Abstract
High glucose reduced the egg-laying rate of the nematode Caenorhabditis elegans and was dependent on serotonergic signaling. Antidiabetic drugs of the biguanide and thiazolidine classes ameliorated the detrimental effect of glucose on egg-laying rate, suggesting the possibility that this quick and easy assay system may be applicable to whole-animal screening for novel antidiabetic drugs, at least, of these classes.
Whole animal assays are required for drug discovery and drug target identification and validation. However, because of the cost, time, and ethical concerns, assays in mammalian models (typically rodents) include only a small number of test subjects and are performed only where absolutely necessary. In order to overcome these limitations, non-mammalian models for drug development are being established. One of the organisms well suited to such models is the nematode Caenorhabditis elegans. It shares most of its fundamental biological processes with humans and has counterparts for many human disease-causing genes.Citation1–4) These features, together with the small size, simple structure, short life cycle, high fecundity, and experimental tractability of the nematode, enable cost-effective and rapid whole animal assays. Indeed, C. elegans has been used extensively to develop alternative or complementary non-mammalian models for human disease and assays to screen for drug candidates.Citation2–10)
Glucose in chronic excess leads to the dysfunction of multiple organ systems, a phenomenon termed glucose toxicity that underlies the development of diabetes and pre-diabetes. C. elegans has been used to study glucose toxicity in relation to aging, because this nematode has an evolutionarily conserved insulin/insulin-like growth factor-1 signaling (IIS) pathway which is involved in both processes. Although IIS activity reduction or antioxidant defense augmentation induced by glucose restriction extends C. elegans lifespan,Citation11) a glucose-enriched diet causes life shortening by up-regulating IIS activity or increasing reactive oxygen species (ROS).Citation11–15) Schlotterer et al.Citation13) suggested that the C. elegans lifespan assay is a good model system to evaluate glucose toxicity and to develop more efficient therapy for diabetes.
Here, we document another phenotype associated with high-glucose feeding, namely, decreased egg-laying rate. The egg-laying assay of C. elegans can be performed more easily than the lifespan assay, and, therefore, might be applicable for large-scale drug screening against glucose toxicity.
The Bristol N2 strain of C. elegans was obtained from the Caenorhabditis Genetic Center (University of Minnesota, MN) and was used as the wild-type. The worms were maintained at 20 °C on Nematode Growth Medium (NGM) culture plates with E. coli OP50 bacteria as a food source. Synchronous populations were generated by bleaching (hypochlorite-NaOH treatment)Citation16) and were used in all experiments. The worms raised on NGM culture plates up to the final larval stage (L4) were placed on NGM containing d-glucose at the indicated concentrations (0–100 mM) and incubated. After further 20 h, the worms were actively egg-laying. We noticed a reduced number of eggs released in the presence of high concentrations of d-glucose (~100 mM), compared with that in the absence of glucose, although glucose had no effect on the onset of egg-laying (data not shown). We, therefore, studied egg-laying behavior precisely using three assay protocols (Fig. ). We first evaluated the egg-laying rate of individual animals. To this end, the worms described above were singly transferred to individual plates containing d-glucose at the indicated concentrations (0–100 mM). The number of eggs released during 6 h was determined. Results were expressed as the mean number of eggs laid per animal per hour. We found that the egg-laying rate of 100 mM d-glucose-fed animals was significantly lower than that of the controls (Fig. (A)). The egg-laying rate of animals fed on 100 mM l-glucose instead of d-glucose was not significantly different from that of the controls (data not shown), demonstrating that the abnormality was not caused by changes in osmotic pressure.
Fig. 1. Glucose decreases the rate of egg laying.
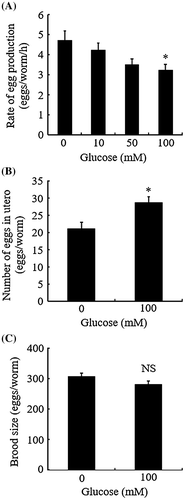
In the second assay, we scored the number of eggs in utero. In this assay, L4 larvae at the white-crescent stage were transferred to culture plates with or without 100 mM d-glucose, and incubated for a further 40 h at 20 °C. Then, the worms were transferred to microtiter wells and treated with 50 μl of 1% sodium hypochlorite for 10 min. The unlaid eggs released by this bleaching treatment were counted. We found that the number of the unlaid eggs in glucose-fed animals was higher than that in control animals (Fig. (B)), suggesting that high-dose glucose did not affect oogenesis, but reduced the speed of egg-laying.
