Abstract
Filamentous fungi are extremely polarized organisms, exhibiting continuous growth at their hyphal tips. The hyphal form is related to their pathogenicity in animals and plants, and their high secretion ability for biotechnology. Polarized growth requires a sequential supply of proteins and lipids to the hyphal tip. This transport is managed by vesicle trafficking via the actin and microtubule cytoskeleton. Therefore, the arrangement of the cytoskeleton is a crucial step to establish and maintain the cell polarity. This review summarizes recent findings unraveling the mechanism of polarized growth with special emphasis on the role of actin and microtubule cytoskeleton and polarity marker proteins. Rapid insertions of membranes via highly active exocytosis at hyphal tips could quickly dilute the accumulated polarity marker proteins. Recent findings by a super-resolution microscopy indicate that filamentous fungal cells maintain their polarity at the tips by repeating transient assembly and disassembly of polarity sites.
Maintaining cell polarity is essential for cells to ensure their proper functions.Citation1,2) Symmetry breaking is often preceded by cytoskeleton-dependent polarization of certain key proteins as observed in epithelial cells with apical–basal polarity, neuronal differentiation from dendrites to axons, and migrating cells. Filamentous fungi are highly polarized eukaryotic cells, which continuously elongate their hyphae at the tips. In distal parts, hyphae can initiate new sites of polar growth in the process of branch formation. The establishment and maintenance of polar growth is a fascinating question in biology.Citation3–5) Filamentous fungi are widely used as a model system for the analysis of the relationship between cell polarity and shape. Some filamentous fungi are pathogen to animals and plants. Their filamentous form is important for the invasion into host cells.Citation6) Other fungi are useful in biotechnology, such as for enzyme production, and fermentation in food industry due to their high ability of enzyme secretion.Citation7,8) Thus, the analysis of polarized growth of filamentous fungi can contribute to the medical, agricultural, and biotechnological fields.
The filamentous ascomycete Aspergillus nidulans has been employed worldwide for more than 60 years as a model organism because it is closely related to clinically and economically important Aspergilli, such as Aspergillus fumigatus, Aspergillus oryzae, Aspergillus niger, etc. The most characteristic cell-type of filamentous fungi is the vegetative hyphae, which consist of multinuclear cells compartmentalized by septa. In single-cell yeasts, such as in budding yeast Saccharomyces cerevisiae and in fission yeast Schizosaccharomyces pombe, polarized growth is restricted to specific time point during the cell cycle, whereas in filamentous fungi cell extension is a continuous and indefinite process. Several overviews have summarized different aspects of polarized growth in fungi.Citation5,9–11) This review will focus mainly on the latest findings on the role of cell cytoskeleton and the mechanism of polarity maintenance during apical membrane extension.
I. The actin cytoskeleton
Actin cytoskeleton plays a central role in cell morphogenesis of filamentous fungi.Citation12,13) There are three high order F-actin structures with distinct functions: actin rings, patches, and cables. The actin rings in cooperation with myosin II function in septum formation.Citation14,15) Actin patches are peripheral punctate structures, where the endocytic machinery is probably located.Citation16) The predominant localization of these patches at subapical regions suggests spatial coupling of apical exocytosis and subapical compensatory endocytosis (Fig. (A)),Citation17) in addition to endocytic recycling of polarized material at the hyphal tip.Citation18) The mutant phenotype of genes involved in endocytosis indicates that endocytosis is essential.Citation16)
Fig. 1. Scheme of tip growth in A. nidulans hyphae.
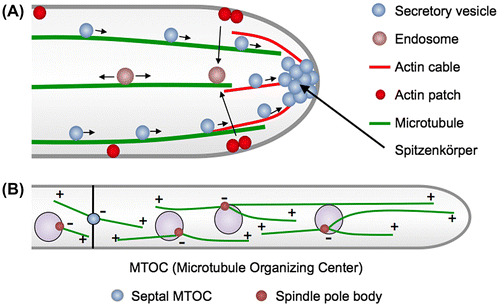
Actin cables are linear bundles of short actin filaments nucleated by formins that are present at the apexes of hyphae. The dynamic actin cables are generally difficult to visualize in filamentous fungi. Phalloidin conjugated to fluorescent dyes has been widely used for imaging F-actin in eukaryotic cells, however, it does not work in most filamentous fungi.Citation19) Recently specific markers, such as Lifeact and tropomyosin, have been developed to visualize actin cables.Citation15,20–22) Actin cables are present at the apex of hyphae and are thought to serve as tracks for myosin V-dependent secretory vesicle transport to the tip (Fig. (A)).Citation12,14,20) The “basic” growth machinery involved in the formation of actin cables, vesicle transport, and exocytosis, such as formin, the polarisome, myosin V, and the exocyst complex, are relatively conserved among eukaryotic cells and localize at the apex of hyphae.Citation23) Before fusion with the plasma membrane, the secretion vesicles accumulate at the so-called “Spitzenkörper” at hyphal tips.Citation24,25) A Spitzenkörper is a special structure in filamentous fungi determining hyphal shape and growth direction.Citation26) Although the exact composition and organization is still not completely understood, one model proposes that the Spitzenkörper acts as a vesicle supply center for growing tips.Citation27)
II. Microtubules
Microtubules (MTs) play a crucial role during mitosis, but also have additional functions in interphase of filamentous fungi. They are important for the distribution of nuclei or other organelles and serve as tracks for endosomes and secretory vesicles, thus they are important for rapid hyphal growth.Citation4,17,28–30)
The rather stable minus end of MTs is located at the MT-organizing center (MTOC), whereas the plus end is facing to the cell periphery with alternating growing and shrinking phases. In filamentous fungi, spindle pole bodies serve as MTOCs (Fig. (B)).Citation31) They contain γ-tubulin, first discovered in A. nidulans, which is required for nucleation of MTs.Citation31,32) Furthermore, areas close to the septa act as MTOCs in A. nidulans (sMTOCs), however, their composition remains elusive.Citation33–35) In the tip compartment of A. nidulans, most MTs are oriented with their dynamic plus ends towards the hyphal tip.Citation36) Nuclei migrate probably along MTs until they reach a certain position. The entire hypha looks therefore very organized with equally spaced nuclei.
