Abstract
Polyketides induce prestalk cell differentiation in Dictyostelium. In the double-knockout mutant of the SteelyA and B polyketide synthases, most of the pstA cells—the major part of the prestalk cells—are lost, and we show by whole mount in situ hybridization that expression of prestalk genes is also reduced. Treatment of the double-knockout mutant with the PKS inhibitor cerulenin gave a further reduction, but some pstA cells still remained in the tip region, suggesting the existence of a polyketide-independent subtype of pstA cells. The double-knockout mutant and cerulenin-treated parental Ax2 cells form fruiting bodies with fragile, single-cell layered stalks after cerulenin treatment. Our results indicate that most pstA cells are induced by polyketides, but the pstA cells at the very tip of the slug are induced in some other way. In addition, a fruiting body with a single-cell layered, vacuolated stalk can form without polyketides.
Graphical abstract
Major part of pstA cells are induced by polyketides and its tip region is induced by another molecule. Single cell layered, vacuolated stalk can be differentiated without polyketides.
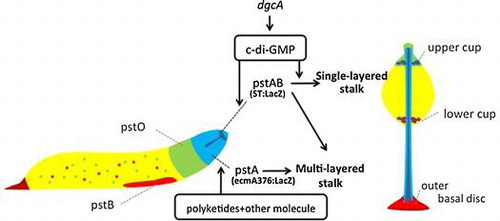
The social amoeba species Dictyostelium discoideum is an excellent model system for studying the regulation of differentiation and pattern formation by secreted molecules. Polyketides are reported to regulate cell-type differentiation in Dictyostelium.Citation1) Its genome contains the largest number of polyketide synthase (PKS) genes from among all known genomes.Citation2) Among the PKS genes, novel hybrid types of PKSs known as SteelyA and SteelyB are utilized to synthesize the spore maturation factor 4-methyl-5-pentylbenzene-1,3-diol (MPBD), and the prestalk and stalk cell differentiation-inducing factor DIF-1, respectively, and thus regulate Dictyostelium development.Citation3–8) Although DIF-1 was identified as the stalk cell inducer by an in vitro monolayer assay and has been reported to account for 95% of total activity,Citation9) the in vivo assay revealed that DIF-1 induced only two prestalk subcell types (prestalk B and O; pstB and pstO) and that prestalkA (pstA) cells differentiated without DIF-1.Citation6,8) Recently, cyclic di-(3,5)-guanosine monophosphate (c-di-GMP) was found to be a stalk cell inducer in both in vivo and in vitro analyses.Citation10,11) Disruption of dgcA, which is responsible for producing c-di-GMP, blocked fructification, and in its knockout mutant slugs, the expression of stalk tube marker genes was also suppressed. However, in the dgcA mutant, the expression of the pstA marker gene was unaffected. Since the pstA cell inducer is not clear yet, we examined the relevance of polyketide in pstA differentiation.
We have previously reported that the stlA/stlB double-knockout mutant made thin, yet vacuolated stalks even though the mutant lost most of its pstA marker gene expression in slugs.Citation12) Since our previous data indicated that MPBD and/or DIF-1 could not recover the pstA marker gene expression in the stlA/stlB double-knockout mutant, it implied that a novel factor induces pstA cells. To examine this, we used cerulenin in vivo to inhibit all PKSs activities.Citation1)
Some previous cerulenin experiments involving D. discoideum have utilized mainly in vitro monolayer assays.Citation1) However, detailed in vivo analysis of differentiation, such as an assay of subcell type marker gene expression, has not been performed.Citation1,13,14) In this study, we used the Steely gene knockout mutant and polyketide synthase inhibitor cerulenin to examine the involvement of polyketides in pstA and stalk cell differentiation.
