and it has been harvested after about 80 days. The blue tomatoes were cultured with irradiation of sunlight, which contains ultraviolet rays, from both above and below using a reflection plate placed on the soil in the greenhouse (Fig. (A)). In this study, the tomato samples were harvested from different plants and 20 fruits were used for analysis. As a result, the entire surface of the tomato peel turned blue probably because of the synthesis of anthocyanins (Fig. (B)).
Fig. 1. The blue tomato used in this study.
Extraction of anthocyanins and carotenoids
The peel, seed, and pulp were separated from the whole blue tomatoes (Fig. (C) and (D)) and frozen immediately in liquid nitrogen. The frozen peel, seed, and pulp were then crushed using Wonder Blender (Osaka Chemical Co., Ltd., Osaka, Japan). Subsequently, each blue tomato piece was added to a solution of 70% ethanol containing 0.5% TFA about 3 times the amount of the piece and then mixed for 30 min to extract anthocyanins.Citation12) The supernatant was recovered by centrifugation at 2600 × g for 10 min. After extracting twice, the precipitates were added to hexane/acetone/ethanol (50:25:25 = v:v:v) solution and mixed to extract the carotenoids, including lycopene and β-carotene.Citation13) The supernatant was recovered by centrifugation at 2600 × g for 10 min. After extracting twice, the blends of ethanol and hexane/acetone/ethanol solution were gathered and dried by evaporation and freeze drying. The extracts were kept at a temperature of 4 °C away from light.
Analysis of anthocyanins by HPLC-DAD
The peel, seed, and pulp extracts of blue tomatoes were first dissolved in 50% ethanol containing 0.5% TFA, and then filtered using a membrane filter (Nacalai Tesque, Inc., Kyoto, Japan). Quantitative determination of anthocyanins in the blue tomatoes was performed by HPLC (Shimadzu, Kyoto, Japan) equipped with a C18 column (Develosil ODS-UG HPLC-5, 4.6 mm i.d. × 250 mm, Nomura Chemical Co., Ltd., Aichi, Japan), according to a modified method reported by Ogawa et al.Citation12) The mobile phase was composed of 0.5% TFA aqueous v/v (A) and acetonitrile containing 0.1% TFA (B). The gradient was as follows: 0 min, 92% A, 8% B; 50 min, 85% A, 15% B; 60 min, 70% A, 30% B; 65 min, 40% A, 60% B; 75 min, 40% A, 60% B. The flow rate was set at 1 mL/min. The injection volume for the extract was 10 µL. The wavelength for detection of anthocyanin was 540 nm, and the column was kept at 25 °C.
Analysis of carotenoids by HPLC-DAD
The extracts of blue tomatoes were dissolved in methanol/acetonitrile (90:10 v/v) solution, and then they were filtered through a membrane filter (Nacalai Tesque, Inc., Kyoto, Japan). Quantitative determination of lycopene and β-carotene in the blue tomatoes was done by HPLC (Shimadzu, Kyoto, Japan) equipped with a C18 column (Develosil ODS-UG-5, 4.6 mm i.d. × 250 mm, Nomura Chemical Co., Ltd., Aichi, Japan) according to the modified method reported by Olives et al.Citation13) The mobile phase was composed of methanol/acetonitrile (90:10 v/v) containing 9 μM of TEA. The flow rate was set at 0.9 mL/min. The injection volume was 10 μL. The wavelength for detection of carotenoids was 470 nm and the column was kept at 30 °C.
