Abstract
Dietary polyphenols are thought to be beneficial for human health by acting as antioxidants. Chlorogenic acid (CGA) is abundant in plant-based foods as an ester of caffeic acid and quinic acid. In this study, we investigated the effects of CGA on mitochondrial protection. Our results demonstrated that pretreatment with CGA ameliorated the intestinal mitochondrial injury induced by H2O2; membrane potential was increased, mitochondrial swelling, levels of reactive oxygen species, contents of 8-hydroxy-2-deoxyguanosine, and cytochrome c released were decreased. The beneficial effects of CGA were accompanied by an increase in antioxidant and respiratory-chain complex I, IV, and V activities. In trinitrobenzene-sulfonic acid-induced colitic rats indicated that CGA supplementation improved mitochondria ultrastructure and decreased mitochondrial injury. Our results suggest a promising role for CGA as a mitochondria-targeted antioxidant in combating intestinal oxidative injury. Daily intake of diets containing CGA, such as coffee and honeysuckle, may be useful for prevention of intestinal diseases.
Graphical abstract
Chlorogenic acid ameliorates intestinal mitochondrial injury in vitro and in vivo and promotes intestinal health.
The intestine is an extremely complex organ with specialized functions in nutrient absorption, barrier formation, immunity, and many others.Citation1,2) However, the intestinal lumen contains numerous noxious stimuli from food and intestinal microorganisms such as antigens, toxins, and chemicals. Frequent exposure to these noxious stimuli may cause intestinal oxidative stress and injury. Oxygen-based free radicals are generated both in the lumen and in the intestinal mucosa. Ingested substances such as alcohol and cholesterol oxides can form a pro-oxidant milieu.Citation3–5) Furthermore, local microbes, infection, or drugs can promote the formation of reactive radicals,Citation6–8) and the influx of leukocytes and lymphocytes to the intestinal mucosa can produce reactive oxygen species (ROS) via respiratory burst.Citation9) Due to these factors, significant oxidative stress is associated with mucosal erosion and plays a causative role in a variety of intestinal diseases such as inflammatory bowel disease (IBD).Citation10)
Mitochondria are special organelles because they are both a target for oxidative stress and a major source of ROS. Decreased respiratory chain functioning and destruction of mitochondrial structure are commonly observed in cases of IBD and oxidative stress,Citation11,12) and impaired mitochondrial respiratory chains promote ROS production.Citation13) In colonic mucus in ulcerative colitis, the activities of mitochondrial respiratory complexes II, III, and IV were decreased by 50–60%.Citation14) Delivery of dinitrophenol into rat ileum resulted in the disruption of epithelial mitochondria,Citation15) and the activities of mitochondrial respiratory complexes I, II, and III were shown to decrease in an iron-ascorbate-induced oxidative stress in Caco-2/15.Citation16) These examples demonstrate the relationship between toxins, intestinal oxidative stress, and respiratory complex activity. Increasing the antioxidant ability and recovering mitochondrial respiratory function may be a positive way to ameliorate mitochondrial and intestinal injury.
Mitochondrial dysfunction can be improved by antioxidant substances.Citation17,18) Chlorogenic acid (CGA) is an ester of caffeic acid and quinic acid found in coffee, honeysuckle, and Eucommia ulmoides and is reported to be one of the most abundant phenolic acids in the human diet.Citation19) CGA has been shown to act as a scavenger of superoxide radicals, hydroxyl radicals, and peroxynitrite in a concentration-dependent manner in vitro.Citation20) CGA was also shown to protect against oxidative stress-related injury in tissues from organs such as the intestine in a number of different pathological settings.Citation21,22) It has been recently reported that CGA has a protective effect on mitochondria in vitro by suppressing mitochondrial membrane depolarization in H2O2-treated PC-12 cells.Citation23) Caffeic acid phenethyl ester (structurally similar to CGA) also ameliorates cadmium-induced mitochondrial injury in kidneys by its antioxidant effects.Citation24)
The daily uptake of CGA by coffee drinkers were up to 0.5–1 g.Citation19) CGA goes into the intestine and exhibits beneficial biological effects for the intestine and the body. In fact, CGA can be hydrolyzed by intestinal microbes.Citation25) The intestine absorbed CGA without hydrolysis and also absorbed its hydrolyzed product such as caffeic acid. Lafay et al.Citation26) demonstrated that CGA was effectively absorbed by the small intestine of rats, about 15–32% of ingested CGA was hydrolyzed into caffeic acid in the cecum, after supplementation for 1.5 h CGA appeared in plasma and urine. Oltof et al.Citation27) reported that about 33% CGA was absorbed in the small intestine. Intestine was a vital metabolic organ in which CGA was metabolited and absorped by intestinal epithelial cells. But, the effect of CGA on intestine was rarely known.
In light, it is hypothesized that CGA can offer protection against intestine injury through mitochondrial mechanism. The present study, therefore, was established to address this issue using isolated rat intestinal mitochondria exposed to H2O2 as an experimental model, as well as trinitrobenzenesulfonic acid (TNBS)-induced rat colitis. Hopefully, it would provide an easy way for promoting intestinal health.
Materials and methods
Experimental protocols
The current investigation consisted of four protocols. The first protocol examined both the effects of H2O2 on intestinal mitochondrial function and also identified the most appropriate dose of H2O2 for use in subsequent protocols. Groups of isolated intestinal mitochondria were incubated with 0, 0.125, 0.25, 0.5, and 1 mol/L H2O2 for 50 min. Alterations in mitochondrial function were evaluated by detection of mitochondrial swelling, ROS production, and membrane potential change.