Materials and methods
Strain, cell culture, development, and β-galactoidase staining and preparation of the cell released materials
In this study, D. discoideum Ax2 and the stlA/stlB double-knockout mutant were used and cultured as described previously.Citation12) The cells were transformed with LacZ markers and grown in HL-5 medium supplemented with 10 μg/ml of blasticidin S and G418. For developmental analysis, cells were washed and grown at 22 °C on phosphate buffer (2.7 mM Na2HPO4/10.7 mM K2HPO4; pH 6.2) agar plates at a density of 1–2 × 106 cells/cm2. Transformed cells with LacZ markers were fixed and stained for β-galactosidase until saturation during migratory slug and culmination stages. The photographs were captured as previously described.Citation12) Cerulenin was purchased from Wako Pure Chemical Industries, Ltd. (Tokyo Japan). For cerulenin treatment, cells were incubated in HL5-medium containing 100 μM cerulenin for 12 h during the vegetative stage and then developed on non-nutrient agar containing 300 μM cerulenin. Cell secretion materials were prepared as described previously without any alterations.Citation12,15)
Gene expression analysis by qRT-PCR
Specific primer sets for qRT-PCR were designed to amplify a unique sequence located near the end of 3′-region of each gene (Table ). BLAST similarity searches were used to confirm that each primer sequence amplified the unique sequence. Before using each primer set for qRT-PCR, genomic DNA was used as a template to evaluate the quality of primer set.
Table 1. Summary of the primer sets used in qRT-PCR.
Total RNA was extracted from cells at the indicated time point using the RNeasy mini kit (Qiagen) according to the manufacturer’s instructions and then the contaminating genomic DNA was eliminated using Recombinant DNase I (TaKaRa). DNase treatment was performed as follows: 50 μg total RNA was mixed with 10 units Recombinant DNase I and DNase I buffer (2 mM Tris-HCl; pH 7.5, 5 mM NaCl, 0.01 mM CaCl2, 5% glycerol) and incubated for 30 min at 37 °C. The reaction was stopped by incubating with 25 mM EDTA at 80 °C for 2 min. cDNA was synthesized by reverse transcription of 1 μg of total RNA with oligo dT15 using ImProm-II Reverse Transcription System (Promega). qRT-PCR was carried out with ABI PRISM 7000 Sequence Detection System (Applied Biosystems) and the amplifications were performed using iTaq Universal SYBR Green Supermix (Bio-Rad). The qRT-PCR program was performed as follows: 1 cycle of 30 s at 95 °C followed by 40 cycles of 15 s at 95 °C, 1 min at 60 °C for annealing, extension, and plate reading. Results of qRT-PCR were analyzed using the comparative CT methodCitation16) with the amplification of ig7 (mitochondrial large rRNA) as a control.
Whole-mount in situ hybridization
Whole-mount in situ hybridization of developmental structures was performed according to the method described by Escalante and SastreCitation17) with minor modifications. Structures were developed on teflon® filters (Omnipore™, Millipore Co., Bradford, MA, USA), fixed and hybridized as described.Citation17) In all, 500 ng/mL of heat-denatured riboprobe were used for hybridization and color reaction was stopped after 2 (streams) to 5 (culminants) hours. Pictures were taken (60×) with a camera (DFC420 Leica Microsystems, Wetzlar, Germany) attached to a stereo microscope (MZ95 Leica Microsystems). Both sense and antisense RNA probes were prepared by in vitro transcription, and digoxigenin labeling was done using a DIG RNA labeling kit (Roche Diagnostics Mannheim, Germany) according to the manufacturer’s protocol. The probes used for in situ hybridization analysis were summarized in Table . Probes of cDNA clone were prepared based on the previous report.Citation18)
Table 2. Summary of probes used for in situ hybridization.
Results and discussion
In our previous work, we showed that the D. discoideum stlA/stlB double-knockout mutant greatly reduced pstA cells in the tip region of slugs by ecmA376:LacZ staining. In contrast, at culmination stage, the intensity of ecmA376:LacZ staining in the tip region was increased in the double-knockout mutant. Based on these data, we hypothesized that pstA cells still have several subcell types and that SteelyA and SteelyB are involved in pstA cell differentiation at the slug stage but less at the culmination stage.Citation12)
Since we used LacZ staining to detect pstA cells, there is a possibility of a technical limitation in detecting very low expression cells and/or fast turnover cells. To overcome this limitation, we analyzed the expression pattern of 17 prestalk marker genes in the double-knockout mutant by in situ hybridization.