Analysis of anthocyanins by HPLC-ESI-MS/MS
The qualitative determination of anthocyanin in each extract of blue tomatoes was analyzed by HPLC-ESI-MS/MS system according to the modified method reported by Ogawa et al.Citation12) A portion of each of the filtrate extract solution was subjected to HPLC on a CAPCELL PAK ACR (2.0 mm i.d. × 250 mm, S-5, 5 μm, Shiseido Co., Ltd., Tokyo, Japan). The columns were maintained at 40 °C. The mobile phase was composed of 2% formic acid aqueous v/v (A) and acetonitrile containing 2% formic acid (B). The gradient was as follows: 0 min, 93% A, 7% B; 40 min, 75% A, 25% B; 55 min, 3% A, 97% B; 65 min, 3% A, 97% B; 66 min, 93% A, 7% B; 75 min, 93% A, 7% B. The flow rate was set at 0.2 mL/min. The injection volume for the extract was 5 μL. The wavelength for detection of flavonoids including anthocyanin was 280 nm, and the column was kept at 40 °C. The extracts were then introduced into the ion-trap electrospray mass spectrometer equipped with ESI (4000 QTRAP®, AB SCIEX, Massachusetts, USA). The mass spectrometer was operated in the positive ion mode under the following conditions: spray voltage, 3000 V; source temperature, 600 °C; curtain gas, 20 psi. The MS/MS acquisition method was an enhanced mass scan [declustering potential (DP), 60 V; collision energy (CE), 10 V; lit fill time, 1 ms] and an enhanced product ion (DP, 60 V; CE, 30 and 50 V; lit fill time, 5 ms).
ABTS radical scavenging activity
The ABTS radical scavenging activity of the blue tomato extracts was determined using the method of Re et al.Citation14) with minor modifications. A volume of 200 μL of ABTS+ solution (absorbance 0.800–0.900) was mixed with 50 μL sample solution comprising blue tomato extract, standard anthocyanidin, anthocyanin, lycopene, and Trolox. An alteration in absorbance was measured at a wavelength of 734 nm after keeping the samples for 5 min at room temperature. ABTS∙+ scavenging value was determined using a standard of Trolox (0–100 μg/mL) and the results were expressed as mg Trolox equivalent per 100 mg extracts (Trolox mg/100 mg) or mg Trolox equivalent per 100 μmol standard anthocyanin (Trolox mg/100 μmol).
ESR measurement
The measurement conditions for ESR (JES-FA 200, JEOL, Tokyo, Japan) were as follows: center field 330 mT, sweep width 1.5 × 10 mT, sweep time 2 min, field modulation width 2 × 0.1 mT, amplitude 4.0 × 100, time constant 0.3 s, microwave power 2.0 mW (DPPH radical). DPPH radical scavenging activities were calculated from the integration values of the ESR spectra corrected with a Mn2+ marker. The conditions were determined by following the procedure of Tanaka et al.Citation15)
Cell culture
Murine photoreceptor cells (661W) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin under a humidified atmosphere of 5% CO2 at 37 °C. Cell passage was ensured by trypsinization every 2 or 3 days.
H2O2-induced cell death assay
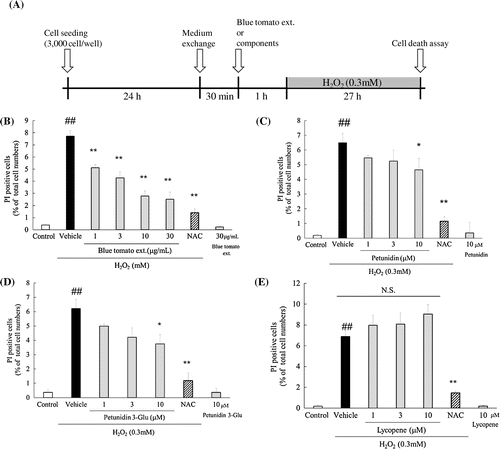
661W (3 × 103 cell/100 μL) were seeded onto a 96-well plate and cultured at 37 °C for 24 h in a humidified atmosphere of 5% CO2. The medium was exchanged for 1% FBS-DMEM. After 30 min, blue tomato extract, petunidin, petunidin-3-glucoside, lycopene, and NAC (1 mM) were added and the cells were incubated for 1 h. H2O2 (0.3 mM) was then added and cells were further cultured for 27 h at 37 °C. After the end of the incubation period, Hoechst 33342 (excitation, 360 nm; emission, 490 nm) and PI were added to the culture medium at final concentrations of 8.1 and 1.5 μM, respectively, and then incubated for another 15 min. Microscopic images through fluorescence filters for Hoechst 33342 (U-MWU, Olympus Co., Tokyo, Japan) and PI (U-MWIG, Olympus) were acquired using a charge-coupled device camera (DP30BW, Olympus). Finally, the number of dead cells was counted from the total number of cells.
