Abstract
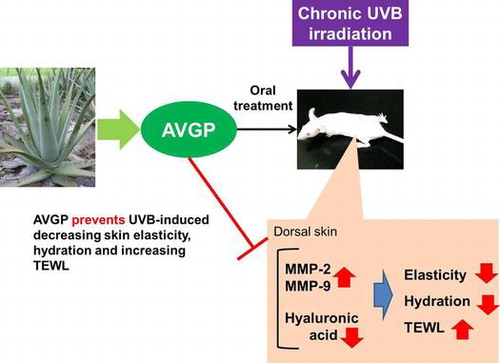
This study reports the effects of oral Aloe vera gel powder (AVGP) containing Aloe sterols on skin elasticity and the extracellular matrix in ultraviolet B (UVB)-irradiated hairless mice. Ten-week-old hairless mice were fed diets containing 0.3% AVGP for 8 weeks and irradiated UVB for 6 weeks. Mice treated with AVGP showed significant prevention of the UVB-induced decrease in skin elasticity. To investigate the mechanism underlying this suppression of skin elasticity loss, we measured the expression of matrix metalloproteinase (MMP)-2, -9, and -13. AVGP prevented both the UVB-induced increases in MMPs expressions. Moreover, we investigated hyaluronic acid (HA) content of mice dorsal skin and gene expression of HA synthase-2 (Has2). In the results, AVGP oral administration prevented UVB-induced decreasing in skin HA content and Has2 expression and attenuates the UVB-induced decrease in serum adiponectin, which promotes Has2 expression. These results suggested that AVGP has the ability to prevent the skin photoaging.
Graphical abstract
AVGP has the potent anti-photoaging activity via suppressing UVB-induced overexpression of MMPs and loss of hyaluronic acid.
The dermis is located under the epidermis, and contains fibroblasts and extracellular matrix (ECM), such as collagen, elastin fibers, fibronectin, hyaluronic acid (HA), and proteoglycans.Citation1,2) These components are produced and secreted from fibroblasts into the extracellular space.Citation2,3) Collagen is the main protein comprising the ECM in dermal tissue, accounting for up to 70% of the dry skin mass.Citation4) Collagen forms the fibrillar structures in dermal tissue, and these are responsible for providing strength and support to the human skin. Elastin fibers are mainly composed of fibronectin and elastin, with a core of cross-linked elastin covered by a layer of fibrillin-rich microfibrils. Elastin fibers comprise approximately 1–2% of the dermal mass, but play an important role in resisting deformational forces and maintaining skin elasticity, by holding the dermal fiber structures in place.Citation5) HA fills the majority of the extracellular space within the tissue.Citation5) These ECM components play a critical role in maintaining the skin integrity and architecture. Therefore, it is thought that changes in the skin, such as the formation of wrinkles, loss of elasticity, sagging, or constitutive dryness, are closely associated with damage to the ECM proteins due to aging.Citation3–5)
Exposure to UV radiation leads to serious effects on dermal tissues, such as formation of wrinkles, loss of skin elasticity, and thickened epithelium; these signs are collectively called cutaneous photoaging.Citation6,7)
Ultraviolet B irradiation to the skin induces the release of proinflammatory factors from epidermal keratinocytes and dermal fibroblasts.Citation8,9) These proinflammatory factors induce the expression of matrix metalloproteinase (MMP)s.Citation10–12) MMPs are a large family of calcium-dependent zinc-containing endopeptidases that are responsible for tissue remodeling and degradation of ECM components, including collagens, elastins, gelatin, and matrix glycoproteins.Citation13) MMP-1 and MMP-13, also known as collagenases, initiate the degradation of collagen fibers by unwinding the triple helix structure and digesting the peptide bonds.Citation13) After digestion, gelatinases such as MMP-2 and MMP-9 further degrade the collagen fragments generated by the collagenases. Furthermore, MMP-2 and MMP-9 also degrade type IV collagen, a constituent of the basal membrane and elastin.Citation13) MMPs are precisely regulated at the level of transcription, and tight regulation of ECM degradation is normally maintained. However, loss of regulation due to a variety of stresses, including UV irradiation, may result in the excessive degradation of the ECM.Citation12)
Aloe barbadensis Miller (Aloe vera) is a species of succulent plant that belongs to the lily family, Liliaceae. Aloe vera is one of the most well-known plants in the world, and has been widely used for centuries.Citation14–16) It is commonly known for its topical use for treating wounds and burns.Citation17) Many studies have reported that topical treatment of Aloe vera gel facilitates wound healingCitation14–17) and promotes collagen synthesis.Citation18) However, there is little knowledge on the effect of Aloe vera ingestion on skin tissue. In previous studies, we have reported that Aloe vera gel administration improved skin dryness and facial wrinkles in a clinical trial.Citation19) In the present report, we investigated the effects of oral administration of Aloe vera gel powder (AVGP) on UV-induced reduction in skin elasticity and dermal extracellular matrix damage in UVB-irradiated hairless mouse skin.
Materials and methods
Preparation of Aloe vera gel powder and animal diets
Dried AVGP was obtained from Aloe vera gel from skinned Aloe leaves by hot-air drying. One gram of AVGP contained approximately 80 μg Aloe sterols, determined by liquid chromatography–tandem mass spectrometry analysis.Citation20) For oral administration, dry AVGP was mixed into the AIN93G mouse chow by Oriental Yeast (Tokyo Japan), the usual supplier of mouse chow for our investigations.