The second protocol explored the impact of CGA on intestinal mitochondrial function under normal conditions and in the presence of H2O2. Seven groups of isolated intestinal mitochondria were included in this protocol. Group 1 served as control, with mitochondria suspended in PBS buffer for 55 min without further treatment. Groups 2 through 6 contained mitochondria treated with 0, 20, 40, 80 and 160 μmol/L CGA, respectively, for 5 min and then 0.5 mol/L of H2O2 was added and incubated for an additional 50 min. In the last group, 160 μmol/L of CGA was added and incubated in PBS for 55 min. The dose of H2O2 used in this protocol was determined by the most appropriate dose achieved from protocol one and was chosen based on the consistent ability of the dose to induce a moderate degree of mitochondrial dysfunction. Mitochondrial swelling, ROS production, and membrane potential changes were evaluated.
The third protocol assessed the contribution of an antioxidant mechanism to the protective potential of CGA. Intestinal mitochondria were assigned to four groups. Group 1 contained untreated mitochondria, Group 2 contained mitochondria treated with H2O2 (the same dose used in protocol 2), Group 3 contained mitochondria treated with CGA (the most effective dose, determined from protocol 2) 5 min prior to H2O2 exposure, and Group 4 contained mitochondria treated with CGA. Markers of mitochondrial injury, antioxidant activity, and activities of respiratory chain complexes were analyzed.
The last protocol explored the effect of CGA on mitochondrial protection in vivo. In this protocol, TNBS-induced colitis rats were investigated. Rats were divided into four groups, which included a control group and CGA-, TNBS-, and CGA + TNBS-treated groups. Microstructure of mitochondria in intestine epithelial cells, anti-oxidation ability, and markers of mitochondrial injury were evaluated.
Isolation of intestinal mitochondria
For both in vitro and in vivo studies, intestinal mitochondrial fractions were prepared from female Sprague-Dawley rats according to the method described by Valoti et al.Citation28) with slight modifications. Intestines were homogenized (1:10, w:v) in 100 mmol/L Tris-HCl buffer, pH 7.5 containing 0.25 mol/L sucrose. The homogenate was centrifuged at 2,000 rpm for 10 min at 4 °C. The supernatant was collected and further centrifuged at 10,000 rpm for 15 min at 4 °C. After centrifugation, the mitochondrial pellets were resuspended in the same buffer used in homogenization. Protein concentrations were determined using a Coomassie Brilliant Blue method that employed bovine albumin as a standard.
Determination of mitochondrial swelling
A light-scattering technique was used to detect mitochondrial swelling.Citation24) This technique monitors the amount of light scattered by the mitochondria, which inversely correlates with mitochondrial size such that an increase in mitochondrial volume results in a decrease in light scattering and measured absorbance. After subjecting mitochondria to various treatments, mitochondrial swelling was assessed by measuring the change in absorbance at 520 nm.
Determination of mitochondrial membrane-potential change
Rhodamine 123 was used to assess mitochondrial membrane-potential changes as previously described with slight modifications.Citation29) Rhodamine 123 is a cell-permeable cationic dye that preferentially enters the mitochondria due to the highly negative mitochondrial membrane potential. Depolarization of the mitochondrial membrane potential due to cell damage results in the loss of Rhodamine 123 from the mitochondria and a decrease in intracellular fluorescence intensity. Mitochondria were washed once in cold PBS and then resuspended in Rhodamine 123 (1 μmol/L) for 30 min in the dark. Fluorescence was measured by flow cytometry (Becton, Dickinson and Company, New Jersey, USA) with an excitation wavelength of 507 nm and an emission wavelength of 529 nm.
Measurement of mitochondrial ROS production
Mitochondrial ROS production was assayed using dihydrorhodamine 123 (DHR) as previously described with slight modifications.Citation30) The measurement is based on ROS-mediated conversion of a non-fluorescent DHR to a highly fluorescent Rhodamine 123. Briefly, mitochondria were incubated with 1 μmol/L DHR at 25 °C for 45 min. Rhodamine 123 fluorescence was measured by 507 nm excitation and 529 nm emission using flow cytometry (Becton, Dickinson and Company, New Jersey, USA). The level of ROS was expressed as arbitrary units of Rhodamine 123 fluorescence intensity.
Determination of cytochrome c and 8-hydroxy-2-deoxyguanosine (8-OHdG) concentrations
The concentrations of cytochrome c were determined in both mitochondrial fractions and supernatants. At the end of the experiment, the reactions were centrifuged at 10,000 rpm for 15 min at 4 °C and the precipitate (mitochondrial fraction) and supernatant were isolated.
The content of cytochrome c in TNBS-induced colitis rats was determined in mitochondria isolated from colonic tissue cytoplasm. The content of cytochrome c present in the cytoplasm was regarded as the cytochrome c that was released from mitochondria. The mitochondrial fractions and cytoplasm were separated by commercial kits (Beyotime Biotechnology, Beijing, China) according to the manufacturer’s instructions.
The content of cytochrome c and 8-OHdG in mitochondrial fractions was determined using the enzyme-linked immunosorbent assay (ELISA) kit (ZSGB-BIO Oorigene, Beijing, China). To perform the assay, the protocol provided by the manufacturer was followed closely. The optical density of each well was determined by the automatic microplate reader set to 450 nm.