Statistical analysis
Data are presented as means ± SEM. Statistical comparisons were made using a one-way analysis of variance followed by Student’s t-test or Dunnett’s multiple-comparison test. A value of p < 0.05 was considered to indicate statistical significance.
Results
Calculation of anthocyanin and carotenoids in the blue tomatoes
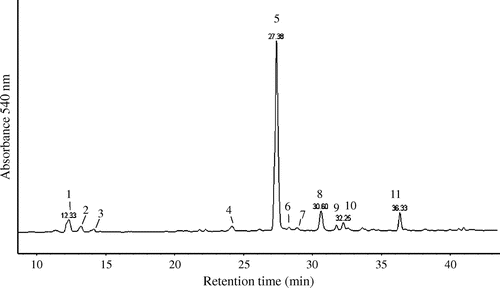
We first separated peel, seed, and pulp from the whole blue tomatoes for separate extraction of anthocyanin and carotenoid. The contents of water, extract, anthocyanin, lycopene, and β-carotene in the blue tomato, used in this study, are summarized in Table . The peel, seed, and pulp were dried and the water content was calculated from the difference in weight. Each freeze-dried solid extract were further extracted with 70% ethanol containing 0.5% TFA to obtain the anthocyanins fraction and with hexane/acetone/ethanol (50:25:25 = v:v:v) to obtain the lycopene and β-carotene fractions (Fig. (A)). The results of HPLC analysis of anthocyanins from the peel, seed, and pulp extracts are shown in Fig. . It was observed that the peel extract contained about ten anthocyanins and lycopene (Fig. (B) and (E)), the seed extract contained both lycopene and β-carotene but no anthocyanins were not detected (Fig. (C) and (F)), and the pulp extract contained two anthocyanins and lycopene (Fig. (D) and (G)). The contents of anthocyanins, lycopene, and β-carotene in each extract were calculated from HPLC analysis using standards, namely cyanidin-3-glucoside, lycopene, and β-carotene. The results revealed that the peel extract contained a large amount of anthocyanins whereas the seed and pulp extracts contained either none or a small amount of anthocyanin (Table ).
Table 1. Blue tomato used in this study and contents of anthocyanin, lycopene, and β-carotene in blue tomato.
Fig. 2. Results of quantitative analyses for anthocyanins, lycopene, and β-carotene in blue tomato.
Identification of anthocyanins in the blue tomatoes
The identification of the peaks was based on HPLC-ESI-MS/MS analysis, and the identity of the anthocyanins was revealed by comparing it with the published data of the previous study because no standard was available.Citation16) The anthocyanins were deduced in the blue tomato peel are summarized in Table . In this study, we successfully predicted anthocyanidins and binding components in the blue tomatoes; however, the structures of anthocyanins could not be determined. Previous reports suggest that the blue tomato contains approximately 11 to over 23 different kinds of anthocyanins, including 3 anthocyanidins (delphinidin, petunidin, and malvidin) binding monomeric or multiple glucose, rutinose, p-coumaric acid, and caffeic acid.Citation16,17) Further, we identified eleven anthocyanins, specifically five delphinidin, four petunidin, and four malvidin-derived anthocyanins from the blue tomato peel by HPLC-ESI-MS/MS analysis (Fig. and Table ). The petunidin-derived anthocyanins, such as petunidin + p-coumaroyl + rutinoside + glycoside (peak 5), were mostly found in the blue tomato peel.
Table 2. Identified anthocyanins in blue tomato peel.
Measurements of antioxidant activity in extracts, anthocyanin, and lycopene
The radical scavenging capacity of the blue tomato extracts and their some components were determined by measuring the ABTS cation radical scavenging activities along with the reducing power activities of each extract in vitro. The ABTS radical scavenging activity is shown in Table . The results were further evaluated by Trolox equivalents antioxidant capacity (TEAC). The blue tomato extracts showed ABTS radical scavenging activity, because of the presence of antioxidants such as petunidin. Highest activity was observed in peel followed by seed and pulp.
Table 3. Antioxidant potency of blue tomato extracts and constituents.