Animals
All animal experiments were conducted with approval of the Animal Research Committee of Morinaga Milk Industry. Nine-week-old female Hos:HR-1 hairless mice were purchased from Japan SLC Inc. (Shizuoka, Japan). The mice were housed in a room with controlled temperature (21–25 °C), humidity (45–55%), and lighting (on at 8 am and off at 8 pm). The mice were fed a normal laboratory diet (AIN93G; Oriental Yeast, Tokyo, Japan) for 1 week to stabilize their metabolic condition. When 10-weeks old, the hairless mice were divided into three groups from the same pool, according to their weight and skin hydration: group 1, UVB irradiation and control diet (UVB (+) control); group 2, UVB irradiation and AVGP-containing diet (UVB (+) AVGP); group 3, no UV treatment and control diet (UVB (−) control). Mice were housed in stainless steel cages (4/cage) under the same condition.
Treatment and UVB irradiation
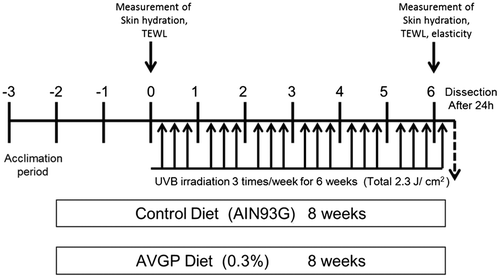
The experimental schedule is shown in Fig. . At 10-weeks old, the mice were started to feed each experimental diet and continued during experimental period. Two weeks after the dietary feeding of AVGP (at 12-weeks old), UVB irradiation was begun, and the hairless mice were dorsally irradiated by UVB three times per week. UVB was supplied by three UVB lamps emitting continues spectrum from 270 to 390 nm, with peak emission at 306 nm (GL20SE; Sankyo Denki, Kanagawa, Japan). The UVB emission was monitored with the Dermaray UV meter DMR-UV-M-2 (Terumo clinical supply Co. Ltd., Gifu, Japan). During UVB irradiation, mice were held in specially designed cages to allow free movement around the cage. The initial dose of UVB was set at 48 mJ/cm2, and was gradually increased every other day of irradiation, for a total dose of 2.3 J/ cm2 over a period of 6 weeks (3rd and 4th days of irradiation: 67 mJ/cm2, 5th and 6th: 86 mJ/cm2, 7th and 8th: 112 mJ/cm2, 9th and 10th: 133 mJ/cm2, 11th and 12th: 156 mJ/cm2, 13th and 14th: 165 mJ/cm2, 15th and 16th: 184 mJ/cm2, 17th and 18th: 200 mJ/cm2). A non-irradiated group of animals was included as a UVB (−) control.
Fig. 1. Experimental schedule. Skin hydration and TEWL were measured at the points of 0 week and 6 weeks.
Twenty-four hours after the last UVB exposure, hairless mouse were euthanized under sevoflurane 24 h after. Blood samples were obtained by cardiac puncture, and serum was prepared by centrifugation of blood at 1000 × g for 10 min at 4 °C. A sample of the skin was isolated and frozen immediately in liquid nitrogen. For histological analysis, some of skin biopsies were fixed in 10% buffered formalin.
Skin hydration and skin TEWL
Skin hydration of the dorsal skin was assessed using a Corneometer CM825 (Courage and Khazaka, Germany) according to the manufacturer’s protocol. Transepidermal water loss (TEWL: g/cm2/hour) was used as a marker of epidermal skin barrier function, and was measured in the dorsal skin using a Tewameter TM300 (Courage and Khazaka, Germany). Both skin hydration and skin TEWL were measured at the points before the UVB irradiation starting and 6 weeks after UVB exposure.
Skin elasticity
A Cutometer Dual MPA580 (Courage & Khazaka, Germany) was utilized for evaluation of the physical properties of the dorsal skin. The instrument was used at a reduced pressure of 200 mbar, with 2 s of suction followed by 1 s of relaxation on the mouse’s skin, with 10 repetitions. Parameters measured were immediate distention (Ue), delayed dictation (Uv), immediate (Uf) and final (Ua) retraction after suction removal. Cutometer-specific R values were determined on these parameters. R2 (Ua/Uf) is overall skin elasticity, R6 (Uv/Ue) is skin viscosity. The skin fatigue area parameter, F3, was analyzed using the Cutometer software, and evaluated as previously described.Citation21) Skin elasticity was measured at the point 6 weeks after UVB exposure.
Histological analysis
Fixed skin tissue was embedded in paraffin blocks, and 3 μm sections were cut. The skin sections were deparaffinized and stained with hematoxylin–eosin (H&E). An image acquisition and analysis system (Olympus, Tokyo, Japan), incorporating an Olympus microscope, was used to capture and analyze the H&E-stained sample at 100 × and 200 × magnification.
Measurement of type I collagen
Type I collagen in the extract from skin samples was quantified with Mouse Type I Collagen Detection Kit (Chondrex, Inc., Redmond, WA, USA). A skin sample was frozen in liquid nitrogen and crushed into a powder using a multibeads shocker (Yasui Kikai, Osaka, Japan). The skin tissue powder treated with pepsin and elastase digestion to measure type I collagen content, according to the manufacturer’s protocol. These levels were determined using a microplate reader (SH-9000, Corona electric, Ibaraki, Japan).