Induction of colitis in rats
The animal experiment was approved by the Nanchang University Animal Experiment Ethics Committee. Thirty-two female Sprague-Dawley rats aged 6 weeks (183 ± 2 g) were randomly distributed into four experimental groups: Control, CGA, TNBS, and CGA + TNBS. Rats were individually housed in an air-conditioned room (22 ± 2 °C) with a 12-h light:12-h dark cycle in the Animal Laboratory of Jiangxi Province Center for Disease Control and Prevention (Nanchang, China). All rats had free access to water and a standard laboratory diet (GB14924.1-2001, China).
TNBS colitis was induced in rats as described previously.Citation31) After a 7-day adaptation period, rats were deprived of food but not water for 24 h before induction of colitis. Fasted animals were lightly anesthetized with 1% pentobarbital sodium and a plastic catheter was inserted rectally into the colon. Next, TNBS was located 8 cm proximal to the anus. One milliliter of TNBS dissolved in 50% ethanol (v/v) was introduced into the lumen of the colon at a dose of 100 mg per kg of body weight through the rubber catheter. Following the administration of TNBS, the animals were kept in a head-down position for 60 s to allow the samples to move through the gastrointestinal tract. In control and CGA groups, rats were injected with sterile water instead of TNBS. In CGA and CGA + TNBS groups, rats were given 60-mg CGA per kg of body weight via oral administration once a day between 9:00–10:00 am
The experimental period was 28 days. After a fasting period of 12 h, animals were anesthetized by intraperitoneal injection of sodium pentobarbital at a dose of 45 mg/kg body weight and were sacrificed by cardiac exsanguination. Rat colons were dissected, weighed, and immediately frozen.
Ultrastructure measurements of mitochondria
The front end of rat colon sections were removed and placed in 2.5% glutaraldehyde in 0.1 mol/L phosphate buffer at pH 7.2 for 2 h. Specimens were then rinsed in the same buffer and fixed in 2% osmium tetroxide for 2 h and dehydrated in graded acetone solutions (30, 50, 70 with 2% uranyl-acetate, 90, and 100%). Specimens were then cleared in propylene oxide and embedded in araldite. Semi-thin sections of 1.5 μm were cut with a diamond knife and stained lightly with 1% toluidine blue. Later, ultrathin 0.08-μm sections were cut with a diamond knife, collected on Formvar-coated single-slot grids, counterstained with 1% uranyl acetate and with Reynold’s lead citrate for 10 min, and examined under a JEM-1200 transmission electron microscope. The images were acquired with an Advanced Microscopy Techniques, Corp.’s Charge-Coupled Device (Danvers, MA) imaging system.
The epitelioglandular mitochondrial damage score of the rats were obtained under the transmission electron microscope and assessed blindly using the methods described by Flameng.Citation32) Each mitochondrion was graded on a 0–4 scale to represent the severity of structural destruction. Mean mitochondrial damage scores of all mitochondria (approximately 100) per rat are as follows: 0 = normal structure with well-preserved mitochondrial granules; 1 = swollen mitochondria with clarification of the matrix; 3 = disruption of mitochondrial crests with clarification as well as condensation of the matrix; 4 = disruption of the crests and loss of integrity of the mitochondrial inner and outer membranes.
Determination of antioxidant, myeloperoxidase, and alkaline phosphatase activity
Spectrophotometry was used to determine the activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), total antioxidant capacity (T-AOC), myeloperoxidase (MPO), and alkaline phosphatase (ALP). The spectrophotometric commercial kits were all purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Activity measurements of mitochondrial respiratory chain complexes Ι, IV, and V
The activities of respiratory chain complexes Ι, IV, and V in mitochondria fractions were determined using commercial kits (Genmed, Shanghai, China).
Statistical analysis
Data values represent the mean ± SE. Comparisons were performed by one-way ANOVA followed by Tukey’s test using SPSS 11.5 software. Values of p < 0.05 were regarded as statistically significant.
Results
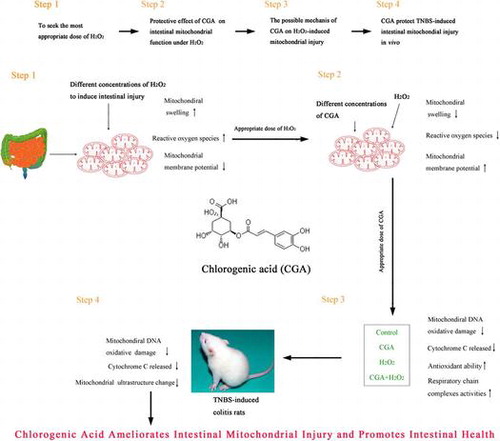
H2O2-induced intestinal mitochondrial injury in a dose-dependent manner
A rapid decrease in mitochondrial absorbance was detected after H2O2 treatment of mitochondria, indicating the presence of mitochondrial swelling. The magnitude of absorbance reduction was shown to have proportional dependence on the concentration of H2O2 (Fig. (A)). Mitochondrial membrane potential was determined by measurements of Rhodamine 123. The lowest concentration of H2O2 (0.125 mol/L) tested caused a slight, albeit not significant, decrease in the fluorescence intensity of Rhodamine 123 compared to that observed in the control group (Fig. (B)). However, a substantial reduction in the fluorescence intensity was evident with increased doses of H2O2, indicating the presence of mitochondrial depolarization. A stepwise increase in the mitochondrial ROS production was also observed with H2O2-concentration increase (Fig. (C)). Because 0.5 mol/L of H2O2 consistently and appropriately induced mitochondrial dysfunction, this dose was used as the optimum dose of H2O2 for the rest of this study.