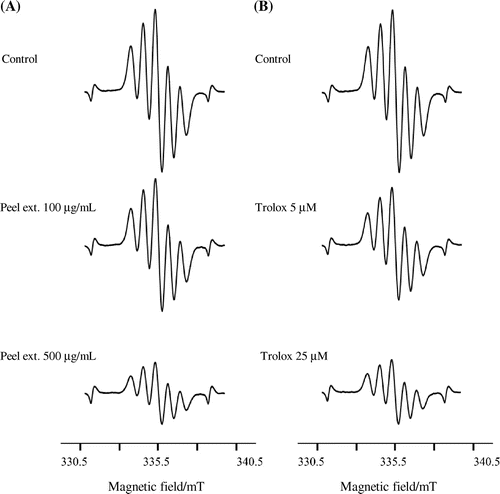
Effects of the blue tomato extract on DPPH radical scavenging activities by ESR
Three different kinds of blue tomato extracts were analyzed for DPPH radical scavenging activity using ESR spectroscopy. The IC50 values of radical scavenging activities in blue tomato peel, pulp, and seed extracts as well as Trolox against DPPH radical are presented in Table and Fig. . We observed that the extracts of peel, pulp, and seed of the blue tomato and Trolox, a typical antioxidant, exhibited scavenging activity against DPPH radical and their IC50 values were 536, 858, 931 μg/mL, and 23 μM, respectively.
Table 4. DPPH radical scavenging activity of blue tomato extracts.
Effect of the blue tomato peel extract, petunidin, petunidin-3-glucoside, and lycopene on H2O2-induced cell death in murine photoreceptor-derived cells (661W)
The protective effects of the blue tomato peel extract and its some components against H2O2-induced death in the 661W cells were investigated. We counted the number of H2O2-induced dead 661W cells exhibiting PI fluorescence and expressed the positive cells as the percentage of PI-positive cells to total cell nuclei, exhibiting Hoechst 33342 fluorescence. As shown in Fig. (A), the blue tomato peel extract significantly reduced the number of apoptotic cells, which was detected by PI labeling in a concentration-dependent manner (1–30 μg/mL). Likewise, petunidin at 10 μM and petunidin-3-glucoside at 10 μM also significantly decreased cell apoptosis (Fig. (B) and (C)). However, lycopene showed no effect on cell death (Fig. (D)).
Fig. 5. Effects of blue tomato peel extract and its components on H2O2-induced cell death in 661W cells.
Discussion
In this study, to our knowledge for the first time, we have analyzed the presence and quantity of anthocyanins in blue tomatoes grown in Japan. In particular, we determined the amounts of anthocyanins and carotenoids, such as lycopene and β-carotene, in the prepared peel, seed, and pulp extracts of the blue tomatoes and found that higher amount of the antioxidants were present in the peel than in the seed and pulp. The anthocyanin and carotenoid contents were analyzed by HPLC, and the results are shown in Table . In a previous study, the amount of anthocyanins in blue tomato peel ranged from 20.6 to 66.5 mg/100 g fresh fruit.Citation17) In another report, the amount of anthocyanins in the peel ranged from 7.79 to 110.79 mg/100 g fresh fruit.Citation16) These results suggest that the blue tomato, used in this study, was found to possess anthocyanins similar to those of shown in the above previous studies. The synthesis of anthocyanins in many fruits and vegetables, which possess capability to produce such pigments, increases mostly in the peel when exposed to ultraviolet light.Citation18) The amount of anthocyanin in the blue tomato may change depending on the environmental conditions of cultivation. We observed that the peel extracts contain not only a high level of anthocyanins but also lycopene compared to that in the other extracts (Table ). Tomatoes are widely known to have several antioxidants in its fruit peel,Citation19) and our results also show that anthocyanin and lycopene are present in the blue tomatoes peel.
We found that other anthocyanins have DPPH radical scavenging activityCitation15) using ESR. Therefore, it seems that the antioxidant activity of the blue tomato peel extract is dependent on the anthocyanin content. In addition, we compared the antioxidant activity between blue tomato and other antioxidant fruits, such as berries. Having compared to the previous reports, antioxidant activity of blue tomato was almost equivalent to the antioxidant activity of strawberry (approximately 1/30–40 of Trolox) and compared to blueberry commonly known as anthocyanin rich containing food was about half.Citation12) This result is considered to have derived from the concentration of antioxidants contained in each extract, including anthocyanins. While peel contained about 170 higher concentration of anthocyanin (Table ), it showed just about 1.5 higher antioxidant activity.