In the third assay, we measured brood size. Consistent with the previous study,Citation14) 100 mM glucose-fed worms showed no significant difference in brood size compared with the controls (Fig. (C)). Taken together, the findings shown in Fig. demonstrate that feeding 100 mM glucose affected egg-laying behavior without altering the rate of gametogenesis or fertilization.
In C. elegans, egg-laying is controlled by the hermaphrodite-specific neurons (HSNs), which form a synapse on the vulval muscles and promote the muscle contraction by serotonergic signaling.Citation4,17) Cholinergic signaling in the HSNs suppresses expression of the essential serotonin (5-HT) biosynthetic gene tph-1, which encodes tryptophan hydroxylase-1, thus inhibiting egg laying.Citation17) Using loss/gain-of-function mutants of the genes involved in this neuromuscular system, we have studied the molecular basis underlying the effect of glucose on the egg-laying behavior of C. elegans. In ongoing experiments, we analyzed tph-1(mg280) null-mutant worms. Egg-laying rate of the mutant worms was determined in the presence or absence of glucose (100 mM), as described above. This 5-HT deficient mutant exhibited an egg-laying defect (Egl) phenotype (Fig. (A)), confirming that serotonergic signaling plays a role in C. elegans egg laying.Citation18) Glucose (100 mM) feeding had no further effect on the egg-laying rate of the mutant worms (Fig. (A)), thus suggesting that the glucose-induced Egl phenotype of N2 worms was dependent on serotonergic signaling.
Fig. 2. Impaired serotonergic signaling is implicated as an underlying mechanism for glucose-induced egg-laying rate reduction.
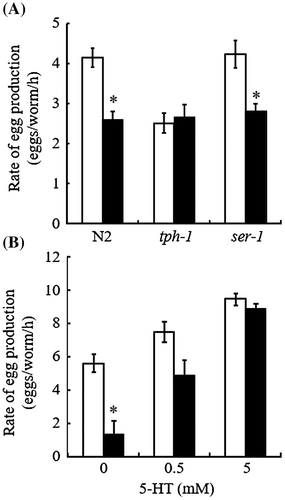
Dempsey et al.Citation19) and Carnell et al.Citation20) demonstrated that of five 5-HT receptors identified in C. elegans,Citation21) SER-1 plays the clearest role in egg-laying behavior. We used the ser-1(ok345) worms to examine the effect of glucose on SER-1 function in egg laying. Notably, ser-1(ok345) worms did not show the Egl phenotype, i.e. there was no significant difference between the basal rates of egg laying in the wild-type and mutant (Fig. (A)). Thus, we confirmed previous results suggesting redundant serotonergic signaling pathways in the regulation of egg laying.Citation21,22) As shown in Fig. (A), a glucose-induced reduction in egg-laying rate was also observed similarly in the wild-type and mutant worms. Because the glucose-induced Egl phenotype of N2 worms was dependent on serotonergic signaling, one possible interpretation of this result was that glucose might affect serotonergic signaling pathways in the vulval muscles mediated via 5-HT receptors other than SER-1. Alternatively, glucose could affect the upstream serotonergic neurotransmission that led to the activation of the vulval muscle function. To distinguish these possibilities, we tested the effect of exogenous 5-HT on the egg-laying behavior of N2 worms in the presence or absence of glucose. As shown in Fig. (B), 5-HT dose-dependently increased the egg-laying rate even in the presence of glucose (100 mM), and completely counteracted the effect of glucose at a concentration of 5 mM. This result suggested that high glucose did not impair the vulval muscle function that consequently influenced egg laying. Taken together, our results shown in Fig. suggested that feeding 100 mM glucose directly or indirectly down-regulated serotonergic signaling which acts upstream of the activation of the vulval muscle 5-HT receptors. However, we do not exclude the possibility that glucose affects neuroendocrine signaling pathways other than serotonergic signaling, which deserves further investigation.