Fig. 1. Effects of different concentrations of H2O2 on mitochondrial function. Intestinal mitochondria were treated with 0.125, 0.25, 0.5, or 1 M H2O2 for 50 min. Mitochondrial swelling (A) was measured by monitoring the absorbance at 540 nm. Mitochondrial membrane potential changes (B) were indicated by Rhodamine 123 fluorescence after staining mitochondria with Rhodamine 123. Mitochondrial ROS production (C) was measured following incubation of mitochondria with DHA with the use of Rhodamine 123 fluorescence.
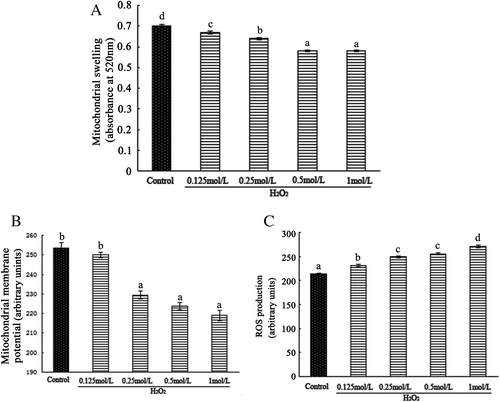
CGA ameliorated H2O2-induced intestinal mitochondrial injury
When CGA was applied to mitochondria prior to H2O2 exposure, CGA dose-dependently attenuated H2O2-induced mitochondrial swelling. At a concentration of 80 μmol/L, CGA significantly decreased mitochondrial swelling compared to H2O2 treatment group (Fig. (A)). The increase in ROS and decline in mitochondrial membrane potential in H2O2-induced mitochondria were reduced upon co-administration of CGA with H2O2 (Fig. (B) and (C)). Only 160 μmol/L CGA was able to restore the membrane-potential change caused by H2O2 to values comparable to the control. CGA at concentrations of 20 and 160 μmol/L decreased mitochondrial ROS levels compared to H2O2 treatment. Compared to the control group, the highest concentration of CGA (160 μmol/L) used in this study caused no significant changes in mitochondrial swelling, membrane potential, and ROS production. Therefore, 160 μmol/L CGA was chosen as the dose to be used in further studies of mitochondrial oxidative stress and mitochondrial respiratory chain complex because this dose demonstrated the greatest beneficial effects.
Fig. 2. CGA alleviated H2O2-induced injury to isolated intestinal mitochondria. Intestinal mitochondria were treated with 0.5 mM H2O2 or a combination of H2O2 and CGA (CGA + H2O2). CGA was added 5 min prior to treatment. Mitochondrial swelling (A), mitochondrial membrane-potential change (B), mitochondrial ROS production (C), content of 8-OHdG in mitochondrial fractions (D), content of cytochrome c in mitochondrial fractions (E), and content of cytochrome c in mitochondrial supernatant (F) are shown.
8-OHdG is a product of oxidative DNA damage.Citation33) The content of 8-OHdG increased in the H2O2 group compared with the control group (p < 0.05) (Fig. (D)). In the CGA + H2O2 group, the levels of 8-OHdG were significantly decreased compared to the H2O2 group (p < 0.05).
Increased levels of cytochrome c were released upon mitochondrial injury. As seen in Fig. (E) and (F), after exposure to H2O2, the level of cytochrome c in mitochondrial fractions was significantly reduced (p < 0.05) (Fig. (E)) and increased in the supernatant compared to the control group (p < 0.01) (Fig. (F)). When 160 μmol/L CGA was administered together with H2O2, the levels of cytochrome c in mitochondrial fractions were increased (p < 0.05) and were decreased in the mitochondrial supernatant compared to the H2O2 group (p < 0.01). No significant differences in 8-OHdG and cytochrome c levels were found between control and 160 μmol/L CGA groups. These results suggest that CGA can prevent H2O2-induced mitochondrial damage.
CGA protection against H2O2-induced oxidant stress
Intestinal mitochondria exposed to H2O2 showed an obvious decrease in antioxidant ability, demonstrated by decreased activities of SOD (p < 0.01), GSH-Px (p < 0.01), and T-AOC (p < 0.01) (Table ). Pretreatment with CGA increased the activities of SOD (p < 0.01), GSH-Px (p < 0.01), and T-AOC (p < 0.01) by 18.70, 89.44, and 45.86%, respectively. None of these parameters were significantly altered in the group of CGA-treated mitochondria compared to those in the control.
Table 1. Effects of CGA on activities of T-AOC, SOD, and GSH-Px in mitochondrial fractions.
CGA protection against H2O2-induced decreases in the activities of mitochondrial respiratory chain complexes
Enzymatic activities of mitochondrial complexes I, IV, and V were assessed in mitochondrial fractions. As observed in Table , the activities of complexes I (p < 0.01), IV (p < 0.01), and V (p < 0.05) were significantly decreased in the H2O2 group compared with the control group. When 160 μmol/L CGA was pre-added and followed by incubation with H2O2, the activities of complexes I (p < 0.01), IV (p < 0.01), and V (p < 0.05) were significantly increased compared to the H2O2 group. No significant differences in activities of mitochondrial complexes I, IV, and V were observed between the control and 160 μmol/L-CGA groups.
Table 2. Effects of CGA on intestinal mitochondrial respiratory chain complexes.
