Abstract
Light is a ubiquitous environmental factor serving as an energy source and external stimulus. Here, I review the conserved molecular mechanism of light-inducible production of carotenoids in three nonphototrophic bacteria: Streptomyces coelicolor A3(2), Thermus thermophilus HB27, and Bacillus megaterium QM B1551. A MerR family transcriptional regulator, LitR, commonly plays a central role in their light-inducible carotenoid production. Genetic and biochemical studies on LitR proteins revealed a conserved function: LitR in complex with adenosyl B12 (AdoB12) has a light-sensitive DNA-binding activity and thus suppresses the expression of the Crt biosynthesis gene cluster. The in vitro DNA-binding and transcription assays showed that the LitR–AdoB12 complex serves as a repressor allowing transcription initiation by RNA polymerase in response to illumination. The existence of novel light-inducible genes and the unique role of the megaplasmid were revealed by the transcriptomic analysis of T. thermophilus. The findings suggest that LitR is a general regulator responsible for the light-inducible carotenoid production in the phylogenetically divergent nonphototrophic bacteria, and that LitR performs diverse physiological functions in bacteria.
Graphical abstract
LitR in complex with AdoB12 represses the transcriptional initiation from carotenoid biosynthesis gene under dark condition. In contrast, the illuminated LitR–AdoB12 converts into inactive form, which allows RNA polymerase to transcribe the carotenoid genes.
Light is a general environmental factor that affects biological functions including photosynthesis, circadian rhythms, and secondary metabolite production, which are observed in various organisms including eukaryotes and phototrophic bacteria.Citation1,2) The molecular mechanism behind transmission of a light signal to gene expression machinery in the above organisms has been reported previously.Citation3−5) In contrast, little is known about the photoresponse phenomena and their regulatory mechanism in nonphototrophic bacteria because of the unavailability of light stimuliCitation2); however, the increased amount of genomic information on diverged bacterial genera revealed wide distribution of a homologous protein similar to the plant-type blue-light receptor LOV, which uses flavin as a light-sensing molecule.Citation1) The common presence of the LOV family suggests that the light-sensing and light response ability also generally occur in nonphototrophic bacteria.
Carotenoids are terpene compounds produced by a wide variety of organisms including plants,Citation6) fungi,Citation7) algae,Citation8) and bacteria.Citation9) Carotenoids have an antioxidant activity and thereby protect the cells from photo-oxidative damage by scavenging harmful agents such as singlet and triplet molecular species produced after illumination.Citation10) The light-inducible carotenoid production is a representative phenotype of the light response present in various organisms including not only plants, fungi, and phototrophic bacteria but also nonphototrophic bacteria.Citation11) Therefore, many organisms use carotenoids to protect the cells from sunlight-derived reactive oxygen species. Thus, induction of carotenoid production by light saves energy in the dark.
In nonphototrophic bacteria, carotenoid production is mainly classified into three types: (i) light-inducible,Citation12) (ii) constitutive,Citation13) and (iii) cryptic.Citation11,14,15) The light-inducible carotenoid production (the focus of this study) is observed in several bacterial species including the gliding Gram-negative bacterium Myxococcus xanthus and Gram-positive bacteria, Mycobacterium spp.Citation16,17) The regulatory mechanism in M. xanthus has been well-studied; two regulators play a central role in the light-inducible carotenoid production: the MerR family transcriptional regulator CarACitation18−20) and extracytoplasmic function (ECF)-type sigma factor CarQ.Citation21−23) Nevertheless, the light-sensing mechanism of this bacterium remained unclear when my colleagues and I started this research. With respect to the light-inducible carotenoid production in Mycobacterium marinum, the detailed molecular mechanism has not been elucidated yet although the involvement of a MarR family-type regulator was suggested.Citation17)
Our work is focused on the regulatory mechanism behind the light-inducible carotenoid production in nonphototrophic bacteria for the following reasons: we are interested in (i) how a light signal is transmitted to the level of gene expression machinery, and (ii) the molecular mechanism underlying the light-inducible carotenoid production, which is poorly understood. We believe that elucidation of the gene expression mechanism will provide new knowledge about bacterial responses to the environment and how bacteria are awoken by illumination or sunlight. This review highlights the current knowledge about the molecular mechanism of the light-inducible carotenoid production in nonphototrophic bacteria.
I. Light-inducible carotenoid production in Streptomyces coelicolor A3(2)
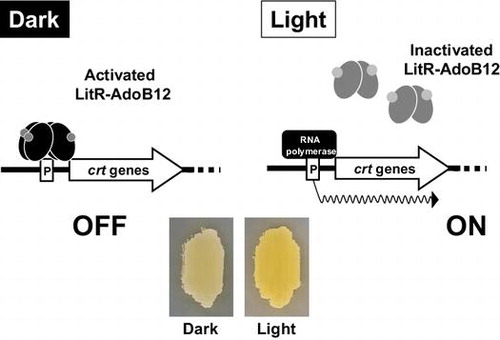
Streptomyces not only has the ability to produce a variety of secondary metabolites including bioactive compounds, such as antibiotics and antitumor drugs, but also has a complex life cycle similar to that of fungi.Citation24,25) Carotenoids are some of the secondary metabolites produced by many Streptomyces spp. and related bacteria.Citation26,27) We originally found that the carotenoid production in S. coelicolor A3(2), a model organism for genetic studies,Citation28) takes place in a light-responsive manner when we studied a strain that is deficient in two pigment antibiotics: actinorhodin and prodigiosin.Citation29) This strain had a yellow color when it was left on top of a desk for several days; this finding prompted us to assume that the pigmentation of S. coelicolor is caused by the illumination. We again incubated the strain on top of the desk and in the drawer of the desk. The strain that was incubated on top of the desk developed a yellow color, whereas the strain in the desk drawer formed white colonies. We assumed that this phenomenon occurs in response to illumination and recognized that carotenoid production had been hidden by the pigment antibiotics.