Western blotting
A sample of the skin was frozen in liquid nitrogen and crushed into a powder using a multibead shocker. The skin tissue powder was dissolved into CelLytic MT (Sigma–Aldrich Japan, Japan), with protease inhibitor cocktail. Skin extract was prepared by centrifugation at 8000 × g, at 4 °C for 10 min, and the supernatant was kept for further experiments. Sample protein concentrations were determined by a BCA protein assay kit (Thermo Fisher Scientific Inc., Waltham, MA, USA). A mixture of equal parts of protein sample and NuPAGE LDS sample buffer in the presence of NuPAGE Sample Reducing Agent (Thermo Fisher Scientific Inc.) was subjected to 10% Bis-Tris-PAGE (Thermo Fisher Scientific Inc.). Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad Trans-Blot Turbo Transfer Pack, 0.22 μM PVDF) using a transfer system (Bio-Rad Trans-Blot Turbo Transfer System). The membranes were blocked for one hour at room temperature in Blocking One (Nakarai, Kyoto, Japan). The membranes were then incubated overnight (approximately 16 h) at 4 °C with the primary antibodies: anti-MMP-2 (Abcam plc, Cambridge, UK) (1:5000), anti-MMP-9 (Abcam) (1:5000), anti-MMP-13 (Abcam) (1:5000), and actin β (Cell signaling Technology, Inc., Danvers, MA, USA). After incubation, the membrane was then incubated at room temperature with the secondary antibody: HRP-conjugated mouse anti-rabbit IgG (Rockland Immunochemicals, Inc., Limerick PA, USA) (1:10000) and HRP-conjugated rat anti-mouse IgG (Rockland Immunochemicals, Inc.) (1:10000). The immunoreactive bands were visualized using ECL Prime western blotting detection reagent (GE Healthcare UK Ltd.), and the signals were detected with the ChemiDoc MP Imaging System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The density was analyzed using Image J software (Bio-Rad Laboratories, Inc.) and normalized with the actin β signal for equal protein loading control in each sample of each experiment.
Gelatin zymography
MMP-2 and MMP-9 enzyme activity were evaluated using Gelatin-zymography kit (Cosmo Bio Co., LTD, Tokyo, Japan) according to the manufacturer’s protocol. The skin extract solution containing 15 g protein was separated by electrophoresis. The stained gelatin-degraded zone was quantified using Image J software.
Measurement of hyaluronic acid
The dry weights of skin samples were determined, and the samples were then digested by lysis buffer, as previously described.Citation22) The Hyaluronic Acid (HA) concentration was determined by HA Quantitative ELISA Assay (Biotech Trading Partners, Encinitas, CA, USA), and normalized to dry weight.
RNA preparation and real-time PCR
Total RNA was isolated from mouse skin using Isogen (Nippon Gene, Tokyo, Japan) and the RNeasy mini kit (QIAGEN, Venlo, Netherlands), according to the manufacturer’s protocol. RNA concentration was determined spectrophotometrically, and a total of 1 μg total RNA was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies Japan, Tokyo, Japan). For analysis, the following primer sets were utilized (Perfect Real Time Primer; Takara Bio Inc., Osaka, Japan): glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [Gapdh; MA162228], hyaluronic acid synthase 1 (Has1) [Has1; MA110796], hyaluronic acid synthase 2 (Has2) [Has2; MA156683], and hyaluronic acid synthase 3 (Has3) [Has3; MA025756].
Serum adiponectin
Serum adiponectin was measured using a mouse/rat high-molecular-weight adiponectin ELISA kit (Shibayagi, Gunma, Japan), according to the manufacturer’s protocols. These levels were determined using a microplate reader.
Data analysis
All data are presented as mean ± SEM. The differences between the UVB-irradiated control and non-irradiated control groups were determined by Student’s t-test. And statistically significant differences between UVB-irradiated control group and UVB-irradiated AVGP group were determined by Student’s t-test. P values of less than 0.05 were considered to be statistically significant. All analyses were performed using SPSS for Windows, version 14.0 (SPSS Inc., Chicago, Illinois).
Results
Effects of oral administration of AVGP on skin barrier function
UVB irradiation induced the reduction in skin hydration and the increase in TEWL significantly. Table shows that AVGP oral administration alleviated significant UVB-induced reduction in skin hydration and increase in TEWL. The UVB-induced epidermal thickness was visualized in histological sections stained with H&E. UVB irradiation significantly increased epidermal thickness in the hairless mice skin. However, the UVB (+) AVGP group showed reduction in the epidermal thickness compared with the UVB (+) control group.
Table 1. Transepidermal water loss (TEWL), skin hydration, and skin fold thickness during the experimental period and after 6 weeks of UVB exposure (n = 8).
AVGP inhibits UVB-induced decreases in mouse skin elasticity
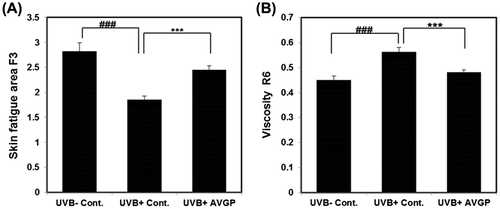
In the UVB (+) control group, there was a significant decrease in skin fatigue F3 and a significant increase in skin viscosity R6 after 6 weeks of UVB irradiation compared with the UVB (−) control group (Fig. (A and B)). On the other hand, F3 of the UVB (+) AVGP group was significantly higher than that of the UVB (+) control group (Fig. (A)). Moreover, the UVB-induced increase in R6 was significantly decreased in the UVB (+) AVGP group, compared with the UVB (+) control group (Fig. (B)). In addition, overall skin elasticity R2 is decreased by UVB exposure; however, R2 of UVB (+) AVGP was tend to be lower than R2 of UVB (+) control (data not shown).
