Abstract
In this study, we investigated the effect of TGF-β1 on cholesterol synthesis in human keratinocytes. TGF-β1 increased the level of cholesterol and the mRNA level of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase in human keratinocytes. These results show that TGF-β1 induces cholesterol synthesis by increasing HMG-CoA reductase mRNA expression in human keratinocytes.
Cholesterol is important in the epidermis as it is required for the lipid lamellae of the stratum corneum, which form the epidermal permeability barrier.Citation1) 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase catalyzes the conversion of HMG-CoA to mevalonic acid and is considered a key enzyme in the regulation of cholesterol production.Citation2) Menon et al.Citation3) reported that acute barrier disruption caused rapid and marked increase in epidermal cholesterol synthesis by the action of HMG-CoA reductase. Topical treatment with either cholesterol or mevalonate markedly improved permeability barrier homeostasis in aged animals.Citation4,5) Our previous studies have demonstrated that high-fat (HF) diets reduced lipid content by decreasing HMG-CoA reductase mRNA expression in the skin.Citation6) However, the molecular mechanism underlying the decrease in the cholesterol level in the skin by HF diets is not well understood.
Transforming growth factor (TGF)-β1 mediates a wide variety of biological functions, such as cell proliferation, differentiation, and extracellular matrix production in the skin.Citation7) It is now clear that TGF-β1 induced diverse cellular responses by binding to and activating specific cell-surface receptors that have intrinsic serine/threonine kinase activity. Our previous studies have demonstrated that HF diets reduced TGF-β1 mRNA expression in the skin.Citation8) Thus, we have focused on the action of TGF-β1 to regulate cholesterol synthesis in the skin following the consumption of HF diets. Therefore, the aim of the present study was to examine the relationship between cholesterol synthesis and the action of TGF-β1 using human keratinocytes.
The human keratinocyte cell line NHEK was obtained from Kurabo Industries, Ltd. (Osaka, Japan), and cultured in HuMedia-KG2 containing insulin (10 μg/mL), human epidermal growth factor (0.1 ng/mL), hydrocortisone (0.5 μg/mL), gentamicin (50 μg/mL), amphotericin B (50 ng/mL), and 0.4% (v/v) bovine pituitary extract (Kurabo Co., Ltd, Osaka, Japan) in a humidified atmosphere of 5% CO2 at 37 °C.
Human keratinocytes were seeded in 6-well plates in culture medium at a density of 3 × 105 cells/well and were incubated for 24 h. Keratinocyte differentiation was performed using a differentiation medium, a mixture of DMEM, and Ham’s F-12 medium (2:1, v/v), supplemented with 10% (v/v) FBS, insulin (10 μg/mL), hydrocortisone (0.4 μg/mL), gentamicin (50 μg/mL) and amphotericin B (50 ng/mL) for total lipid extraction. The cells were treated with TGF-β1 (0, 10, or 30 ng/mL) (Peprotech, Rocky Hill, NJ) for 9 days. Total lipids in human keratinocytes were extracted using chloroform/methanol (1:1 vol/vol). Lipid samples from human keratinocytes were prepared according to the Folch method.Citation9) Cholesterol levels in the lipid samples were measured with Cholescolor Liquid Kit (Toyobo Co, Ltd, Osaka, Japan).
Human keratinocytes were seeded in 96-well plates in culture medium at a density of 1 × 104 cells/well and were incubated for 24 h. The cells were treated with TGF-β1 (0 or 30 ng/mL) for 24 h. Total RNA was extracted and cDNA was prepared using a TaqMan® Gene Expression Cells-to-CT™ Kit according to the manufacturer’s instructions. The amplified products were detected using the TaqMan universal PCR master mix core reagent kit and TaqMan Gene Expression Assays kit (Applied Biosystems, Foster City, CA). The mRNA levels of HMG-CoA reductase and GAPDH were measured using quantitative PCR with an ABI Prism 7300 apparatus (Applied Biosystems, Foster City, CA). The levels were expressed as values relative to those of human GAPDH. Amplification reactions were performed under the following conditions: 2 min at 50 °C and 10 min at 95 °C, followed by 50 cycles of 15 s at 95 °C and 1 min at 60 °C.
Results are expressed as mean ± SD. The means of multiple groups were compared using the Student–Newman–Keuls test. *p < 0.05 and **p < 0.01 indicate values that are significantly different from the vehicle.