The putative crt biosynthesis gene cluster of S. coelicolor is composed of two convergent operons: crtEIBV and crtYTU (Fig. ). Our preliminary chemical analysis showed that the yellow pigment is isorenieratene, a carotenoid previously found in other actinomycetes such as S. griseus and Mycobacterium smegmatis. According to the results of transcriptional analysis, the light-induced phenomenon is regulated at the transcriptional level; the transcription of crtE and crtY, the first gene of the operon, was induced by illumination, whereas their transcription was extremely weak in the dark. These findings suggested that this bacterium retained a regulatory system for light signal transduction.
Fig. 1. The proposed regulatory mechanism of light-inducible carotenoid production in S. coelicolor A3(2).
Two transcriptional regulatory proteins are encoded in the flanking region of the crt biosynthesis gene cluster of S. coelicolor: (i) a MerR family transcriptional regulator (designated as LitR; light-inducible transcriptional regulator) and (ii) ECF-type sigma factor (designated as LitS). LitR, consisting of 330 amino acid residues, contains a DNA-binding domain at the N terminus and vitamin B12 (synonym cobalamin, Cbl)-binding domain at the C terminus. Generally, MerR family proteins serve as positive or negative regulators whose activity is regulated by the association with a ligand that is directly related to the genes that are regulated by the MerR regulator.Citation30,31) Therefore, it appears that Cbl serves as a ligand for LitR. In contrast, LitS is involved in the promoter recognition by the RNA polymerase holoenzyme.
Fig. depicts a hypothetical regulatory model of the light-inducible carotenoid production in S. coelicolor according to our transcriptional analyses. Such an analysis with S1 nuclease mapping showed that the transcription of crtE, crtY, and litS is induced by illumination, while the litR transcription is slightly increased by illumination. The knockout of litR caused constitutive expression of litR itself but abrogated the transcription of crtE, crtY, and litS. This result indicates that LitR represses the litR gene but enhances the expression of litS. Our experiments were hampered by the difficulties with preparation of an active recombinant LitR protein: biochemical analysis of the LitR protein is necessary for elucidation of its properties.
The litS knockout abrogated the light induction of the crtE and crtY genes but had no effect on the expression of litR or litS itself. This finding suggested that σLitS serves as a sigma factor directing the promoter recognition by crtE and crtY. We successfully reproduced the σLitS function by an in vitro runoff transcriptional assay: the RNA polymerase holoenzyme containing σLitS specifically directs the transcription initiated from the promoters preceding crtE and crtY. The alignment of these two promoters provided the consensus sequence recognized by σLitS, a possible consensus sequence, GCAT(G/C)-(16 or 17 bp)-C(G/C)(G/C)AA(G/C) in the region -35 to -10.
The central role of LitR and σLitS in the regulatory system was also supported by the genetic analysis involving a heterologous host, S. griseus, a streptomycin producer.Citation32) This bacterium is a suitable host because of its inability to produce carotenoids despite retaining the crt biosynthesis gene cluster on the chromosome.Citation14) The light-inducible transcription was monitored with a reporter plasmid, which carries litR, litS, and melanin biosynthesis genes melC1-melC2 fused with the light-inducible promoter. An S. griseus transformant carrying the reporter plasmid showed melanin (brown pigment) production during illumination, whereas melanin was not produced during culturing in the dark. The light-dependent melanin production was abrogated by the deletion of litR or litS from the reporter plasmid. This evidence points to two things: (i) the light-inducible transcription is activated only by two proteins including σLitS-RNA polymerase holoenzyme and LitR, and (ii) LitR is a transcriptional regulator with a photosensory function because illumination has no effect on the activity of the σLitS-RNA polymerase holoenzyme in the in vitro transcription assay.
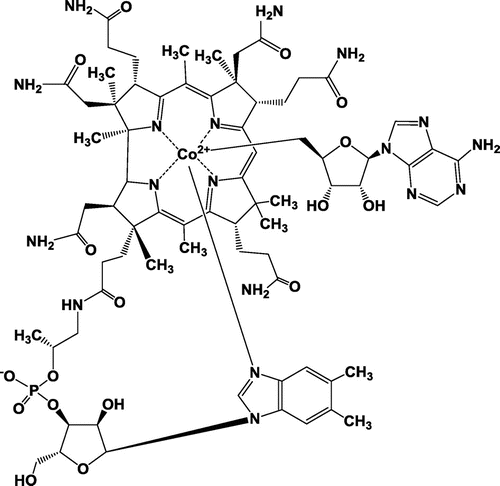
The existence of a putative B12-binding domain at the C terminus of LitR suggests that Cbl serves an antenna molecule that receives the light signal. Cbl is a cobalt (Co)-containing tetrapyrrole that absorbs blue and green light (Fig. ). Our gene disruption experiment revealed the involvement of Cbl in the regulatory mechanism: an S. coelicolor mutant deficient in cobDQN, which encodes enzymes involved in a later step of Cbl biosynthesis, showed constitutive carotenoid production at a low level, and the transcription of crt biosynthesis genes was weak, in line with the phenotype.Citation33) Addition of 0.1 μM MeCbl to the medium and genetic complementation both restored the ability to perform light-induced carotenogenesis in the mutant. This result shows that Cbl serves as a light-sensing cofactor of LitR by binding to the C-terminal domain. In addition, the mutation in cbl genes causes a defect in the morphological differentiation and in production of two pigment antibiotics in S. coelicolor, although the mutation mostly did not affect the viability in a rich culture medium.Citation34) The pleiotropic role of Cbl in the development of Streptomyces indicates that Cbl participates in primary metabolism and serves as a signaling compound involved in the signal transduction supporting the complex life cycle.
Fig. 2. Chemical structure of Adenosyl B12 (AdoB12).