Another possible explanation of the double-mutant phenotype is that a PKS other than SteelyA or SteelyB may supply a polyketide required for full induction of pstA cell differentiation at the slug stage. To examine this hypothesis, we used the specific inhibitor of PKSs, cerulenin, and examined the staining pattern of ecmA376:LacZ.
The expression pattern of prestalk marker genes in the stlA/stlB double-knockout mutant by in situ hybridization
We chose genes that have expression specific to the prestalk regions by referring to the Atlas in situ hybridization database (http://togodb.biosciencedbc.jp/togodb/view/dicty_atlas_db/#ja). In the stlA/stlB double-knockout mutant, most of the genes that are expressed in the prestalk region were suppressed (Table , Fig. ); however, little difference in gene expression could be detected in either the stlA and stlB single-knockout mutants (data not shown).
Table 3. Summary of whole mount in situ hybridization results.
Fig. 1. Comparison of prestalk specific gene expression in Ax2 and the stlA/stlB double-knockout mutant strains.
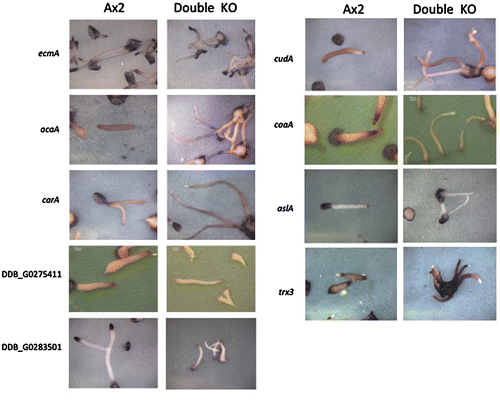
Seven out of ten genes that express in the pstA/AO region were suppressed in the stlA/stlB double-knockout mutant. These genes include ecmA and acaA. In the case of carA, the expression region was changed. CarA originally is expressed in whole pstA/AO and rear region in the wild-type slug. In the double-knockout mutant, carA was expressed in the pstO region but not the pstA region. Most of genes expressed in pstO and pstAB regions showed low expression intensity in the double-knockout mutant. It should be noted that mrfA (in pstO region) and cudA (in pstAB region) were also suppressed (Table ). Based on these data, we conclude that the absence of the major part of pstA cells in the stlA/stlB double-knockout mutant was not due to the detection limit of LacZ staining, but rather due to change in gene expression during development.
Polyketides regulate a major part of pstA cell differentiation in the slug stage
We next examined the involvement of polyketides in pstA cell differentiation using commonly used PKS inhibitor, cerulenin.Citation1,14) Since cerulenin is also known to inhibit fatty acid synthaseCitation13,19), we examined the influence of this inhibition. We compared the development of cerulenin treated Ax2 cells with and without 200 μM palmitic acid and detected no developmental difference between the two (data not shown). This result agrees well with the previous report.Citation14)
Fig. (a) and (b) shows the ecmA376:LacZ staining patterns in the slug stage with or without cerulenin treatment. The expression of ecmA376:LacZ was largely reduced by cerulenin (Fig. (a) and (b)) and this is consistent with the stlA/stlB double-knockout mutant. This result suggests that polyketides play an important role for the expression of the pstA marker gene ecmA, and that two kinds of Steelys are responsible in inducing pstA cells.
Fig. 2. EcmA376:LacZ expression pattern in the slug stage.

To elucidate the involvement of PKS other than SteelyA and SteelyB, the stlA/stlB double-knockout mutant cells were treated with cerulenin. Compared to the untreated double-knockout mutant as control (Fig. (c)), cerulenin-treated double-knockout mutant showed less ecmA376:LacZ staining in the tip region, although stained cells remained in the very tip region in the slug (Fig. (d) arrowheads). This indicates the involvement of other polyketides for pstA induction and the presence of another subcell type in the pstA region. The residual stained pstA cells might be induced by the signal other than polyketide. Since the phenotypic feature of cerulenin treated wild-type cells was resemble to that of double-knockout mutant, cerulenin treatment is strong enough to inhibit PKS activities completely. Meanwhile, these are several alternative explanations for residual stained cells in the tip regions. One possibility is due to the difference of intrinsic sensitivity to differentiation-inducing molecules, as is shown in DIF-1.Citation20) If there are extremely biased cells in prestalk region, they may differentiate into stalk cells without any signal. The other possibility is the limit of cerulenin permeability to the multicellular structures.