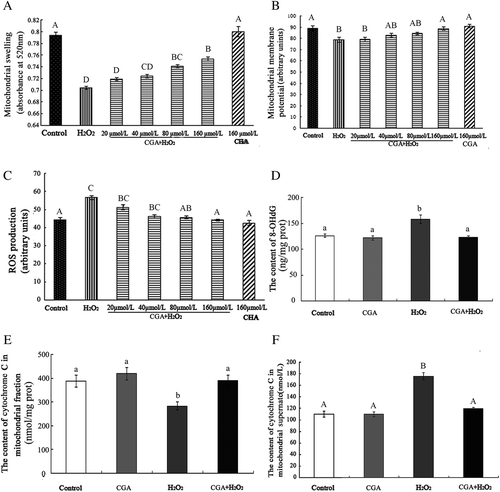
CGA ameliorates colonic injury in TNBS-induced colitis rat
Histological evaluation by staining of specimens from colons of control- and CGA-group rats revealed integrated villi and a compactly arrayed epithelium (Fig. (A)). The histological appearance of the intestine demonstrated a marked infiltration of inflammatory cells by TNBS administration. The CGA + TNBS group was found to prevent inflammatory cell infiltration. Based on the histological injury score, the histological injury score in the CGA + TNBS group was decreased compared to the colitis rats (TNBS group) (Fig. (B)).
Fig. 3. CGA ameliorates colonic injury in TNBS-induced colitis rat. Histological findings of proximal colon samples stained with hematoxylin and eosin (A). Arrows indicate inflammatory cells. Histological injury scoring (B) indicated a grading scale for histological assessment of inflammation in colitis. The length of colon (C), weight of colon (D), activity of MPO (E), and ALP (F).
Compared to normal animals, the length of colon (p < 0.05) was decreased, while the length of colon (p < 0.05) was increased in the TNBS group (Fig. (C) and (D)). MPO, a constitutive component of neutrophil azurophilic granules, is a good marker of inflammation and tissue injury.Citation34) ALP promotes the resolution of inflammation through detoxification of endotoxin.Citation35) In this study, the activity of MPO (p < 0.01) was increased, and the activity of ALP (p < 0.01) was decreased in TNBS-induced colitis rats, compared with normal rats (Fig. (E) and (F)). These changes were ameliorated by CGA supplementation. Compared to colitis rats, the length of colon (p < 0.05) and the activity of ALP (p > 0.05) in CGA + TNBS group were increased, the weight of colon (p < 0.05) and the activity of MPO (p < 0.05) were decreased in the CGA + TNBS group.
Effects of CGA on mitochondrial ultrastructure in colonic epithelial cells in TNBS-induced colitis rats
Electron microscopy indicated that untreated mitochondria in the control and CGA groups had a classical ultrastructure containing densely packed cristae and matrix (Fig. (A)). Following TNBS exposure, mitochondria appeared swollen with loss of cristae and matrix density. From results of mitochondrial damage score, the mitochondrial damage score in CGA + TNBS group was decreased (p < 0.05), as compared with colitis rats (TNBS group) (Fig. (B)). These structural alterations induced by TNBS were ameliorated in mitochondria treated with CGA.
Fig. 4. CGA alleviated TNBS-induced mitochondrial injury and ultrastructural changes. Ultrastructure of mitochondria (A), arrows in control and CGA groups show the normal mitochondrial ultrastructure. Arrows in the TNBS group show mitochondrial ultrastructural changes, such as swelling and lost cristae. Arrows in the CGA + TNBS group indicate amelioration of mitochondrial ultrastructural changes. Mitochondrial damage score (B), the content of 8-OHdG (C) and cytochrome c (D) in mitochondria, and cytochrome c in cytoplasm (E) are shown.
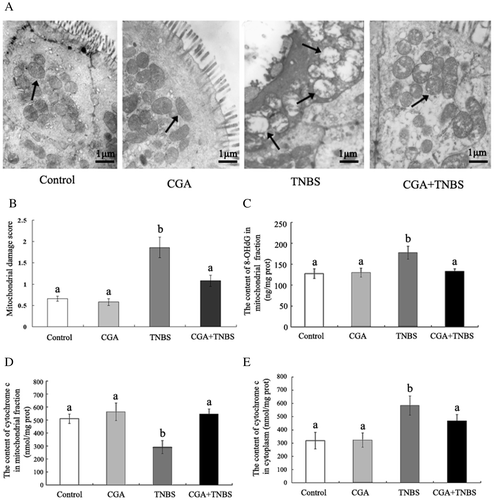
In colitis animals, the content of 8-OHdG in mitochondria (Fig. (C)) and the release of cytochrome c from mitochondria (Fig. (E)) were increased, and the content of cytochrome c in mitochondria (Fig. (D)) was decreased compared with normal animals. These changes were ameliorated by CGA administration; the content of 8-OHdG in mitochondrial fractions decreased from 177.49 ± 15.52 ng/mg protein in colitis rats to 132.85 ± 6.21 ng/mg protein (p < 0.05), released cytochrome c decreased by 19.80% (585.05 ± 72.55 nmol/mg protein in the TNBS group vs. 469.20 ± 46.37 nmol/mg protein in the CGA + TNBS group) (p < 0.05), and cytochrome c in mitochondria increased (p < 0.05).