A protein homologous to LitR is found in almost every Streptomyces spp. whose genome has been completely sequenced; however, ~60% of Streptomyces species do not produce carotenoids in a light-dependent manner (our unpublished observation). The same is true for Bacillus as described below. The inconsistency between retention of litR and the phenotype suggests that the role of LitR is not confined to the control of carotenoid production and indicates that LitR has diverse functions.
II. Wide distribution of litR homologs in nonphototrophic bacteria
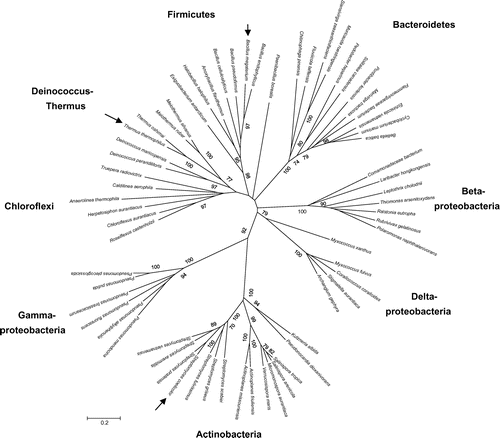
The genomic information on phylogenetically diverged bacteria is publicly available; hence, a comparative genomics approach is a powerful tool for the exploration of bacterial functions in silico.Citation35) Our genomic search revealed wide distribution of litR homologs in phylogenetically diverged bacterial genera including not only Gram-positive but also Gram-negative bacteria (Fig. ).Citation32) Approximately 30% of the bacterial genera retain the gene encoding a litR homolog in their genomes, including the bacteria studied in the field of applied microbiology, for example, Thermus, Bacillus, and Pseudomonas, and in clinical research, for example, P. aeruginosa and Burkholderia known as pathogens. The common presence of a LitR homolog indicates that nonphototrophic bacteria generally are equipped with the light-responsive system.
Fig. 3. The neighbor-joining phylogenetic tree based on amino acid sequence of the B12-binding domain of LitR homologs from Gram-positive and Gram-negative bacteria. Arrowheads indicate LitR proteins characterized in our study.
Photoresponse-related genes are located in close proximity to the litR homolog. These genes are crt biosynthesis gene cluster, phr, and bacteriorhodopsin in Thermus sp. CCB_US3_UF1Citation36) and LOV in Pseudomonas spp.Citation37) Phr is a DNA photolyase that repairs pyrimidine dimers (generated by UV) using light as an energy source. LOV is a homolog of a plant blue-light sensor, phototropin. Proteorhodopsin, a homolog of bacteriorhodopsin, associates with a retinal molecule and serves as a light-driven proton pump.Citation38,39) In contrast to the diversity of photoresponse-related proteins, the litR homolog is conserved. This situation indicates that LitR may serve as a light sensor to regulate their transcription after illumination.
The other interesting finding is multiplicity of LitR homologs in a single bacterial genome: three homologs in Pseudomonas spp., two homologs in Actinosynnema mirum, a nocardicin producerCitation39,40); three homologs in Kitasatospora setae,Citation41) a setamycin producer; and five homologs in Amycolatopsis mediterranei,Citation42) a rifamycin producer. LitR lies outside the crt biosynthesis operon or cluster; this situation is suggestive of the diversity of LitR functions. The bacteria retaining multiple litR homologs are mainly soil habitants; this finding suggests that litR genes provide an advantage for survival in the soil niche.
Of these litR-retaining bacteria, we selected Thermus thermophilus HB27Citation43,44) and Bacillus megaterium QM B1551Citation45,46) as representatives of Gram-negative and Gram-positive bacteria, respectively, because both strains play an important role in the field of applied microbiology. Our intensive studies on the regulatory mechanism are described below.
III. LitR from the Gram-negative bacterium T. thermophilus HB27
T. thermophilus HB27 is a high-GC Gram-negative thermophilic bacterium isolated from hot springs whose proteins have been used for the studies of enzyme properties and protein structure because of their high stability. This bacterium harbors a 1.85-Mb chromosome and a large (0.26-Mb) circular plasmid pTT27. The large plasmid pTT27 carries the genes of a LitR homolog and crt biosynthesis enzymes in an adjacent region.Citation47) The litR gene is a part of an operon structure with a gene encoding the cyclic AMP (cAMP) receptor protein (CRP)/fumarate and nitrate reduction regulator (FNR) family transcriptional regulator (designated as LdrP; light-dependent regulatory protein), whose function as a positive regulator is activated by the binding of cAMP.Citation48) The gene organization suggests that LdrP serves as a positive regulator like LitS in S. coelicolor.
T. thermophilus produces a yellow pigment called thermozeaxanthin, a zeaxanthin derivative (glycosylated).Citation49) The biosynthesis is known to be constitutive; however, our research based on the presence of a litR homolog in the genome of T. thermophilus HB27 revealed that this bacterium produces thermozeaxanthin in a light-responsive manner. It is likely that the light-dependent phenomena had been overlooked owing to the leaky regulation of expression of the corresponding genes. Our gene disruption experiment showed that crt genes, which are located far from litR, are responsible for the synthesis of thermozeaxanthin. The light-inducible carotenoid production was also observed in phylogenetically close bacteria belonging to the Thermus genus; these findings indicate that the capacity for light sensing is widespread within this genus.