Histological analysis
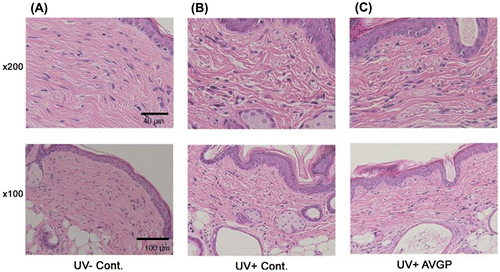
A histological evaluation was performed on the hairless mice exposed to UVB. Fig. shows the most representative results of the histological study. Image of mouse skin from each group is visualized using H&E staining. There was the space change in dermal fibrous tissue of the UVB-irradiated control group. However, in the AVGP-treated group, the decrease in the space change in dermal fibrous tissue was observed.
Type I collagen level in HR mouse skin tissue
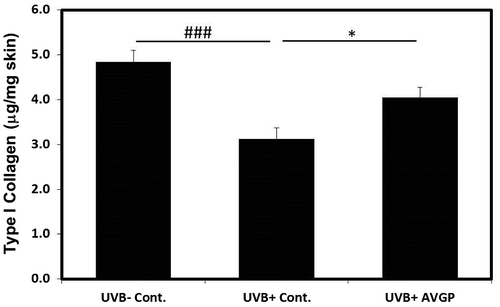
We measured the content of type I collagen extracted from skin samples by ELISA. Chronic UVB irradiation of mouse dorsal skin decreased the content of type I collagen. However, in UVB (+) AVGP group, UVB-induced decrease in type I collagen was inhibited compared with UVB (+) control group (Fig. ).
AVGP reduced UVB-induced increase in MMP-13, MMP-2, and MMP-9 expression
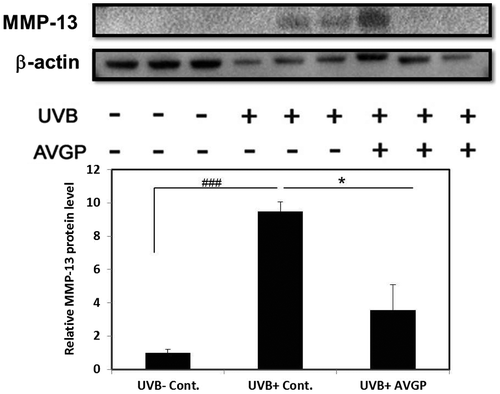
Many studies reported that UVB irradiation induced MMPs expression in skin and MMP is closely linked to photoaging. We examined the effect on the UVB-induced expressions of MMPs. The mice dorsal skin protein expression of MMP-13 in UVB (+) control group significantly increased 9.5-fold (p < 0.001) by comparison with those in UVB (−) control group (Fig. ). On the other hand, MMP-13 expression of the UVB (+) AVGP group showed significant suppression of the UVB-induced increase in MMP-13 by 38.9 % (p < 0.05) (Fig. ).
Fig. 5. Protein levels of MMP-13 after 6 weeks of UVB irradiation.
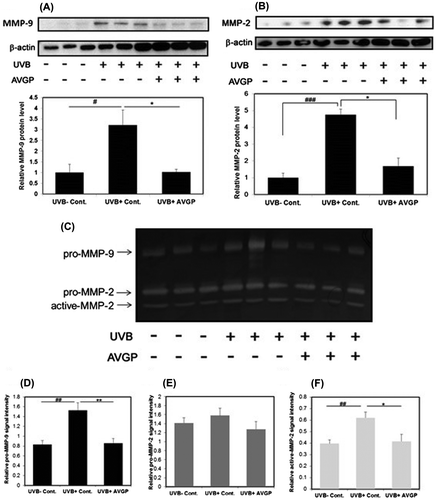
AVGP significantly prevented increase in total protein expression of MMP-2 (active and pro) and MMP-9 (active and pro) in UVB irradiation mice (Fig. (A and B)). The levels of pro-MMP-9 and MMP-2 expression in UVB (+) control group significantly increased 1.8-fold (p < 0.04) and 1.6-fold (p < 0.01) by comparison with those in UVB (−) control group (Fig. (D and F)). However, the UVB (+) AVGP group showed significant suppression of the UVB-induced increase in pro-MMP-9 and MMP-2 protein expression, by 67.7% (p < 0.03) and 56.2% (p < 0.01), respectively, compared with the UVB (+) control group (Fig. (D and F)). Pro-MMP-2 expression did not differ between groups (Fig. (E)). Activated MMP-9 expression could not be detected in this study.
Fig. 6. Expression and gelatinolytic activity of MMP-9 and MMP-2 after 6 weeks of UVB irradiation.
AVGP suppresses UVB-induced decrease in skin hyaluronic acid and reduction in Has2 mRNA expression
Because AVGP showed suppression of hydration loss from the stratum corneum, we next measured the HA content in hairless mouse dorsal skin. Compared with the UVB (−) control, the skin HA content of the UVB (+) control group was significantly reduced (Fig. (A)). On the other hand, the extent of the UVB-induced reduction in dermal HA was decreased in the UVB (+) AVGP group (Fig. (A)).