Discussion
The major question addressed in this study is whether CGA is capable of preventing or ameliorating the functional and ultrastructural disorders of intestinal mitochondria injury. The effects of CGA on H2O2-induced mitochondrial injury in vitro and TNBS-induced colitis rats in vivo were investigated. The outcomes of the study demonstrate the mitochondrial protective effect of CGA and further suggest that this benefit is mediated, at least in part, by its potential to increase antioxidant and mitochondrial respiratory-complex activities.
In the in vitro studies, H2O2-induced intestinal dysfunction was accompanied by a substantial rise in mitochondrial ROS and 8-OHdG, a decrease in total antioxidant, SOD, and GSH-Px activities, indicating a role of oxidative stress in mediating the toxic effects of H2O2. The surge in ROS may trigger the opening of the inner membrane anion channel and allow the release of superoxide anions from the mitochondrial matrix, resulting in mitochondrial membrane depolarization.Citation36) The release of ROS via inner membrane anion channel opening may then trigger the inner membrane anion channel openings of neighboring mitochondria.Citation37) This chain effect may further increase levels of ROS within mitochondria, thereby exacerbating the damaging effects and causing increased release of cytochrome c from mitochondria. Mitochondrial DNA is prone to oxidative damage due to direct contact with the ROS produced in the mitochondria, and 8-OHdG is the product of oxidative DNA.Citation33,38,39) Thus, 8-OHdG and cytochrome c levels present inside of mitochondria, and cytochrome c levels released from mitochondria, are markers of mitochondrial damage. Treatment with CGA decreased H2O2-induced ROS production in a dose-dependent manner, increased the mitochondrial membrane potential, and decreased the content of 8-OHdG and release of cytochrome c from the mitochondria. T-AOC, SOD, and GSH-Px activities were also increased. These results indicate that CGA ameliorated the H2O2-induced injury to isolated intestinal mitochondria and increased antioxidant ability.
The mitochondrial respiratory chain, which includes the five multimeric protein complexes I, II, III, IV, and V, is considered the powerhouse of cells.Citation40) The decreased activity of mitochondrial complexes alters the normal electron flow through the respiratory chain, and damage to the mitochondrial respiratory chain is the main source of ROS.Citation41) Mitochondrial function during oxidative stress and exposure to H2O2 has been extensively investigated.Citation42–44) H2O2 treatment of retinal pigment epithelial cells resulted in significantly increased mitochondrial DNA damage in vitro.Citation42) Treatment of isolated rat heart mitochondria with H2O2 resulted in complex II inactivity.Citation12) Current literature presents conflicting findings regarding the inhibitory effects of H2O2 on mitochondrial respiration complexes in isolated intestinal mitochondria. The inhibitory effects of H2O2 on complexes I, IV, and V were in a dose-dependent manner (Supplementary Table S1). The electron flow inhibition decreased the mitochondrial membrane potential and promoted ROS production, leading to an increase in the mitochondrial permeability through the transport pore and an increase in cytochrome c release and oxidative stress. Pretreatment with CGA increased the activities of complexes I, IV, and V in vitro. These results indicate that CGA ameliorated the H2O2-induced mitochondrial injury, at least in part, by increasing the activities of mitochondrial respiratory complexes.
Dysfunction of mitochondrial respiratory activity may, therefore, contribute to disruption of mitochondrial ultrastrucutre. Mitochondrial dysfunction in the pathogenesis of IBD has been reported in clinical cases and animal studies.Citation14, 45–48) Sifroni et al.Citation14) reported that complex IV activity decreased by 50–60% in colonic mucosal cells in UC patients. Tirosh et al.Citation46) suggested that mitochondria played a pivotal role in TNBS-induced colitis and that TNBS would promote mitochondrial damage directly through mitochondrial DNA depletion and loss of tissue respiration. Rectal biopsy specimens from control subjects and patients with colitis showed evidence of colitis-associated mitochondrial damage including swelling, outer- and inner-membrane partition with loss of integrity, and a decrease and loss of organization in cristae.Citation11) In our study, CGA supplementation restored normal mitochondrial structure and reduced swelling, as well as increased the activities of SOD, and mitochondrial respiratory complexes I and V, and inhibited lipid peroxidant (Supplementary Table S2 and S3). Thus, the protective effect of CGA on the structure of mitochondria may be due to increased mitochondrial antioxidant activity and modulated respiratory complexes activity.
However, CGA could be metabolized and transformed by intestinal microbes. Plumb et al.Citation49) reported there was no evidence of hydrolytic enzyme for CGA in the intestine, liver or plasma extracts, and CGA ingestion by human is most likely cleaved into caffeic acid and quinic acid by an esterase enzyme provided by the colonic microflora. Rat supplementation with CGA (250 μmol/L/day) for 8 days, 57.4% of the CGA was metabolited by microbial.Citation25) On the other hand, intestinal epithelial cells absorped CGA and its hydrolyzed product. The mitochondrial protective effect of CGA may be due to CGA or/and its hydrolyzed product, such as caffeic acid. The effect of CGA metabolizm on mitochondria needs to be further investigated.
Treatment with CGA alone did not show any significant effects on the tested parameters or mitochondrial ultrastructure when applied to isolated mitochondria in vitro or to normal rats, confirming the safety of CGA. The observed effects in this study were attributed to improved antioxidant abilities and mitochondrial respiratory chain complex activities, thereby protecting intestinal mitochondria from injury.
Conclusions
The present study revealed that CGA supplementation protected intestinal mitochondria from oxidative stress in vitro and in vivo. Daily intake of diets containing CGA such as coffee, honeysuckle, Eucommia ulmoides, and cabbage, may be useful for the prevention of intestinal diseases such as IBD.