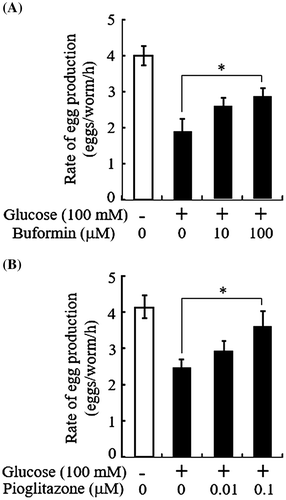
Schlotterer et al.Citation13) proposed that the lifespan assay of C. elegans could be used to evaluate glucose toxicity. The egg-laying assay is less time-consuming and labor-intensive than the lifespan assay, and thereby can be performed on a large scale. Thus, the glucose-induced reduction in egg-laying rate might be applicable to whole-animal screening of drugs against glucose toxicity. We tested this possibility by examining whether well-known anti-diabetic drugs could counteract the effect of glucose in this assay. We used two different classes of oral antidiabetic drugs, buformin and pioglitazone, which are of the biguanide class and the thiazolidine class, respectively. Although neither buformin nor pioglitazone showed significant effects on the egg-laying rate of control worms grown on the plates without glucose (data not shown), both drugs ameliorated the glucose-induced impairment of egg-laying rate (Fig. ). It has been shown using C. elegans that high glucose increases ROS leading to a reduction in the activity of glyoxalase-1, the major enzyme detoxifying methylglyoxal.Citation13) Methylglyoxal is a precursor of advanced glycation endproducts (AGEs). The ROS-induced accumulation of molecular damage including AGE formation is well-known to contribute to numerous human diseases, including diabetes.Citation23) Accordingly, it is a plausible hypothesis that high glucose induced ROS overproduction, which might increase the formation of AGEs in neuroendocrine cells, contributing to the detrimental effect of glucose on the serotonergic signaling, which plays a role in egg-laying behavior. If the hypothesis is correct, the glucose-lowering action of buformin and pioglitazone may decrease the formation of ROS and AGEs, thereby ameliorating egg-laying rate. Alternatively, adenosine monophosphate-activated protein kinase (AMPK), which mediates primarily or partially the noted action of these drugs, may be directly involved in serotonergic signalingCitation24–26) and its implication in egg-laying behavior. The present results anticipate that this simple and rapid in vivo assay system could be potentially useful for a screening of novel antidiabetic drug candidates. Meanwhile, we could not exclude the possibility that this assay system might also detect non-antidiabetic agents which improve egg-laying rate by up-regulating vulval function directly or indirectly via other neuroendocrine signaling pathways.
Fig. 3. Antidiabetic drugs counteract glucose-induced egg-laying rate reduction.
Authors contribution
All authors were responsible for the study concept and design. E.T. and K.M. carried out the study. H.T. shared responsibility for the writing of the manuscript with E.T. and K.M.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was partially supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI [grant number 24659469 to H.T.].
Acknowledgments
We thank the Caenorhabditis Genetics Center, which is funded by a grant from the National Institute of Health for Research Resources, for providing wild-type and mutant worms, and Dr. Y. Ohshima for helpful comments on the experiments.
Notes
Abbreviations: IIS, insulin/insulin-like growth factor-1 signaling; ROS, reactive oxygen species; NGM, nematode growth medium; AGEs, advanced glycation endproducts.