Fig. 7. (A) Measurement of HA content and (B) mRNA levels of hyaluronic acid synthases, Has2 in hairless mouse skin extract after 6 weeks of UVB irradiation.
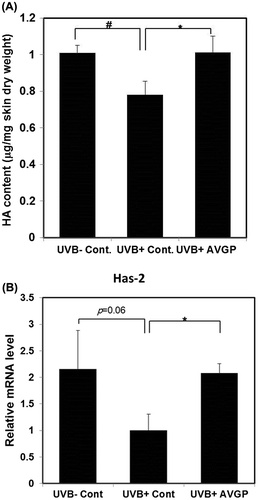
Fig. (B) shows that the mRNA expression of Has2 tended to be decreased in response to UVB irradiation (p = 0.06); however, AVGP treatment inhibited the UVB-induced decrease in Has2 mRNA expression (p < 0.05). By contrast, the mRNA expressions of Has1 and Has3 were not changed in response to either UVB irradiation or AVGP treatment (data not shown).
AVGP attenuates the UVB-induced decrease in serum adiponectin
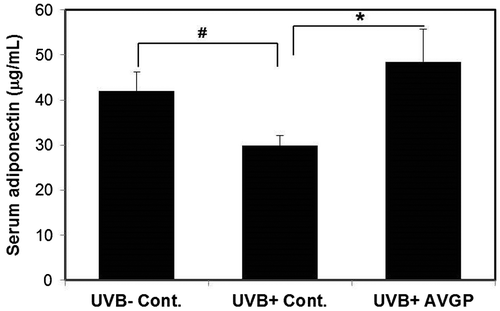
Some studies have reported that adiponectin increase production of HA and the expression of Has2, we measured the serum adiponectin level. The serum adiponectin level of the UVB (+) control group was decreased compared with the UVB (−) control group (p < 0.05). On the other hand, the serum adiponectin level of the UVB (+) AVGP group was increased (p < 0.05) compared with that of the UVB (+) control group (Fig. ).
Discussion
Generally, skin aging can be classified into two mechanisms, chronological aging and photoaging.Citation6) Repeated exposure to UVB causes the premature aging of the skin, clinically characterized by fine and coarse wrinkles, dyspigmentation, loss of elasticity, and dryness.Citation1,5,23) Many studies report that ECM changes, such as collagen fiber fragmentation, degradation of elastin fiber, and decrease in HA synthesis, are observed in the skin tissue as signs of photoaging.Citation22–25) Therefore, it is thought that ECM degradation in the dermal tissue is one of the main causes of photoaging.
In the present study, we investigated the effects of AVGP on the prevention of photoaging. AVGP was observed to prevent the reduction in skin fatigue area F3 and the increase in skin viscosity R6, in photoaging model animals (Fig. ). Some studies have reported that repeated UV irradiation on hairless mice caused decreases in skin strain and tensile strength.Citation26–30) It has also been reported that the F3 parameter in human cheek and upper arm skin declines with aging.Citation22,31) In addition, skin elasticity R2 decreased and skin viscosity R6 increased with aging in human forearm skin.Citation31) These changes in skin elasticity are not affected by skin thickening, and are thought to be associated with degradation of dermal ECM.Citation26) We evaluated the ECM changes of the dermis by observation of tissue sections (Fig. ). In UVB-irradiated mouse skin tissue sections, we observed the change of ECM structure, the dermal fibrous degeneration, in the dermis. However, in UVB with AVGP treatment group, the UVB-induced change in ECM structure was reduced. We measured the content of type I collagen and AVGP prevented UVB-induced decrease in type I collagen content (Fig. ). We attempt to measure the type III and IV collagens, other components of dermal fibrous tissue by western blotting; however, these were not detected in this study. Accordingly, these data suggest that AVGP oral administration might prevent the UVB-induced degeneration or change in dermal structure by suppressing the decrease in type I collagen.
Many studies have indicated that UV irradiation upregulates the expression of MMPsCitation10–12,32,33), and the abnormal increase in MMPs causes structural and functional alterations of the dermis. Some study has demonstrated that the topical application of an MMP inhibitor, CGS27023A in mice,Citation34) as well as a CGS27023A-treated 3D skin cell model,Citation35) showed suppression of both dermal collagen degradation and destruction of the epidermal basement membrane.
This study also demonstrated that UVB-irradiation evoked significant elevation of expression of MMP-13. And we found that AVGP oral administration suppressed UVB-induced increase in the expression (Fig. ). Moreover, AVGP prevented the increase in active-MMP-2 and pro-MMP-9 expressions (Fig. ). MMP-2 is activated by MMP-13, MMP-3, and other MMPs.Citation36) In this study, AVGP treatment suppressed the increase in active-MMP-2 might be attributed to the decrease in MMP-13 expression.
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors of nuclear hormone receptor superfamily comprising the following three subtypes: PPARα, PPARγ, and PPARβ/δ. Barlaka et al. reported that PPARβ/δ agonist suppressed the increase in MMP-2 and -9 expressions in adult rat cardiac myocytes under the stress of reactive oxygen species.Citation37) Moreover, Lee et al. reported that PPARγ agonist reduces MMP-9 activity in focal cerebral ischemia model.Citation38) Our previous study showed that some Aloe sterols isolated from AVGP have ligand activity of PPARs.Citation39) Therefore, we hypothesized that Aloe sterols containing AVGP may regulate MMP-9 expression and MMP-2 expression and activation pathway via regulating transcriptional activity of PPARs.