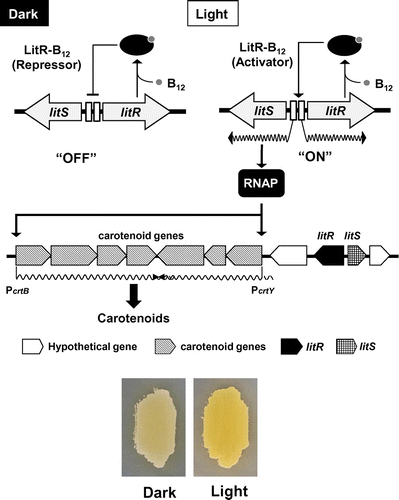
Fig. shows a proposed regulatory model of the light-inducible carotenoid production in T. thermophilus HB27. Our transcriptional analysis revealed that the light-inducible carotenoid production is regulated at the transcriptional level. Hence, the transcription of crtB encoding a phytoene synthase is remarkably induced by illumination, whereas the light induction does not occur in the dark. Genetic analysis of knockout mutants revealed that two tandem transcriptional regulator genes, litR and ldrP, play a central role in the light-responsive regulation. Disruption of ldrP causes a loss of crtB transcription but does not affect the transcription initiated from the litR promoter. This result leads to two conclusions: (i) LdrP is not involved in the expression of litR and ldrP itself and (ii) LdrP is an activator specific to crtB transcription. On the other hand, the litR knockout leads to constitutive transcription of crtB and litR itself indicating that LitR serves as a transcriptional repressor for both genes.
Fig. 4. The working model of the light-inducible carotenoid production in T. thermophilus HB27.
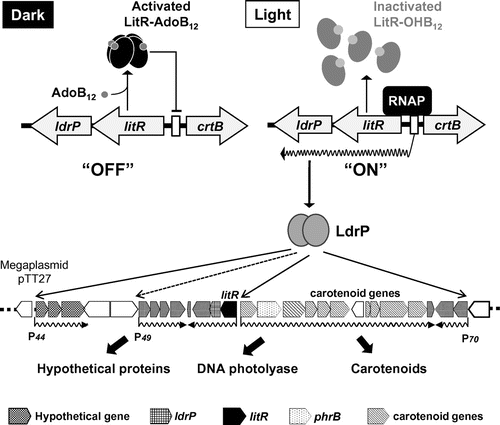
The function of the LdrP protein was successfully demonstrated with an in vitro runoff transcriptional assay: Thermus RNA polymerase holoenzyme specifically directs the crtB transcription in the presence of a recombinant LdrP protein but does not in the absence of LdrP. The CRP/FNR family proteins generally require cAMP to assume the active form; however, the transcriptional activation by LdrP occurs in the absence of cAMP. This result is supported by three-dimensional structure of LdrP reported by Agari et al.Citation50) this structure does not afford sufficient space for the incorporation of cAMP, and the structure of LdrP is similar to that of the Escherichia coli CRP–cAMP protein complex.Citation51) These findings suggest that LdrP is a cAMP-independent CRP/FNR family protein and is in the active form constitutively.
As described above, we identified the mechanism behind the transcriptional activation of crtB, but it is still unclear how the light induction of crtB transcripts takes place. The knockout of litR caused the constitutive transcription of crtB and litR, indicating that the fundamental function of LitR is a repressor that inhibits crtB transcription in the dark. Hence, we assumed that it was important to elucidate the mechanism underlying LitR inactivation by illumination. We used a recombinant LitR protein prepared in an E. coli expression system. In agreement with the fact that LitR retains a B12-binding domain at the C terminus, LitR has an ability to associate with B12 derivatives including hydroxocobalamin (OHB12), methyl Cbl, and 5′-deoxyadenosylcobalamin (AdoB12). Among these B12 derivatives, LitR that is associated with AdoB12 showed a strong and specific DNA-binding activity toward the intergenic promoter region of litR and crtE in the dark. On the other hand, the DNA-binding activity of LitR–AdoB12 during illumination drastically decreases.
In parallel with our study, Ortiz-Guerrero et al.Citation52) reported that a chimeric protein—CTt2 consisting of the N-terminal DNA-binding domain of CarH and the C-terminal Cbl-binding domain of LitR of T. thermophilus HB8—is a light-sensitive DNA-binding protein, according to the function of the ligand: AdoB12. Light causes photolysis of AdoB12 bound to CTt2, leading to the conversion of AdoB12 to OHB12 due to the chemical cleavage of the Co–C bond of AdoB12, which abrogates its DNA-binding activity. The photolysis of AdoB12 also induces a dramatic subunit dissociation of CTt2; the tetramer of CTt2–AdoB12 assumes monomeric structure in response to illumination. Furthermore, Díez et al.Citation53) reported that the full-length native protein TtCarH (a LitR homolog: a single-residue substitution) from T. thermophilus HB8 has a light-sensitive DNA-binding activity. These results clearly showed that LitR/CarH family proteins are novel coenzyme B12-based photosensors.
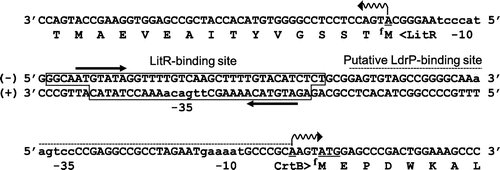
Our analyses provided detailed information on the main switch for the light-inducible transcription, which is located in the intergenic region between litR and crtB. As shown in Fig. , the LitR–AdoB12 complex directly binds to the promoter region -55 to -94 relative to the transcriptional start site of crtB. The putative sequences of the region -35 to -10 of litR promoter and crtB promoter are similar to the σA-dependent promoter of T. thermophilus. In contrast, LdrP activation requires the region -51 to + 1 relative to the transcriptional start site of crtB, which contains a potential binding sequence (AGTGT[N7]GCAAAA) that has partial similarity with the consensus [WWGTGA(N5–7)ACACWW] for the cAMP-independent CRP/FNR regulator of T. thermophilus. This finding suggests that the LitR–AdoB12 complex binds to a single site, regulating the bidirectional transcription of litR itself and crtB.
Fig. 5. The main switch for the light-inducible gene expression in Thermus thermophilus HB27.
IV. LitR of the Gram-positive bacterium Bacillus megaterium QM B1551
B. megaterium is a low-GC, endospore-forming Gram-negative soil bacterium.Citation45,46) The genetic research on the Bacillus genus has been extensive owing to the ability of these bacteria to form endospores and produce useful enzymes and secondary metabolites.Citation54) The carotenoid production in this genus has also been observed, and several chemical structures were elucidatedCitation55−57); however, there are no reports on the light responsiveness. During our screening of light-responsive soil-dwelling bacteria, we originally found that a number of strains of B. megaterium, Bacillus sphaericus, and Bacillus pumilus produce a carotenoid-like yellow pigment in response to illumination.Citation58) This phenomenon suggests that a light-responsive regulatory mechanism also exists in this genus.