Authors Contribution
Z. Ruan was in charge of the whole project and involved in the designing of the study and revised the paper. Y. Zhou conducted the animal trial and wrote a part of paper. L. Zhou carried out a part of the experiment. S. Mi, M. Jiang, and X. Li assisted with in vitro study. X. Wu, Z. Deng and Y. Yin assisted with discussion.
Funding
This research was financially supported by the Natural Science Foundation of Jiangxi province [20151BAB204036], the Open Project Program of State Key Laboratory of Food Science and Technology, Nanchang University [SKLF-ZZB-201515], [SKLF-KF-201414], [SKLF-KF-201405], [SKLF-KF-201416], the Excellent Young Scientist Award by Jiangxi Province [20153BCB23026].
Supplemental materials
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2015.1127130.
Disclosure statement
No potential conflict of interest was reported by the authors.
supplementary_file-11-5.doc
Download MS Word (50 KB)Notes
Abbreviations: 8-OhdG, 8-hydroxy-2-deoxyguanosine; ALP, alkaline phosphatase; CGA, chlorogenic acid; dihydrorhodamine 123 (DHR); GSH-Px, glutathione peroxidase; MPO, myeloperoxidase; ROS, reactive oxygen species; SOD, superoxide dismutase; T-AOC, total antioxidant capacity; TNBS, 2,4,6-trinitrobenzenesulfonic acid.
References
- Shimizu M. Interaction between food substances and the intestinal epithelium. Biosci. Biotechnol. Biochem. 2010;74:232–241.10.1271/bbb.90730
- Turner JR. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9:799–809.10.1038/nri2653
- Young IS, Woodside JV. Antioxidants in health and disease. J. Clin. Patho. 2001;54:176–186.10.1136/jcp.54.3.176
- Parks DA. Oxygen radicals: mediators of gastrointestinal pathophysiology. Gustroenterology. 1989;30:293–298.10.1136/gut.30.3.293
- Mazalli MR, Bragagnolo N. Increase of cholesterol oxidation and decrease of PUFA as a result of thermal processing and storage in eggs enriched with n-3 fatty acids. J. Agr. Food. Chem. 2009;57:5028–5034.10.1021/jf901187j
- Sánchez S, Martín MJ, Ortiz P, et al. Effects of dipyrone on inflammatory infiltration and oxidative metabolism in gastric mucosa: comparison with acetaminophen and diclofenac. Digest Dis. Sci. 2002;47:1389–1398.10.1023/A:1015395103160
- Parks DA, Williams TK, Beckman JS. Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am. J. Physiol. 1988;254:G768–G774.
- Biswas K, Bandyopadhyay U, Chattopadhyay I, et al. A novel antioxidant and antiapoptotic role of omeprazole to block gastric ulcer through scavenging of hydroxyl radical. J. Biolog. Chem. 2003;278:10993–11001.10.1074/jbc.M210328200
- Babbs CF. Oxygen radicals in ulcerative colitis. Free Radical Bio. Med. 1992;13:169–181.10.1016/0891-5849(92)90079-V
- Pravda J. Radical induction theory of ulcerative colitis. World J. Gastroentero. 2005;11:2371–2384.10.3748/wjg.v11.i16.2371
- Hsieh SY, Shih TC, Yeh CY, et al. Comparative proteomic studies on the pathogenesis of human ulcerative colitis. Proteomics. 2006;6:5322–5331.10.1002/(ISSN)1615-9861
- Nulton-Persson AC, Szweda Li. Modulation of mitochondrial function by hydrogen peroxide. J. Biol. Chem. 2001;276:23357–23361.10.1074/jbc.M100320200
- Chen Q, Vazquez EJ, Moghaddas S, et al. Production of reactive oxygen species by mitochondria: central role of complex III. J. Biol. Chem. 2003;278:36027–36031.10.1074/jbc.M304854200
- Sifroni KG, Damiani CR, Stoffel C, et al. Mitochondrial respiratory chain in the colonic mucosal of patients with ulcerative colitis. Mol. Cell Biochem. 2010;342:111–115.10.1007/s11010-010-0474-x
- Nazli A, Yang PC, Jury J, et al. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am. J. Pathol. 2004;164:947–957.10.1016/S0002-9440(10)63182-3
- Taha R, Seidman E, Mailhot G, et al. Oxidative stress and mitochondrial functions in the intestinal Caco-2/15 cell line. PLoS One. 2010;5:e11817.10.1371/journal.pone.0011817
- Carrasco-Pozo C, Mizgier ML, Speisky H, et al. Differential protective effects of quercetin, resveratrol, rutin and epigallocatechin gallate against mitochondrial dysfunction induced by indomethacin in Caco-2 cells. Chem-Biol. Interact. 2012;195:199–205.10.1016/j.cbi.2011.12.007
- Valenti D, De Rasmo D, Signorile A, et al. Epigallocatechin-3-gallate prevents oxidative phosphorylation deficit and promotes mitochondrial biogenesis in human cells from subjects with Down’s syndrome. BBA-Mol. Basis Dis. 2013;1832:542–552.10.1016/j.bbadis.2012.12.011
- Clifford MN. Chlorogenic acids and other cinnamates–nature, occurrence and dietary burden. J. Sci. Food Agr. 1999;79:362–372.10.1002/(ISSN)1097-0010
- Kono Y, Kobayashi K, Tagawa S, et al. Antioxidant activity of polyphenolics in diets: rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. BBA-Gen Subjects. 1997;1335:335–342.10.1016/S0304-4165(96)00151-1
- Graziani G, D’argenio G, Tuccillo C, et al. Apple polyphenol extracts prevent damage to human gastric epithelial cells in vitro and to rat gastric mucosa in vivo. Gustroenterology. 2005;54:193–200.