We tested to detect the expressions of TIMP-1 and TIMP-2, and there was no difference in TIMP-1 expression (supplemental Fig. 1), and TIMP-2 expression was not detected in this study. These data suggested that AVGP oral treatment inhibited that type I collagen degradation in dermis via regulating MMPs expression and activation, not regulating TIMP-1 mediated pathway.
Keratinocytes in the epidermis and fibroblasts in the dermis exposed to UV radiation produce inflammatory cytokines, such as IL-1α, IL-6, and TNFα.Citation23) These cytokines stimulate keratinocytes and fibroblasts to express MMPs, leading to the digestion of the ECM.Citation2,24) Moreover, UV irradiation of human skin induces reactive oxygen species (ROS), leading to activation of ROS-sensitive signaling kinases in keratinocytes and fibroblasts.Citation1,2,9,23) It is generally accepted that Aloe vera has anti-inflammatory and anti-oxidant effects.Citation15,16) AVGP may also have such anti-inflammatory and anti-oxidant effects, and further investigation is warranted.
Some study shows that UVB-induced EBM damage is related with wrinkle formation.Citation40,41) Moreover, MMP inhibitors were shown to inhibit the UVB irradiation-induced wrinkle formation and epidermal hyperplasia, Citation41) suggesting that the inhibition of MMPs may prevent skin photoaging. Moreover, Cho et al. reported that oral Aloe vera gel supplement improves wrinkles and elasticity in photodamaged skin, the decrease in MMP-1 mRNA of buttock skin in clinical trial.Citation42) Our study also shows AVGP oral administration inhibited the UVB irradiation-induced decrease in skin elasticity. These studies suggested that due to its inhibitory effect of ECM breakdown via suppressing expression of MMPs, oral treatment of Aloe vera gel may prevent photoaging like as the loss of skin elasticity and UVB-induced wrinkle formation.
Cho et al. also reported that Aloe vera gel oral treatment improves the increase in protype I collagen mRNA.Citation42) In our previous study, we also observed that Aloe sterols stimulated collagen production in human dermal fibroblasts.Citation19) From the results of the increase in type I collagen in AVGP treatment group, we can suggest the possibility that AVGP has not only the potency of regulating MMPs expression, but also improves the production of collagen I in this study. This is a subject for future analysis.
The present results demonstrated that AVGP prevents significant UVB irradiation-induced decreases in skin hydration and elasticity in mouse dorsal skin. The reduction in HA is thought to be one mechanism associated with such skin changes.Citation24,40,41) Reduction in HA in UVB-irradiated skin has been reported to be partially attributed to the downregulation of HAS expression.Citation25,43) HA is synthesized at the plasma membrane by HA synthesis (Has) isoenzyme 1, 2, and 3, and Has2 is the major producer of HA in the dermis. In the present study, chronic UVB irradiation decreased both the amount of HA and Has2 mRNA expression in mouse skin; however, mice fed AVGP showed a reduction in the irradiation-induced loss of HA and Has2 mRNA levels. Has expression is regulated by several factors, such as growth factors, hormones, and cytokines. Some studies have reported that Has2 expression is upregulated by adiponectin.Citation42,44) Further studies have reported that adiponectin blood concentration was decreased in HR-1 mice in response to UVB irradiation.Citation44,45) In a previous study, we have shown that oral administration of Aloe sterols increased blood adiponectin levels in a diabetes animal model.Citation46) In the present study, we evaluated the blood adiponectin level, and observed that the UVB-induced decrease in blood adiponectin levels was prevented by oral AVGP treatment (Fig. ). In our previous study, we reported that Aloe sterols act as a ligand of PPAR α and γ,Citation47) which are known to regulate the expression of adiponectin.Citation48) Many studies have shown that PPARγ ligands, such as thiazolidinediones (TZDs), increase plasma adiponectin levels.Citation48) Accordingly, Aloe sterols contained in AVGP may act as a PPARγ ligand, inducing an increase in serum adiponectin levels. On the other hand, in the previous study, we also observed that Aloe sterols stimulated HA production in human dermal fibroblasts.Citation19) Further studies are required for clarification of the mechanism governing AVGP promotion of HA synthesis.
In summary, the present results demonstrated that dietary feeding of AVGP containing Aloe sterols suppressed the UVB-induced decreases in skin elasticity and hydration in hairless mice. AVGP may exert its effects via two mechanisms: first, AVGP inhibited UVB irradiation-induced upregulation of MMP expression, and might prevent the degradation of the ECM; second, AVGP suppressed the UVB irradiation-induced decrease in Has2 expression via attenuating the UVB-induced decrease in serum adiponectin level. Thus, the present results suggested that AVGP containing Aloe sterols may be a promising material for the prevention of photoaging.
Author contribution
MS, MT, and EM conceived and designed the experiments. MS, EM, RY, and KN performed the experiments. MS, MT, and EM analyzed the data. KY and FA discussed about this study and reviewed the paper. YY and FF reviewed the data analysis and interpretation. MS wrote the paper. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental materials
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2016.1156480.