The genome of B. megaterium QM B1551 retains a litR homolog (BMQ_4356) and a putative crt biosynthesis gene cluster consisting of two operons, crtI1-crtI2-crtB (BMQ_0654–0656) and BMQ_2952–2949. Unlike in S. coelicolor and T. thermophilus, the litR gene in Bacillus is located in a locus far from crt genes. LitR of B. megaterium QM B1551 consists of 303 amino acid residues, showing 28% amino acid sequence identity to LitR of T. thermophilus. Our preliminary genetic analysis using the knockout mutant revealed that (i) the LitR homolog negatively regulates light-inducible carotenoid production, (ii) a cbl knockout mutant causes constitutive and weak carotenoid production, and (iii) crtI1-crtI2-crtB represents the carotenoid biosynthesis genes. Our evidence suggests that the LitR homolog also plays a crucial role in the light-dependent carotenoid production in this bacterium, and this function depends on AdoB12. With respect to the chemical structure of the carotenoid produced by B. megaterium QM B1551, we were unable to identify this compound owing to its high light sensitivity, although the litR mutation results in 10-fold greater amounts of the carotenoid compared with the wild type.
Our biochemical study revealed that the Bacillus LitR recombinant protein has many properties similar to those of T. thermophilus: (i) LitR associates with AdoB12, (ii) AdoB12–LitR is a photosensitive DNA-binding protein, (iii) LitR–AdoB12 directly binds to the promoter region of crt biosynthesis genes and litR itself, (iv) LitR–AdoB12 serves as a transcriptional repressor, (v) LitR–AdoB12 forms a homo-oligomer, and (iv) LitR–AdoB12 undergoes subunit dissociation in response to illumination. The characteristic of this LitR that is different is the subunit structure: the tetramer of Bacillus LitR–AdoB12 dissociates into dimers, whereas the tetramer of Thermus LitR–AdoB12 dissociates into monomers. This result was confirmed by two independent analytical methods: gel filtration chromatography and analytical ultracentrifugation.
Fig. represents a working model illustrating the molecular mechanism underlying light-inducible carotenoid production in B. megaterium QM B1551 according to our genetic and biochemical analysis. AdoB12-LitR (recombinant protein) specifically binds to the promoter region of crt and litR, repressing their transcription in the dark. Exposure to light transforms the LitR–AdoB12 complex to the inactive form, and this event leads to dissociation from the target promoter. Subsequently, the RNA polymerase holoenzyme directs transcription of carotenoid biosynthesis genes, and finally, the expressed crt enzymes catalyze carotenoid biosynthesis. Our analysis also revealed detailed promoter structures preceding the crtI1 and litR genes. The DNA site bound by LitR was determined by DNase I footprint analysis: for the litR promoter, the region -36 to -67 of the sense strand and region -27 to -69 of the antisense strand relative to the transcriptional start site; for crtI1 promoter: the region -13 to + 22 of the sense strand and region -15 to + 18 of the antisense strand. Alignment of two LitR-binding sequences yields a definitive consensus sequence, 5′-TG(T/A)A(C/T)A-N16-T(G/A)TA(C/T)A-3′. Determination of the transcriptional start point showed that the regions -10 and -35 of crt and litR promoters are similar to that of the promoter of σA, an essential sigma factor.
Fig. 6. The working model of light-inducible carotenoid production in B. megaterium QM B1551.
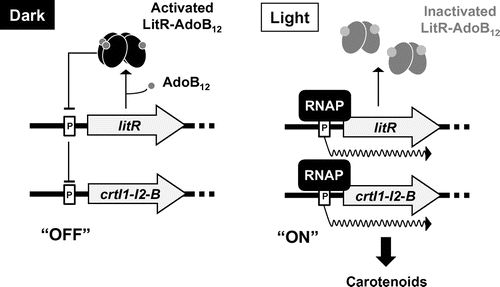
The proposed regulatory model of light-inducible carotenoid production in B. megaterium seems to be simpler than that of other bacteria such as S. coelicolor and T. thermophilus. This is because a gene encoding a transcriptional regulator or sigma factor is not located in the region adjacent to litR. The simplicity of the light-inducible regulatory mechanism in B. megaterium was demonstrated by two experiments: (i) in vivo genetic analysis of Bacillus subtilis 168 and (ii) an in vitro runoff transcriptional assay involving minimal components. In the in vivo analysis of B. subtilis 168, a heterologous host that lacks a litR homolog and crt genes, the B. subtilis transformant retaining the litR gene showed light-inducible transcription. This result indicates that LitR can be responsible for the light dependence of the crt transcription without other regulatory proteins. Subsequently, an in vitro runoff transcriptional assay reproduced the light-dependent transcription observed in vivo. The presence of a non-illuminated LitR–AdoB12 complex strongly and specifically inhibits the mRNA synthesis that starts from the crt promoter and is directed by the σA-containing RNA polymerase holoenzyme. Meanwhile, the illuminated LitR–AdoB12 allows the σA-RNA polymerase to synthesize mRNA. This is the first in vitro evidence showing that a member of the LitR/CarH family, LitR-AdoB12, serves as a light-sensitive repressor.
The in vitro runoff assay also provided information on the light wavelength and type of Cbl required for the LitR repressor activity. Among Cbl derivatives, AdoB12 was the only Cbl that conferred the repressor activity on LitR. In contrast, light wavelengths of 365, 450, and 530 nm inhibit the LitR-repressor activity, whereas illumination at 633 nm does not affect the activity. The light wavelengths that inactivate AdoB12–LitR activity are consistent with the absorption spectrum of AdoB12. Collectively, these results show that the AdoB12–LitR complex serves as a blue and green light-sensitive repressor.