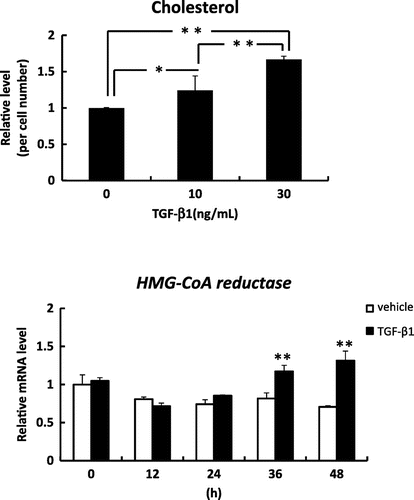
In the present study, the levels of cholesterol were increased in human keratinocytes treated with TGF-β1 (10, or 30 ng/mL) for 9 days (Fig. (A)). TGF-β1 affected the cell numbers of keratinocytes (data not shown). Upper layer of skin has a high cholesterol content and an accumulation of apoptotic keratinocytes. TGF-β1 may induce cholesterol synthesis at upper layer of skin. HMG-CoA reductase mRNA expression increased in a time-dependent manner following the addition of TGF-β1 (30 ng/mL), and significantly increased after 36 and 48 h in comparison to the cells without TGF-β1 (Fig. (B)). TGF-β1 mediates a wide variety of biological functions by binding to its high affinity type II receptor to form a tetrameric complex with type I receptor.Citation10) The action of TGF-β1 is mainly mediated by the Smad family of proteins and mitogen-activated protein kinase (MAPK).Citation11) Smad2/3 complexes translocate into the cell nucleus and affect the transcriptional status of target genes in co-operation with DNA binding cofactors. We also observed that TGF-β1 promoted Smad3 phosphorylation in human keratinocytes (data not shown). Yakymovych et al.Citation12) showed that protein kinase C, a major component in the signaling pathways downstream of TGF-β1 receptors, phosphorylated Smad3 but not Smad2. These data suggest that the action of protein kinase C is involved in TGF-β1-promoted Smad3 phosphorylation in human keratinocytes. The detailed mechanism for the up-regulation of HMG-CoA reductase gene expression by TGF-β1 signaling will be clarified by future studies, including the promoter analyses of HMG-CoA reductase.
Fig. 1. Effects of TGF-β1 on the level of cholesterol and the mRNA level of HMG-CoA reductase in human keratinocytes.
In summary, we have shown that TGF-β1 induces cholesterol synthesis by increasing HMG-CoA reductase mRNA expression in human keratinocytes. Kawai et al.Citation13) demonstrated that adiponectin upregulated the gene expression of TGF-β1 in keratinocytes. A significant negative correlation exists between the serum adiponectin concentration and body fat mass, and adiponectin concentrations decrease after the consumption of a HF diet.Citation14,15) Additionally, high-fat diets reduced the level of type I tropocollagen and hyaluronan in the skin by suppressing the action of adiponectin.Citation8) Taken together, our results suggest that such dietary patterns may impair the skin barrier function and lead to skin dysfunction and various cutaneous diseases.
Authors contribution
TY and YO conceived and designed the study. AM and MS performed the experiments. TY wrote the paper. KK and YO reviewed and edited the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jack SM, Wood LC, Lauer S, et al. Effect of cutaneous permeability barrier disruption on HMG-CoA reductase, LDL receptor, and apolipoprotein E mRNA levels in the epidermis of hairless mice. J. Lipid. Res. 1992;33:1307–1314.
- Proksch E, Elias PM, Feingold KR. Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity in murine epidermis. Modulation of enzyme content and activation state by barrier requirements. J. Clin. Invest. 1990;85:874–882.10.1172/JCI114514
- Menon GK, Feingold KR, Moser AH, et al. De novo sterologenesis in the skin. II. Regulation by cutaneous barrier requirements. J. Lipid Res. 1985;26:418–427.
- Ghadially R, Brown BE, Hanley K, et al. Decreased epidermal lipid synthesis accounts for altered barrier function in aged mice. J. Invest. Dermatol. 1996;106:1064–1069.10.1111/1523-1747.ep12338692
- Zettersten EM, Ghadially R, Feingold KR, et al. Optimal ratios of topical stratum corneum lipids improve barrier recovery in chronologically aged skin. J. Am. Acad. Dermatol. 1997;37:403–408.10.1016/S0190-9622(97)70140-3
- Yamane T, Kobayashi-Hattori K, Oishi Y. A high-fat diet reduces ceramide synthesis by decreasing adiponectin levels and decreases lipid content by modulating HMG-CoA reductase and CPT-1 mRNA expression in the skin. Mol. Nutr. Food Res. 2011;55:S186–S192.10.1002/mnfr.v55.2s
- Kim MS, Song HJ, Lee SH, et al. Comparative study of various growth factors and cytokines on type I collagen and hyaluronan production in human dermal fibroblasts. J. Cosmet. Dermatol. 2014;13:44–51.10.1111/jocd.2014.13.issue-1
- Yamane T, Kobayashi-Hattori K, Oishi Y, et al. High-fat diet reduces levels of type I tropocollagen and hyaluronan in rat skin. Mol. Nutr. Food Res. 2010;54:S53–S61.10.1002/mnfr.v54.4s
- Folch J, Lees M. Sloane Stanley G H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509.
- Shi Y, Massagué J. Mechanisms of TGF-β Signaling from cell membrane to the nucleus. Cell. 2003;113:685–700.10.1016/S0092-8674(03)00432-X
- Leivonen SK, Kähäri VM. Transforming growth factor-β signaling in cancer invasion and metastasis. Int. J. Mol. Cancer. 2007;121:2119–2124.10.1002/(ISSN)1097-0215
- Yakymovych I, Ten Dijke P, Heldin CH, et al. Regulation of Smad signaling by protein kinase C. FASEB J. 2001;15:553–555.
- Kawai K, Kageyama A, Tsumano T, et al. Effects of adiponectin on growth and differentiation of human keratinocytes—Implication of impaired wound healing in diabetes. Biochem. Biophys. Res. Commun. 2008;374:269–273.10.1016/j.bbrc.2008.07.045
- Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941–946.10.1038/90984
- Akagiri S, Naito Y, Ichikawa H, et al. A mouse model of metabolic syndrome; Increase in visceral adipose tissue precedes the development of fatty liver and insulin resistance in high-fat diet-fed male KK/Ta mice. J. Clin. Biochem. Nutr. 2008;42:150–157.10.3164/jcbn.2008022