- Yun N, Kang JW, Lee SM. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012;23:1249–1255.10.1016/j.jnutbio.2011.06.018
- Park JB. Isolation and quantification of major chlorogenic acids in three major instant coffee brands and their potential effects on H2O2-induced mitochondrial membrane depolarization and apoptosis in PC-12 cells. Food Funct. 2013;4:1632–1638.10.1039/c3fo60138b
- Kobroob A, Chattipakorn N, Wongmekiat O. Caffeic acid phenethyl ester ameliorates cadmium-induced kidney mitochondrial injury. Chem.-Biol. Interact. 2012;200:21–27.10.1016/j.cbi.2012.08.026
- Gonthier MP, Verny MA, Besson C, et al. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr. 2003;133:1853–1859.
- Lafay S, Morand C, Manach C. Absorption and metabolism of caffeic acid and chlorogenic acid in the small intestine of rats. Brit. J. Nutr. 2006;96:39–46.10.1079/BJN20061714
- Olthof MR, Hollman PC, Katan MB. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001;131:66–71.
- Valoti M, Morón J, Benocci A, et al. Evidence of a coupled mechanism between monoamine oxidase and peroxidase in the metabolism of tyramine by rat intestinal mitochondria. Biochem. Pharmacol. 1998;55:37–43.10.1016/S0006-2952(97)00379-1
- Wang M, Ruan Y., Chen Q., et al. Curcumin induced HepG2 cell apoptosis-associated mitochondrial membrane potential and intracellular free Ca2+ concentration. Eur. J. Pharmacol. 2011;650:41–47.10.1016/j.ejphar.2010.09.049
- Li J, Baud O, Vartanian T, et al. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc. Natl. Acad. Sci. USA. 2005;102:9936–9941.10.1073/pnas.0502552102
- Ruan Z, Lv Y, Fu X, et al. Metabolomic analysis of amino acid metabolism in colitic rats supplemented with lactosucrose. Amino Acids. 2013;45:877–887.10.1007/s00726-013-1535-8
- Flameng W, Andres J, Ferdinande P, et al. Mitochondrial function in myocardial stunning. J. Mol. Cell. Cardiol. 1991;23:1–11.10.1016/0022-2828(91)90034-J
- Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutation Res./Rev. Mutation Res. 1997;387:147–163.10.1016/S1383-5742(97)00035-5
- Bradley PP, Priebat DA, Christensen RD, et al. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78:206–209.10.1111/jid.1982.78.issue-3
- Poelstra K, Bakker WW, Klok PA, et al. Dephosphorylation of endotoxin by alkaline phosphatase in vivo. Am. J. Pathol. 1997;151:1163.
- Aon MA, Cortassa S, Marban E, et al. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 2003;278:44735–44744.10.1074/jbc.M302673200
- Zorov DB, Filburn CR, Klotz LO, et al. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000;192:1001–1014.10.1084/jem.192.7.1001
- Santos JH, Mandavilli BS, Van Houten B. Measurement of oxidative mtDNA damage and repair using quantitative PCR. Methods Mol. Biol. 2002;197:159–176.
- Sawyer DE, Van Houten B. Repair of DNA damage in mitochondria. Mutat. Res. 1999;434:161–176.10.1016/S0921-8777(99)00027-0
- DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. New Engl. J. Med. 2003;348:2656–2668.10.1056/NEJMra022567
- Shokolenko I, Venediktova N, Bochkareva A, et al. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids. Res. 2009;37:2539–2548.10.1093/nar/gkp100
- Ballinger SW, Houten BV, Jin GF, et al. Hydrogen peroxide causes significant mitochondrial DNA damage in human RPE Cells. Exp. Eye Res. 1999;68:765–772.10.1006/exer.1998.0661
- Nulton-Persson AC, Szweda Li. Modulation of mitochondrial function by hydrogen peroxide. J. Biol. Chem. 2001;276:23357–23361.10.1074/jbc.M100320200
- Maechler P, Jornot L, Wollheim CB. Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic beta cells. J. Biol. Chem. 2009;274:27905–27913.
- Santhanam S, Rajamanickam S, Motamarry A, et al. Mitochondrial electron transport chain complex dysfunction in the colonic mucosa in ulcerative colitis. Inflamm. Bowel Dis. 2012;18:2158–2168.10.1002/ibd.22926
- Tirosh O., Levy E., Reifen R. High selenium diet protects against TNBS-induced acute inflammation, mitochondrial dysfunction, and secondary necrosis in rat colon. Nutrition. 2007;23:878–886.10.1016/j.nut.2007.08.019
- Vanderborght M, Nassogne MC, Hermans D, et al. Intractable ulcerative colitis of infancy in a child with mitochondrial respiratory chain disorder. J. Pediatr. Gastroenterol. Nutr. 2004;38:355–357.10.1097/00005176-200403000-00023
- Restivo NL, Srivastava MD, Schafer IA, et al. Mitochondrial dysfunction in a patient with crohn disease: possible role in pathogenesis. J. Pediatr. Gastroenterol. Nutr. 2004;38:534–538.10.1097/00005176-200405000-00014
- Gonthier MP, Verny MA, Besson C, et al. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr. 2003;133:1853–1859.