V. Light-inducible gene cluster of T. thermophilus HB27
To elucidate the diverse functions of LitR, we performed transcriptomic analysis in T. thermophilus and identified 19 LitR-dependent genes that showed 2.0- to 27.5-fold greater transcriptional activity in the litR mutant than in the wild-type strain in the dark.Citation59) As shown in Fig. , LitR-dependent genes that are clustered around litR itself form four putative operons. The genes encoding a protein with known functions are the five following genes: a methyltransferase (TT_P0045), the α/β-hydrolase fold protein (TT_P0046), short-chain dehydrogenases/reductases (TT_P0049), flavin-containing oxidoreductase (TT_P0050), and YceI-like protein from E. coli (TT_P0051). The other products of the light-inducible gene are hypothetical proteins; therefore, the relations of these gene products with light are not known. Therefore, these genes may be novel light-inducible genes. The 30% genes retained by the megaplasmid encode photoresponse-related proteins; 45 of 251 genes are litR, ldrP, crt genes, a B12 biosynthesis gene cluster, and the aforementioned novel light-inducible genes. This finding suggests that the major role of the megaplasmid is to protect the cell from light stress.
Our transcription experiments in vivo and in vitro demonstrated that a recombinant LdrP protein directly activates the crtB promoter and TT_P0044 promoter (P44 in Fig. ). We also identified the minimal region (in the promoter) that is required for the transcriptional activation by LdrP in this same assay. The nucleotide sequence alignment and the mutation analysis of conserved nucleotides yielded a predicted consensus sequence: 5′-(A/G)(T/C)(T/G)TNGCCN(T/G)NG(G/C)NNA-3′, for the LdrP recognition site. The present evidence indicates that the light-dependently expressed LdrP, which is regulated by LitR, serves as a master switch for the set of light-inducible genes.
VI. Concluding remarks and future perspectives
Our research revealed that LitR serves as a general photosensory transcriptional regulator in the light-inducible carotenoid production of nonphototrophic bacteria including not only Gram-negative but also Gram-positive bacteria. The wide distribution of litR among bacterial species also indicates that the light stress response during illumination is essential for survival in the harsh soil environment. A major lesson from our study is that light as a general environmental factor can activate a dormant gene responsible for a useful bacterial function. Our research identified novel light-inducible genes in the above bacteria; this means that the LitR family has diverse functions. The progress of our research may uncover a novel light-sensing mechanism and its diversity among bacterial species. This research is also expected to elucidate the interaction of bacteria and sunlight based on the wide distribution and divergent functions of LitR and its homologs.
Funding
This study was partly supported by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology and by various other grants mentioned in the related publications.
Acknowledgments
I am most grateful to Prof. Emeritus Teruhiko Beppu and Prof. Kenji Ueda for their continuing encouragement, helpful suggestions, and considerable support of this research. I would like to acknowledge constant help and suggestions from late Prof. Sueharu Horinouchi and Prof. Yasuo Ohnishi (The University of Tokyo). I am deeply indebted to Prof. Haruo Ikeda (Kitasato University), Dr. Akeo Shinkai, Prof. Hideaki Nojiri, and Prof. Akira Nakamura for helpful discussions and for teaching me many valuable experimental methods. I would like to express my appreciation for support during this work to all members of the Laboratory of Biotechnology, Life Science Research Center, College of Bioresource Sciences, Nihon University. I thank H. Takano and S. Sumi for construction of the phylogenetic tree.
Disclosure statement
No potential conflict of interest was reported by the author.
Notes
This review was written in response to the author’s receipt of the JSBBA Award for Young Scientists in 2015.
References
- Herrou J, Crosson S. Function, structure and mechanism of bacterial photosensory LOV proteins. Nat. Rev. Microbiol. 2011;9:713–723.10.1038/nrmicro2622
- Purcell EB, Crosson S. Photoregulation in prokaryotes. Curr. Opin. Microbiol. 2008;11:168–178.10.1016/j.mib.2008.02.014
- Van der Horst MA, Hellingwerf KJ. Photoreceptor proteins, “star actors of modern times”: a review of the functional dynamics in the structure of representative members of six different photoreceptor families. Acc. Chem. Res. 2004;37:13–20.10.1021/ar020219d
- Falciatore A, Bowler C. The evolution and function of blue and red light photoreceptors. Curr. Top. Dev. Biol. 2005;68:317–350.
- Herrera-Estrella A, Horwitz BA. Looking through the eyes of fungi: molecular genetics of photoreception. Mol. Microbiol. 2007;64:5–15.10.1111/mmi.2007.64.issue-1
- Nisar N, Li L, Lu S, et al. Carotenoid metabolism in plants. Mol. Plant. 2015;8:68–82.10.1016/j.molp.2014.12.007
- Avalos J. Biological roles of fungal carotenoids. Curr. Genet. 2015;61:309–324.10.1007/s00294-014-0454-x
- Takaichi S. Carotenoids in algae: distributions, biosyntheses and functions. Mar. Drugs. 2011;9:1101–1118.10.3390/md9061101
- Vachali P, Bhosale P, Bernstein PS. Microbial carotenoids. Methods Mol. Biol. 2012;898:41–59.10.1007/978-1-61779-918-1
- Edge R, McGarvey DJ, Truscott TG. The carotenoids as anti-oxidants–a review. J. Photochem. Photobiol. B. 1997;41:189–200.10.1016/S1011-1344(97)00092-4
- Takano H, Asker D, Beppu T, et al. Genetic control for light-induced carotenoid production in non-phototrophic bacteria. J. Ind. Microbiol. Biotechnol. 2006;33:88–93.10.1007/s10295-005-0005-z
- Koyama Y, Kato F, Yazawa Y. Effect of light on the pigmentation of bacteria in Actinomycetales. In: Arai T, editor. Actinomycetales, the boundary microorganisms. Tokyo: Toppan; 1976. p. 65–85.