In the culmination stage, wild-type cells and single Steely knockout mutant cells showed clear lacZ staining in both the tip region and the stalk. Conversely, the stlA/stlB double-knockout mutant and cerulenin-treated cells showed very limited staining in the tip region and almost no staining in the stalk (Fig. S1).
Addition of Ax2 cell secretions restored pstA marker gene expression in cerulenin treated cells
To examine the pstA cell differentiation inducer, we analyzed the expression of ecmA376:LacZ in cerulenin-treated cells in the presence of wild-type cell secretions. As described in a previous report,Citation12) ecmA376:LacZ expression in the stlA/stlB double-knockout mutant was almost completely recovered in the presence of wild-type cell secretions (Fig. (d)). This result strongly suggests that there are ecmA inducing factors in wild-type cell secretions.
Fig. 3. Ax2 cell secretions restored ecmA376:LacZ staining.
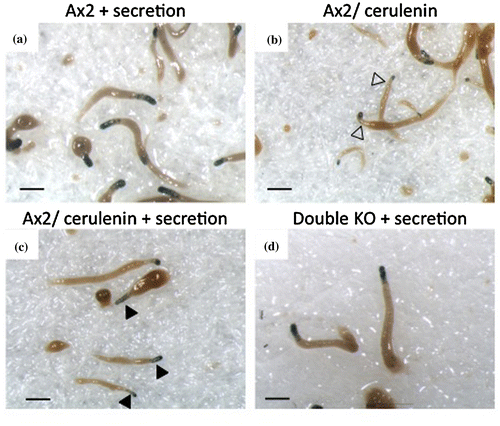
The addition of wild-type cell secretions to cerulenin treated cells recovered light ecmA376:LacZ staining in the anterior 20% of the slugs, though the staining intensity was not recovered to the degree seen in the double-knockout mutant (Fig. (c) and (d)). This difference as indicated in Fig. (c) and (d) also suggests the involvement of PKS(s) other than SteelyA and SteelyB for pstA differentiation. Another interpretation of these results could be that there is a signal relaying system of PKSs whose products such as those of PKS 1 and PKS 2, influence or regulate other PKSs.
Stalk cell differentiates without polyketides
Interestingly, even with extremely low pstA marker gene expression, the cerulenin-treated cells and the stlA/stlB double-knockout mutant cells could form fruiting bodies, although they looked very fragile compared to untreated or wild-type cells. In most cases, wild-type cells of D. discoideum form fruiting bodies with a three-cell layer or a thicker layered stalk around the basal disc. In the cerulenin-treated cells and the stlA/stlB double-knockout mutant cells; however, we observed that more than 50% of the fruiting bodies have single-cell layered, thin stalks (Fig. (b) and (e)). Microscopic analysis showed these stalks were normally vacuolated in the case of double-knockout mutant, this single-layered stalk was most obvious when cells were developed at slightly lower cell density (1 × 106 cells/cm2).
Fig. 4. Single-cell layered stalk differentiation without polyketides.

The stlA single-knockout mutant showed normal, thick stalks (Fig. (c)), while the stlB single-knockout mutant lost basal disks and had stalks that were thinner than the Ax2 cells, but thicker than the stlA/stlB double-knockout mutant or cerulenin-treated cells (Fig. (d)). We used cerulenin at a high enough concentration, that is 300 μM, for PKS inhibitionCitation14) and still found vacuolated stalk cells in fruiting bodies, suggesting this single-cell layered stalk differentiated without polyketides.