Anthcoyanin might be partially linked the activity, but the contribution of other compounds contained the extracts should be considered. Treatment of the blue tomato extract containing petunidin and petunidin-3-glucoside protected 661W from damage induced by H2O2. It is the first report that cell protective effect of blue tomatoes anthocyanin. In a previous study, the mechanism of H2O2-induced 661W cell death has been reported to be an apoptosis-dependent pathway that involves production of reactive oxygen species (ROS).Citation20) Cone-cell degeneration is primarily responsible for the gradual constriction of the central visual field, which ultimately leads to blindness. Therefore, visual acuity is dependent on the rate of cone-cell degeneration. Our studies demonstrated that the blue tomato peel extract and petunidin-derived anthocyanin have potent scavenging activities against ABTS radical. These findings indicate that the mechanism by which the blue tomato extract suppresses apoptosis is related to the inhibition of ROS production. However, the IC50 values of radical scavenging activity in the blue tomato peel extract were much higher than effective concentrations in vitro. Thus, the function of the blue tomato peel extract in cell survival pathway is still unknown, and this hypothesis needs further investigation. On the other hand, lycopene had no significant effect on the cell death against H2O2-induced cell damage. There is experimental evidence that lycopene has superoxide radical and hydroxyl radical scavenging activity,Citation21) and there are several reports that lycopene exhibits a cell protective effect.Citation22,23) However, in this study, no such cell protective effect of lycopene on 661W cell viability under oxidative stress was observed. The reason could be due to a difference in cell types.
Conclusion
In conclusion, we have performed a thorough analysis of the Japanese cultured blue tomato and have predicted a total of 11 types of anthocyanins in the peel extract. We showed that the blue tomato peel extract, which specifically contains petunidin-derived anthocyanin, exert protective effects against H2O2-induced retinal photoreceptor cell damage in vitro model. We suggest that intake of the blue tomato extract may prove to be useful for a prophylactic treatment, because oxidative stress is one of the major causes of various eye diseases.
Author contributions
Emi Ooe, Kenjirou Ogawa, Tadashi Horiuchi, Masamitsu Shimazawa, and Hideaki Hara conceived and designed the experiments. Emi Ooe, Kenjirou Ogawa, Hiroyuki Tada, and Hiromi Murase performed the experiments. Emi Ooe, Kenjirou Ogawa, and Hiroyuki Tada analyzed the data. Tadashi Horiuchi and Hideaki Hara contributed reagents/material/analysis tools. Emi Ooe, Kenjirou Ogawa, Kazuhiro Tsuruma, and Hideaki Hara wrote the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
We thank Mr. Kenjiro Ishii (British Seed Ltd., Gifu, Japan) for gifting the Japanese blue tomato and Dr. Junji Tanaka for technical support.
Notes
Abbreviations: ESI, electrospray ionization; TFA, trifluoroacetic acid; TEA, triethylamine; ESR, electron spin resonance.
References
- Daniels J-A, Mulligan C, McCance D, et al. A randomised controlled trial of increasing fruit and vegetable intake and how this influences the carotenoid concentration and activities of PON-1 and LCAT in HDL from subjects with type 2 diabetes. Cardiovasc. Diabetol. 2014;13:16.10.1186/1475-2840-13-16
- Dwyer JH, Navab M, Dwyer KM, et al. Oxygenated carotenoid lutein and progression of early atherosclerosis: the Los Angeles atherosclerosis study. PLoS ONE. 2001, 19;103:2922–2927.
- Chen J, O’Donoghue A, Deng YF, et al. The effect of lycopene on the PI3K/Akt signalling pathway in prostate cancer. Anticancer Agents Med Chem. 2014;14:800–805.10.2174/1871520614666140521121317
- Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–488.10.3390/nu6020466
- Gao JX, Li Y, Zhang HY, et al. Lycopene ameliorates erectile dysfunction in streptozotocin-induced diabetic rats. Pharmazie. 2012;67:256–259.