- Netzer R, Stafsnes MH, Andreassen T, et al. Biosynthetic pathway for γ-cyclic sarcinaxanthin in Micrococcus luteus: heterologous expression and evidence for diverse and multiple catalytic functions of C(50) carotenoid cyclases. J. Bacteriol. 2010;192:5688–5699.10.1128/JB.00724-10
- Lee HS, Ohnishi Y, Horinouchi S. A sigmaB-like factor responsible for carotenoid biosynthesis in Streptomyces griseus. J. Mol. Microbiol. Biotechnol. 2001;3:95–101.
- Kato F, Hino T, Nakaji A, et al. Carotenoid synthesis in Streptomyces setonii ISP5395 is induced by the gene crtS, whose product is similar to a sigma factor. Mol. Gen. Genet. 1995;247:387–390.10.1007/BF00293207
- Ramakrishnan L, Tran H, Federspiel N, et al. A crtB homolog essential for photochromogenicity in Mycobacterium marinum: isolation, characterization, and gene disruption via homologous recombination. J. Bacteriol. 1997;179:5862–5868.
- Gao L, Groger R, Cox J, et al. Transposon mutagenesis of Mycobacterium marinum identifies a locus linking pigmentation and intracellular survival. Infect. Immun. 2003;71:922–929.10.1128/IAI.71.2.922-929.2003
- López-Rubio JJ, Padmanabhan S, Lázaro JM, et al. Operator design and mechanism for CarA repressor-mediated down-regulation of the photoinducible CarB operon in Myxococcus xanthus. J. Biol. Chem. 2004;279:28945–28953.10.1074/jbc.M403459200
- Pérez-Marín MC, López-Rubio JJ, Murillo FJ, et al. The N terminus of Myxococcus xanthus CarA repressor is an autonomously folding domain that mediates physical and functional interactions with both operator dna and antirepressor protein. J. Biol. Chem. 2004;279:33093–33103.10.1074/jbc.M405225200
- León E, Navarro-Avilés G, Santiveri CM, et al. A bacterial antirepressor with SH3 domain topology mimics operator DNA in sequestering the repressor DNA recognition helix. Nucleic Acids Res. 2010;38:5226–5241.10.1093/nar/gkq277
- Whitworth DE, Bryan SJ, Berry AE, et al. Genetic dissection of the light-inducible carqrs promoter region of Myxococcus xanthus. J. Bacteriol. 2004;186:7836–7846.10.1128/JB.186.23.7836-7846.2004
- Browning DF, Whitworth DE, Hodgson DA. Light-induced carotenogenesis in Myxococcus xanthus: functional characterization of the ECF sigma factor CarQ and antisigma factor CarR. Mol. Microbiol. 2003;48:237–251.10.1046/j.1365-2958.2003.03431.x
- Gorham HC, McGowan SJ, Robson PR, et al. Light-induced carotenogenesis in Myxococcus xanthus: light-dependent membrane sequestration of ECF sigma factor CarQ by anti-sigma factor CarR. Mol. Microbiol. 1996;19:171–186.10.1046/j.1365-2958.1996.360888.x
- Chandra G, Chater KF. Developmental biology of Streptomyces from the perspective of 100 actinobacterial genome sequences. FEMS Microbiol. Rev. 2013;38:345–379.
- Liu G, Chater KF, Chandra G, et al. Molecular regulation of antibiotic biosynthesis in streptomyces. Microbiol. Mol. Biol. Rev. 2013;77:112–143.10.1128/MMBR.00054-12
- Houssaini-Iraqui M, Lazraq MH, Clavel-Sérès S, et al. Cloning and expression of Mycobacterium aurum carotenogenesis genes in Mycobacterium smegmatis. FEMS Microbiol. Lett. 1992;90:239–244.10.1111/fml.1992.90.issue-3
- Krügel H, Krubasik P, Weber K, et al. Functional analysis of genes from Streptomyces griseus involved in the synthesis of isorenieratene, a carotenoid with aromatic end groups, revealed a novel type of carotenoid desaturase. Biochim. Biophys. Acta. 1999;1439:57–64.10.1016/S1388-1981(99)00075-X
- Bentley SD, Chater KF, Cerdeno-Tarraga AM, et al. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature. 2002;417:141–147.10.1038/417141a
- Takano H, Obitsu S, Beppu T, et al. Light-induced carotenogenesis in Streptomyces coelicolor a3(2): identification of an extracytoplasmic function sigma factor that directs photodependent transcription of the carotenoid biosynthesis gene cluster. J. Bacteriol. 2005;187:1825–1832.10.1128/JB.187.5.1825-1832.2005
- Brown NL, Stoyanov JV, Kidd SP, et al. The MerR family of transcriptional regulators. FEMS Microbiol. Rev. 2003;27:145–163.10.1016/S0168-6445(03)00051-2
- Hobman JL. MerR family transcription activators: similar designs, different specificities. Mol. Microbiol. 2007;63:1275–1278.10.1111/mmi.2007.63.issue-5
- Takano H, Beppu T, Ueda K. The CarA/LitR-family transcriptional regulator: Its possible role as a photosensor and wide distribution in non-phototrophic bacteria. Biosci. Biotechnol. Biochem. 2006;70:2320–2324.10.1271/bbb.60230
- Takano H, Hagiwara K, Ueda K. Fundamental role of cobalamin biosynthesis in the developmental growth of Streptomyces coelicolor A3 (2). Appl. Microbiol. Biotechnol. 2015;99:2329–2337.10.1007/s00253-014-6325-z
- Takano H, Nishiyama T, Amano SI, et al. Streptomyces metabolites in divergent microbial interactions. J. Ind. Microbiol. Biotechnol. 2016;43:143–148.10.1007/s10295-015-1680-z
- Medema MH, Fischbach MA. Computational approaches to natural product discovery. Nat. Chem. Biol. 2015;11:639–648.10.1038/nchembio.1884
- Teh BS, Lau NS, Ng FL, et al. Complete genome sequence of the thermophilic Thermus sp. CCB_US3_UF1 from a hot spring in Malaysia. Stand. Genomic Sci. 2015;10:289.10.1186/s40793-015-0053-6
- Jentzsch K, Wirtz A, Circolone F, et al. Mutual exchange of kinetic properties by extended mutagenesis in two short LOV domain proteins from Pseudomonas putida. Biochemistry. 2009;48:10321–10333.10.1021/bi901115z
- Bamann C, Bamberg E, Wachtveitl J, et al. Proteorhodopsin. Biochim. Biophys. Acta. 1837;2014:614–625.