References
- Hulme SE, Whitesides GM. Chemistry and the worm: Caenorhabditis elegans as a platform for integrating chemical and biological research. Angew. Chem. Int. Ed. Engl. 2011;50:4774–4807.10.1002/anie.v50.21
- Rodriguez M, Snoek LB, De Bono M, et al. Worms under stress: C. elegans stress response and its relevance to complex human disease and aging. Trends Genet. 2013;29:367–374.10.1016/j.tig.2013.01.010
- Aitlhadj L, Stürzenbaum SR. Caenorhabditis elegans in regenerative medicine: a simple model for a complex discipline. Drug Discov. Today. 2014;19:730–734.10.1016/j.drudis.2014.01.014
- Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006;5:387–399.10.1038/nrd2031
- Epstein HF, Benian GM. Paradigm shifts in cardiovascular research from Caenorhabditis elegans muscle. Trends Cardiovasc. Med. 2012;22:201–209.10.1016/j.tcm.2012.07.021
- Lublin AL, Link CD. Alzheimer’s disease drug discovery: in vivo screening using Caenorhabditis elegans as a model for β-amyloid peptide-induced toxicity. Drug Discov. Today Technol. 2013;10:e115–e119.10.1016/j.ddtec.2012.02.002
- Arvanitis M, Glavis-Bloom J, Mylonakis E. C. elegans for anti-infective discovery. Curr. Opin. Pharmacol. 2013;13:769–774.10.1016/j.coph.2013.08.002
- Sin O, Michels H, Nollen EA. Genetic screens in Caenorhabditis elegans models for neurodegenerative diseases. Biochim. Biophys. Acta. 2014;1842:1951–1959.10.1016/j.bbadis.2014.01.015
- Leung CK, Wang Y, Malany S, et al. An ultra high-throughput, whole-animal screen for small molecule modulators of a specific genetic pathway in Caenorhabditis elegans. PLoS One. 2013;8:e62166.10.1371/journal.pone.0062166
- O’Reilly LP, Luke CJ, Perlmutter DH, et al. C. elegans in high-throughput drug discovery. Adv. Drug. Deliv. Rev. 2014;69–70:247–253.10.1016/j.addr.2013.12.001
- Schulz TJ, Zarse K, Voigt A, et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293.10.1016/j.cmet.2007.08.011
- Lee SJ, Murphy CT, Kenyon C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab. 2009;10:379–391.10.1016/j.cmet.2009.10.003
- Schlotterer A, Kukudov G, Bozorgmehr F, et al. C. elegans as model for the study of high glucose- mediated life span reduction. Diabetes. 2009;58:2450–2456.10.2337/db09-0567
- Mondoux MA, Love DC, Ghosh SK, et al. O-linked-N-acetylglucosamine cycling and insulin signaling are required for the glucose stress response in Caenorhabditis elegans. Genetics. 2011;188:369–382.10.1534/genetics.111.126490
- Choi SS. High glucose diets shorten lifespan of Caenorhabditis elegans via ectopic apoptosis induction. Nutr. Res. Pract. 2011;5:214–218.10.4162/nrp.2011.5.3.214
- Stiernagle T. Maintenance of C. elegans. In: Hope IA, editor. C. elegans: a practical approach. London: Oxford University Press; 1999. p. 51–67.
- Tanis JE, Moresco JJ, Lindquist RA, et al. Regulation of serotonin biosynthesis by the G proteins Galphao and Galphaq controls serotonin signaling in Caenorhabditis elegans. Genetics. 2008;178:157–169.10.1534/genetics.107.079780
- Sze JY, Victor M, Loer C, et al. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–564.10.1038/35000609
- Dempsey CM, Mackenzie SM, Gargus A, et al. Serotonin (5HT), fluoxetine, imipramine and dopamine target distinct 5HT receptor signaling to modulate Caenorhabditis elegans egg-laying behavior. Genetics. 2005;169:1425–1436.
- Carnell L, Illi J, Hong SW, et al. The G-protein-coupled serotonin receptor SER-1 regulates egg laying and male mating behaviors in Caenorhabditis elegans. J. Neurosci. 2005;25:10671–10681.10.1523/JNEUROSCI.3399-05.2005
- Hapiak VM, Hobson RJ, Hughes L, et al. Dual excitatory and inhibitory serotonergic inputs modulate egg laying in Caenorhabditis elegans. Genetics. 2009;181:153–163.
- Xiao H, Hapiak VM, Smith KA, et al. SER-1, a Caenorhabditis elegans 5-HT2-like receptor, and a multi-PDZ domain containing protein (MPZ-1) interact in vulval muscle to facilitate serotonin-stimulated egg-laying. Dev. Biol. 2006;298:379–391.10.1016/j.ydbio.2006.06.044
- Nowotny K, Jung T, Höhn A, et al. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5:194–222.10.3390/biom5010194
- Cunningham KA, Bouagnon AD, Barros AG, et al. Loss of a neural AMP-activated kinase mimics the effects of elevated serotonin on fat, movement, and hormonal secretions. PLoS Genet. 2014;10:e1004394.10.1371/journal.pgen.1004394
- Laporta J, Peters TL, Merriman KE, et al. Serotonin (5-HT) affects expression of liver metabolic enzymes and mammary gland glucose transporters during the transition from pregnancy to lactation. PLoS One. 2013;8:e57847.10.1371/journal.pone.0057847
- Cunningham KA, Hua Z, Srinivasan S, et al. AMP-activated kinase links serotonergic signaling to glutamate release for regulation of feeding behavior in C. elegans. Cell Metab. 2012;16:113–121.10.1016/j.cmet.2012.05.014