TBBB_1156480_Supplementary_Figure.doc
Download MS Word (73.5 KB)References
- Cerimele D, Celleno L, Serri F. Physiological changes in ageing skin. Br. J. Dermatol. 1990;122:13–20.10.1111/bjd.1990.122.issue-s35
- Hwang K, Yi B, Choi K. Molecular mechanisms and in vivo mouse models of skin aging associated with dermal matrix alterations. Lab. Anim. Res. 2011;27:1–8.10.5625/lar.2011.27.1.1
- Rossetti D, Kielmanowicz MG, Vigodman S, et al. A novel anti-ageing mechanism for retinol: induction of dermal elastin synthesis and elastin fibre formation. Int. J. Cosmet. Sci. 2011;33:62–69.10.1111/ics.2011.33.issue-1
- Gniadecka M, Nielsen OF, Wessel S, et al. Water and protein structure in photoaged and chronically aged skin. J. Inves. Dermatol. 1998;111:1129–1133.10.1046/j.1523-1747.1998.00430.x
- Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (II): protein, glycosaminoglycan, water, and lipid content and structure. Skin. Res. Technol. 2006;12:145–154.10.1111/srt.2006.12.issue-3
- Nishimori Y, Edwaeds C, Pearse A, et al. Degenerative alterations of dermal collagen fiber bundles in photodamaged human skin and UV-irradiated hairless mouse skin: possible effect on decreasing skin mechanical properties and appearance of wrinkle. J. Invest. Dermatol. 2001;117:1458–1463.
- Takahashi Y, Ishikawa O, Okada K, et al. Disaccharide analysis of human skin glycosaminoglycans in sun-exposed and sun-protected skin of aged people. J. Dermatol. Sci. 1996;11:129–133.10.1016/0923-1811(95)00430-0
- Yaar M, Gilchrest BA. Photoageing: mechanism, prevention and therapy. Br. J. Dermatol. 2007;157:874–887.10.1111/bjd.2007.157.issue-5
- Jenkins G. Molecular mechanisms of skin ageing. Mech. Aging Dev. 2002;123:801–810.10.1016/S0047-6374(01)00425-0
- Fisher GJ, Voorhees JJ. Molecular mechanisms of photoaging and its prevention by retinoic acid ultraviolet irradiation induce Ap-1-regulated matrix metalloproteinases that degrade human skin in vivo. J. Investig. Dermatol. Symp. Proc. 1998;3:61–68.
- Fligiel SEG, Varani J, Datta SC, et al. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J. Invest. Dermatol. 2003;120:842–848.10.1046/j.1523-1747.2003.12148.x
- Quan T, Little E, Quan H, et al. Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: impact of altered extracellular matrix microenvironment on dermal fibroblast function. J. Invest. Dermatol. 2013;133:1362–1366.10.1038/jid.2012.509
- Verma RP, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioog. Med. Chem. 2007;15:2223–2268.10.1016/j.bmc.2007.01.011
- Reynolds T, Dweck AC. Aloe vera leaf gel: a review update. J. Ethnopharmacol. 1999;68:3–37.10.1016/S0378-8741(99)00085-9
- Hamman JH. Composition and applications of Aloe vera leaf gel. Molecules. 2008;13:1599–1616.10.3390/molecules13081599
- Rodríguez ER, Martín JD, Romero CD. Aloe vera as a functional ingredient in foods. Crit. Rev. Food Sci. Nutrition. 2010;50:305–326.10.1080/10408390802544454
- Maenthaisong R, Chaiyakunapruk N, Niruntraporn S, et al. The efficacy of Aloe vera used for burn wound healing: a systematic review. Burns. 2007;33:713–718.10.1016/j.burns.2006.10.384
- Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on collagen characteristics in healing dermal wounds in rat. Mol. Cell Biochem. 1998;181:71–76.10.1023/A:1006813510959
- Tanaka M, Misawa E, Yamauchi K, et al. Effects of plant sterols derived from Aloe vera gel on human dermal fibroblasts in vitro and on skin condition in Japanese women. Clin. Cosmet. Investig. Dermatol. 2015;8:95–104.10.2147/CCID
- Misawa E, Tanaka M, Nabeshima K, et al. Administration of dried aloe vera gel powder reduced body fat mass in diet-induced obesity (DIO) rats. J. Nutr. Sci. Vitaminol. 2012;58:195–201.10.3177/jnsv.58.195
- Ohshima H, Kinoshita S, Oyobikawa M, et al. Use of cutometer area parameters in evaluating age-related changes in the skin elasticity of the cheek. Skin Res. Tech. 2013;19:e238–e242.10.1111/j.1600-0846.2012.00634.x
- Rock K, Meusch M, Fuchs N, et al. Estradiol protects dermal hyaluronan/versican matrix during photoaging by release of epidermal growth factor from keratinocytes. J. Biol. Chem. 2012;287:20056–20069.10.1074/jbc.M112.353151
- Ichihashi M, Ando H, Yoshida M, et al. Photoaging of the skin. Anti. Aging Med. 2009;6:46–59.10.3793/jaam.6.46
- Rock K, Grandoch M, Majora M, et al. Collagen fragments inhibit hyaluronan synthesis in skin fibroblasts in response to ultraviolet B (UVB): new insights into mechanisms of matrix remodeling. J. Biol. Chem. 2011;286:18268–18276.10.1074/jbc.M110.201665
- Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin. Am. J. Pathol. 2006;168:1861–1868.10.2353/ajpath.2006.051302
- Takema Y, Imokawa G. The effects of UVA and UVB irradiation on the viscoelastic properties of hairless mouse skin in vivo. Dermatology. 1998;196:397–400.10.1159/000017931
- Jeong H. Skin elasticity and sea polyohenols. Seanol Sci. Center Rev. 2010;1:1–10.