DgcA expression in the stlA/stlB double-knockout mutant and cerulenin treated cells
In 2012, the prokaryote-signaling molecule c-di-GMP was reported to trigger stalk cell differentiation in Dictyostelium.Citation10) To examine the involvement of DgcA in stalk cell differentiation in the absence of polyketides, we quantified its expression with qRT-PCR and Lac Z staining at the slug stage in the wild type and stlA/stlB double mutants. The expression levels of dgcA in cerulenin-treated cells and in the stlA/stlB double-knockout mutant cells were higher than in wild-type cells and stlA or stlB single-knockout mutant cells (Fig. (a)). The celurenin-treated cells and the double-knockout mutant cells showed very low levels of ecmA376:LacZ staining and higher levels of dgcA expression. In both cells, mature stalk marker ST:LacZ staining showed in the proper tip site position and showed stronger LacZ staining than in Ax2 cells (Fig. (b)–(d)).
Fig. 5. Expression of dgcA and spatial expression of stalk cell specific ST:LacZ in the slug stage.
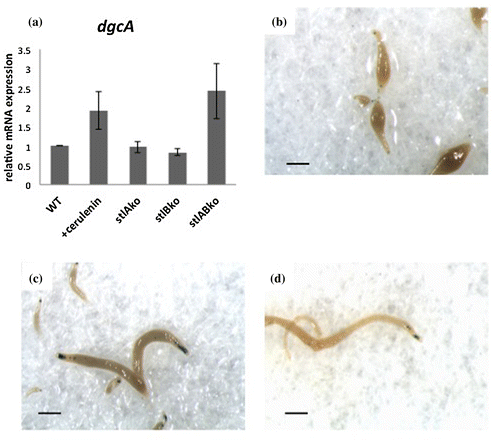
DgcA in Dictyostelium was found to synthesize c-di-GMP and was expressed maximally at the slug stage when cells start to develop prestalk–prespore patterning. We found that the expression of dgcA is upregulated in both the stlA/stlB double-knockout mutant and cerulenin-treated cells. Therefore, fragile stalk differentiation in cerulenin-treated cells and double-knockout mutant cells may be due to higher expression of dgcA.
c-di-GMP requires cooperation with DIF-1 for inducing vacuolization in vitroCitation21); whereas our previous results have shown stlB-dependent DIF-1 is responsible for the development of a basal disc and a lower cup in the fruiting body in vivo.Citation6) One possible explanation for this inconsistency may be due to the difference between in vivo and in vitro conditions. There are three DIF-1 biosynthesis gene knockout mutants (stlB-, chlA-, and dmtA-) that all lack DIF-1 but form vacuolated stalks in vivo.Citation6–8) An in vitro stalk cell assay system may contain artificial conditions that make the assay suitable for the analysis of prestalk cell inducing factors, but not for stalk cell inducing factors. It is also possible that there is another, unknown stalk cell inducer that can produce vacuolated stalk cells without DIF-1 in vivo.
Author contributions
Y. Sato and T. Saito conceived and designed the study. Y. Sato and T. Suarez performed the experiments. Y. Sato and T. Saito wrote the manuscript and all authors approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This study was supported by JSPS KAKENHI [grant number 15K01807] to TS. SASAKAWA SCIENTIFIC RESEARCH GRANT [No.24-535] to YS supported this study.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2016.1162087.
Fig.S1-revjpg.jpg
Download JPEG Image (101.1 KB)Acknowledgments
The authors appreciate Dr. T. Araki (Sophia University) for his critical reading of this manuscript.
Notes
Abbreviations: MPBD, 4-methyl-5-pentylbenzene-1,3-diol; DIF-1, 1-(3,5-dichloro-2,6-dihydroxy-4-methoxyphenyl) hexan-1-on; c-di-GMP, cyclic di-(3,5)-guanosine monophosphate; PKS, polyketide synthase; pstA, prestalkA.