- Gajendragadkar PR, Hubsch A, Mäki-Petäjä KM, et al. Effects of oral lycopene supplementation on vascular function in patients with cardiovascular disease and healthy volunteers: a randomised controlled trial. PLoS ONE. 2014;9:e99070.10.1371/journal.pone.0099070
- Matsunaga N, Chikaraishi Y, Shimazawa M, et al. Vaccinium myrtillus (Bilberry) extracts reduce angiogenesis in vitro and in vivo. Evid Based Complement Altern. Med. 2010;7:47–56.10.1093/ecam/nem151
- Matsunaga N, Imai S, Inokuchi Y, et al. Bilberry and its main constituents have neuroprotective effects against retinal neuronal damage in vitro and in vivo. Mol. Nutr. Food Res. 2009;53:869–877.10.1002/mnfr.v53:7
- Matsunaga N, Tsuruma K, Shimazawa M, et al. Inhibitory actions of bilberry anthocyanidins on angiogenesis. Phytother. Res. 2010;24,S1:S42–S47.10.1002/ptr.v24.1s
- Ogawa K, Tsuruma K, Tanaka J, et al. The protective effects of bilberry and lingonberry extracts against UV light-induced retinal photoreceptor cell damage in vitro. J. Agric. Food Chem. 2013;61:10345–10353.10.1021/jf402772h
- Ogawa K, Kuse Y, Tsuruma K, et al. Protective effects of bilberry and lingonberry extracts against blue light-emitting diode light-induced retinal photoreceptor cell damage in vitro. BMC Complement Altern. Med. 2014;14:120.10.1186/1472-6882-14-120
- Ogawa K, Sakakibara H, Iwata R, et al. Anthocyanin composition and antioxidant activity of the crowberry (Empetrum nigrum) and other berries. J. Agric. Food Chem. 2008;56:4457–4462.10.1021/jf800406v
- Barba AI, Hurtado M, Mata MC, et al. Application of a UV–vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006;95:328–336.10.1016/j.foodchem.2005.02.028
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237.10.1016/S0891-5849(98)00315-3
- Tanaka J, Kadekaru T, Ogawa K, et al. Maqui berry (Aristotelia chilensis) and the constituent delphinidin glycoside inhibit photoreceptor cell death induced by visible light. Food Chem. 2013;139:129–137.10.1016/j.foodchem.2013.01.036
- Peter JM, Peter B, James RM. Characterization of tomatoes expressing anthocyanin in the fruit. J. Amer. Soc. Hort. Sci. 2008;133:262–269.
- Jones CM, Mes P, Myers JR. Characterization and inheritance of the anthocyanin fruit (Aft) tomato. J. Hered. 2003;94:449–456.10.1093/jhered/esg093
- Arakawa O, Hori Y, Ogata R Relative effectiveness and interaction of ultraviolet-B, red and blue light in anthocyanin synthesis of apple fruit. Physiol. Plant. 1985;64:323–327.10.1111/ppl.1985.64.issue-3
- Ramandeep KT, Geoffrey PS. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005;38:487–497.
- Kunchithapautham K, Rohrer B. Apoptosis and autophagy in photoreceptors exposed to oxidative stress. Autophagy. 2007;3:433–441.10.4161/auto.4294
- Trevithick-Sutton CC, Foote CS, Collins M, et al. The retinal carotenoids zeaxanthin and lutein scavenge superoxide and hydroxyl radicals: a chemiluminescence and ESR study. Mol. Vis. 2006;30:1127–1135.
- Tang X, Yang X, Peng Y, et al. Protective effects of lycopene against H2O2-induced oxidative injury and apoptosis in human endothelial cells. Cardiovasc. Drugs Ther. 2009;23:439–448.10.1007/s10557-009-6206-3
- Li Y, Xue F, Xu SZ, et al. Lycopene protects bone marrow mesenchymal stem cells against ischemia-induced apoptosis in vitro. Eur. Rev. Med. Pharmacol. Sci. 2014;18:1625–1631.