- Pyun HB, Kim M, Park J, et al. Effects of collagen tripeptide supplement on photoaging and epidermal skin barrier in UVB-exposed hairless mice. Prev. Nutr. Food Sci. 2012;17:245–253.10.3746/pnf.2012.17.4.245
- Sugimoto S, Ishii Y, Izawa N, et al. Photoprotective effects of Bifidobacterium breve supplementation against skin damage induced by ultraviolet irradiation in hairless mice. Photodermathol. Photo. 2012;28:312–319.10.1111/phpp.2012.28.issue-6
- Oba C, Ohara H, Morifuji M, et al. Collagen hydrolysate intake improves the loss of epidermal barrier function and skin elasticity induced by UVB irradiation in hairless mice. Photodermathol. Photo. 2013;29:204–211.10.1111/phpp.2013.29.issue-4
- Gerhardt LC, Lenz A, Spencer ND, et al. Skin-textile friction and skin elasticity in young and aged persons. Skin Res. Technol. 2009;15:288–298.10.1111/srt.2009.15.issue-3
- Fligiel SEG, Varani J, Datta SC, et al. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J. invest. Dermatol. 2003;120:842–848.10.1046/j.1523-1747.2003.12148.x
- Yokose U, Hachiya A, Sriwiriyanont P, et al. The endogenous protease inhibitor TIMP-1 mediates protection and recovery from cutaneous photodamage. J. Invest. Dermatol. 2012;132:2800–2809.10.1038/jid.2012.204
- Inomata S, Matsunaga Y, Amano S, et al. Possible involvement of gelatinase in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J. Invest. Dermatol. 2003;120:128–134.
- Amano S, Ogura Y, Akutsu N, et al. Protective effect of matrix metalloproteinase inhibitors against epidermal basement membrane damage: skin equivalents partially mimic photoageing process. Br. J. Dermatol. 2005;153:37–46.10.1111/bjd.2005.153.issue-s2
- Chakraborti S, Mandal M, Das S, et al. Regulation of matrix metalloproteinases: an overview. Mol. Cell Biochem. 2003;253:269–285.10.1023/A:1026028303196
- Barlaka E, Görbe A, Gáspár R, et al. Activation of PPARβ/δ protects cardiac myocytes from oxidative stress-induced apoptosis by suppressing generation of reactive oxygen/nitrogen species and expression of matrix metalloproteinases. Pharmacol. Res. 2015;95–96:102–110.10.1016/j.phrs.2015.03.008
- Lee SR, Kim HY, Hong JS, et al. PPARγ agonist pioglitazone reduces matrix metallpproteinase-9 activity and neuronal damage after focal cerebral ischemia. BBRC. 2009;380:17–21.
- Nomaguchi K, Tanaka M, Misawa E, et al. Aloe vera phytosterols act as ligands for PPAR and improve the expression levels of PPAR target genes in the livers of mice with diet-induced obesity. Obes. Res. Clin. Pract. 2011;5:e190–201.
- Takahashi Y, Ichikawa O, Okada K, et al. Disaccharide analysis of human skin glycosaminoglycans in sun-exposed and sun-protected skin of aged people. J. Dermal. Sci. 1996;11:129–133.10.1016/0923-1811(95)00430-0
- Averbeck M, Gedhardt CA, Voigt S, et al. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J. Invest. Dermatol. 2006;127:687–697.
- Cho S, Lee S, Lee MJ, et al. Dietary Aloe vera supplementation improves facial wrinkles and elasticity and it increases the type I procollagen gene expression in human skin in vivo. Ann. Dermatol. 2009;21:6–11.10.5021/ad.2009.21.1.6
- Dai G, Freudenberger T, Zipper P, et al. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am. J. Pathol. 2007;171:1451–1461.10.2353/ajpath.2007.070136
- Meeran SM, Singh T, Nagy TR, et al. High-fat diet exacerbates inflammation and cell survival signals in the skin of ultraviolet B-irradiated C57BL/6 mice. Toxicol. Appl. Pharm. 2009;241:303–310.10.1016/j.taap.2009.09.003
- Matsui S, Yamane T, Kobayashi-Hattori K, et al. Ultraviolet B irradiation reduces the expression of adiponectin in ovarial adipose tissues through endocrine actions of calcitonin gene-related peptide-induced serum amyloid A. PLoS ONE. 2014;9:e98040.10.1371/journal.pone.0098040
- Yamane T, Kobayashi-Hattori K, Oishi Y. Adiponectin promotes hyaluronan synthesis along with increases in hyaluronan synthase 2 transcripts through an AMP-activated protein kinase/peroxisome proliferator-activated receptor-α-dependent pathway in human dermal fibroblasts. Biochem. Biophys. Res. Commun. 2011;415:235–238.10.1016/j.bbrc.2011.09.151
- Misawa E, Tanaka M, Nomaguchi Kouji, et al. Oral ingestion of Aloe vera phytosterols alters hepatic gene expression profiles and ameliorates obesity-associated metabolic disorders in Zucker diabetic fatty rats. J. Agr. Food Chem. 2012;60:2799–2806.10.1021/jf204465j
- Kadowaki T, Yamauchi T, Kubota N, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 2006;116:1784–1792.10.1172/JCI29126
- Ezure T, Amano S. Adiponectin and leptin up-regulate extracellular matrix production by dermal fibroblasts. BioFactors. 2007;31:229–236.10.1002/biof.v31:3/4