References
- Serafimidis I, Kay RR. New prestalk and prespore inducing signals in Dictyostelium. Dev. Biol. 2005;282:432–441.10.1016/j.ydbio.2005.03.023
- Eichinger L, Pachebat JA, Glöckner G, et al. The genome of the social amoeba Dictyostelium discoideum. Nature. 2005;435:43–57.10.1038/nature03481
- Austin MB, Saito T, Bowman ME, et al. Biosynthesis of Dictyostelium discoideum differentiation-inducing factor by a hybrid type I fatty acid-type III polyketide synthase. Nat. Chem. Biol. 2006;2:494–502.10.1038/nchembio811
- Narita TB, Koide K, Morita N, et al. Dictyostelium hybrid polyketide synthase, SteelyA, produces 4-methyl-5-pentylbenzene-1,3-diol and induces spore maturation. FEMS Microbiol. Lett. 2011;319:82–87.10.1111/fml.2011.319.issue-1
- Anjard C, Su Y, Loomis WF. The polyketide MPBD initiates the SDF-1 signaling cascade that coordinates terminal differentiation in Dictyostelium. Eukaryotic Cell. 2011;10:956–963.10.1128/EC.05053-11
- Saito T, Kato A, Kay RR. DIF-1 induces the basal disc of the Dictyostelium fruiting body. Dev. Biol. 2008;317:444–453.10.1016/j.ydbio.2008.02.036
- Neumann CS, Walsh CT, Kay RR. A flavin-dependent halogenase catalyzes the chlorination step in the biosynthesis of Dictyostelium differentiation-inducing factor 1. Proc. Natl. Acad. Sci. USA. 2010;107:5798–5803.10.1073/pnas.1001681107
- Thompson CR, Kay RR. The role of DIF-1 signaling in Dictyostelium development. Mol. Cell. 2000;6:1509–1514.10.1016/S1097-2765(00)00147-7
- Kay RR. DIF signalling. In: Maeda Y, Inouye K, Takeuchi I, editors. Dictyostelium – a model system for cell and developmental biology. Tokyo: Universal Academy Press; 1996. p. 279–292.
- Chen ZH, Schaap P. The prokaryote messenger c-di-GMP triggers stalk cell differentiation in Dictyostelium. Nature. 2012;488:680–683.10.1038/nature11313
- Chen ZH, Schaap P. Secreted cyclic-di-GMP induces stalk cell differentiation in the eukaryote Dictyostelium discoideum. J. Bacteriol. 2015;198:27–31. doi:10.1128/JB.00321-15
- Sato YG, Kagami HN, Narita TB, et al. Steely enzymes are involved in prestalk and prespore pattern formation. Biosci. Biotechnol. Biochem. 2013;77:2008–2012.10.1271/bbb.130294
- Chance K, Hemmingsen S, Weeks G. Effect of cerulenin on the growth and differentiation of Dictyostelium discoideum. J. Bacteriol. 1976;128:21–27.
- Kay RR. The biosynthesis of differentiation-inducing factor, a chlorinated signal molecule regulating Dictyostelium development. J. Biol. Chem. 1998;273:2669–2675.10.1074/jbc.273.5.2669
- Motohashi KA, Morita N, Kato A, et al. Identification of Des-methyl-DIF-1 methyltransferase in Dictyostelium purpureum. Biosci. Biotechnol. Biochem. 2012;76:1672–1676.10.1271/bbb.120183
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408.10.1006/meth.2001.1262
- Escalante R, Sastre L. Investigating gene expression: in situ hybridization and reporter genes. In: Eichinger L, Rivero F, editors. Dictyostelium discoideum protocols. Totowa, NJ: Humana Press; 2006. p. 230–247.
- Maeda M, Kuwayama H, Yokoyama M, et al. Developmental changes in the spatial expression of genes involved in myosin function in Dictyostelium. Dev. Biol. 2000;223:114–119.10.1006/dbio.2000.9736
- Ōmura S. Cerulenin. Methods Enzymol. 1981;72:520–532.10.1016/S0076-6879(81)72041-X
- Thompson CR, Kay RR. Cell-fate choice in Dictyostelium: intrinsic biases modulate sensitivity to DIF signaling. Dev. Biol. 2000;227:56–64.10.1006/dbio.2000.9877
- Song Y, Luciani MF, Giusti C, et al. c-di-GMP induction of Dictyostelium cell death requires the polyketide DIF-1. Mol. Biol. Cell. 2015;26:651–658.10.1091/mbc.E14-08-1337