- Brown LS. Eubacterial rhodopsins — unique photosensors and diverse ion pumps. Biochim. Biophys. Acta. 2014;1837:553–561.10.1016/j.bbabio.2013.05.006
- Land M, Lapidus A, Mayilraj S, et al. Complete genome sequence of Actinosynnema mirum type strain (101T). Stand. Genomic Sci. 2009;1:46–53.10.4056/sigs.21137
- Ichikawa N, Oguchi A, Ikeda H, et al. Genome sequence of Kitasatospora setae nbrc 14216t: an evolutionary snapshot of the family Streptomycetaceae. DNA Res. 2010;17:393–406.10.1093/dnares/dsq026
- Zhao W, Zhong Y, Yuan H, et al. Complete genome sequence of the rifamycin SV-producing Amycolatopsis mediterranei U32 revealed its genetic characteristics in phylogeny and metabolism. Cell Res. 2010;20:1096–1108.10.1038/cr.2010.87
- Henne A, Brüggemann H, Raasch C, et al. The genome sequence of the extreme thermophile Thermus thermophilus. Nat. Biotechnol. 2004;22:547–553.10.1038/nbt956
- Brüggemann H, Chen C. Comparative genomics of Thermus thermophilus: plasticity of the megaplasmid and its contribution to a thermophilic lifestyle. J. Biotechnol. 2006;124:654–661.10.1016/j.jbiotec.2006.03.043
- Eppinger M, Bunk B, Johns MA, et al. Genome sequences of the biotechnologically important Bacillus megaterium strains QM B1551 and DSM319. J. Bacteriol. 2011;193:4199–4213.10.1128/JB.00449-11
- Bunk B, Schulz A, Stammen S, et al. A short story about a big magic bug. Bioeng Bugs. 2010;1:85–91.10.4161/bbug.1.2.11101
- Takano H, Kondo M, Usui N, et al. Involvement of CarA/LitR and CRP/FNR family transcriptional regulators in light-induced carotenoid production in Thermus thermophilus. J. Bacteriol. 2011;193:2451–2459.10.1128/JB.01125-10
- Green J, Scott C, Guest JR. Functional versatility in the CRP-FNR superfamily of transcription factors: FNR and FLP. Adv. Microb. Physiol. 2001;44:1–34.10.1016/S0065-2911(01)44010-0
- Yokoyama A, Shizuri Y, Hoshino T, et al. Thermocryptoxanthins: novel intermediates in the carotenoid biosynthetic pathway of Thermus thermophilus. Arch. Microbiol. 1996;165:342–345.10.1007/s002030050336
- Agari Y, Kuramitsu S, Shinkai A. X-ray crystal structure of TTHB099, a CRP/FNR superfamily transcriptional regulator from Thermus thermophilus HB8, reveals a DNA-binding protein with no required allosteric effector molecule. Proteins. 2012;80:1490–1494.10.1002/prot.24049
- Popovych N, Tzeng SR, Tonelli M, et al. Structural basis for cAMP-mediated allosteric control of the catabolite activator protein. Proc. Natl. Acad. Sci. USA. 2009;106:6927–6932.10.1073/pnas.0900595106
- Ortiz-Guerrero JM, Polanco MC, Murillo FJ, et al. Light-dependent gene regulation by a coenzyme B12-based photoreceptor. Proc. Natl. Acad. Sci. USA. 2011;108:7565–7570.10.1073/pnas.1018972108
- Díez AI, Ortiz-Guerrero JM, Ortega A, et al. Analytical ultracentrifugation studies of oligomerization and DNA-binding of TtCarH, a Thermus thermophilus coenzyme B12-based photosensory regulator. Eur. Biophys. J. 2013;42:463–476.10.1007/s00249-013-0897-x
- Mondol MA, Shin HJ, Islam MT. Diversity of secondary metabolites from marine bacillus species: chemistry and biological activity. Mar. Drugs. 2013;11:2846–2872.10.3390/md11082846
- Maeda I. Genetic modification in Bacillus subtilis for production of C30 carotenoids. Methods Mol. Biol. 2012;892:197–205.10.1007/978-1-61779-879-5
- Steiger S, Perez-Fons L, Fraser PD, et al. Biosynthesis of a novel C30 carotenoid in Bacillus firmus isolates. J. Appl. Microbiol. 2012;113:888–895.10.1111/jam.2012.113.issue-4
- Khaneja R, Perez-Fons L, Fakhry S, et al. Carotenoids found in Bacillus. J. Appl .Microbiol. 2010;108:1889–1902.
- Takano H, Mise K, Hagiwara K, et al. Role and function of LitR, an Adenosyl B 12 -bound light-sensitive regulator of bacillus megaterium QM b1551, in regulation of carotenoid production. J. Bacteriol. 2015;197:2301–2315.10.1128/JB.02528-14
- Takano H, Agari Y, Hagiwara K, et al. LdrP, a cAMP receptor protein/FNR family transcriptional regulator, serves as a positive regulator for the light-inducible gene cluster in the megaplasmid of Thermus thermophilus. Microbiology. 2014;160:2650–2660.10.1099/mic.0.082263-0
- Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739.10.1093/molbev/msr121