III. Vesicle transport through the cell cytoskeleton
Two classes of MT-dependent motors, the minus end-directed dynein and the plus end-directed kinesins, are involved in the positioning of organelles and transport of membranes. Whereas genomes of filamentous fungi contain a single dynein motor, they usually encode 10–12 kinesins.Citation37) The function of kinesin-3 and the dynein motor in the transport of early endosomes has been extensively studied.Citation4,38–40)
The deletion of conventional kinesin (kinesin-1) in different fungi decreased the growth rate, and caused defects in Spitzenkörper stability, protein secretion, and pathogenicity.Citation41–46) These results suggest a possible conserved role in vesicle transportation similar to higher eukaryotic cells. Secretory vesicles are thought to be transported by kinesin-1 along MTs for long distances toward hyphal tips in filamentous fungi, although the localization of ER and Golgi close to hyphal tips raises questions about the function and cargoes of kinesin-1.Citation47,48) Therefore, long distance transport of secretion vesicles could be less important and actin-dependent movement could be rather sufficient for polarized growth. Indeed, hyphal extension can occur long time without functional MTs, but is immediately stopped if the integrity of the actin cytoskeleton is disturbed.Citation13,29) Although the dependency on MT and actin cytoskeleton could be diverse in different fungi, vesicle movement and delivery to the tip plasma membrane likely depends on the cooperation of actin and MT-dependent motors.Citation14,45,49,50)
IV. Polarity markers
Polar cell extension depends on spatially defined insertion of new materials controlled by polarity marker proteins that localize to the plasma membrane. Polarity markers on the plasma membrane determine the polarity site, where actin cables are organized and secretory vesicles are delivered along the actin cables (Fig. (A)). In general, membrane-associated polarity markers are transported via secretory vesicles and supplied to the polarity site by exocytosis. In S. cerevisiae, exocytosis takes place at sites of the Rho GTPase Cdc42, a polarity marker.Citation51) Two positive feedback loops are thought to contribute to Cdc42 polarization. One pathway involves recruiting GEFs (guanine nucleotide exchange factors) and effector complexes from the cytoplasm in a cytoskeleton-independent manner.Citation52) The other one is a vesicle-recycling feedback loop, where Cdc42 orients actin cables, which in turn deliver Cdc42 as cargo on secretory vesicles.Citation53)
Fig. 2. Relationship between polarity marker and exocytosis.
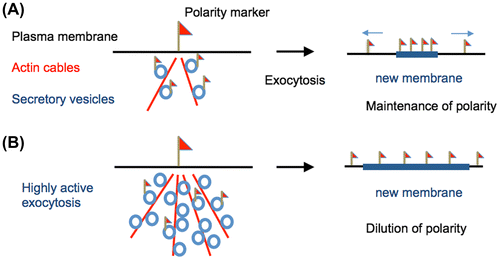
As a consequence of exocytosis at the polarity site, the added membrane may result in dilution or dispersion of the membrane-associated polarity markers (Fig. (B)).Citation54) Recent mathematical models predict that, under certain simulated conditions with highly active exocytosis (8–32 times faster than in budding yeast), membrane insertion via exocytosis can almost eradicate concentrated Cdc42 polarity complexes.Citation55) The dilution of polarity marker due to exocytic membrane insertion has been shown to be essential for effective chemotropism in yeast.Citation56)
Rho type GTPases, Cdc42, and Rac1 (ModA and RacA), have also been found in A. nidulans.Citation57) Both Cdc42 and Rac1 share at least one overlapping function that is required for polarity establishment. The combination of Δcdc42 with Δrac1 appeared synthetically lethal in A. nidulans.Citation57) Besides the Rho GTPases, the so-called “cell-end markers” (TeaA and TeaR) function as polarity markers. The cell-end markers localize at the tip of the hyphae and control growth direction (Fig. (A)).Citation58) TeaA, is delivered specifically to the apex by growing MTs, and is anchored to the membrane by direct interaction with another cell-end marker at the plasma membrane, TeaR.Citation58,59) TeaR has CaaX motif for post-translational lipid modification and anchors to the cell membrane. The interaction of TeaA and TeaR at the apical membrane initiates the recruitment of additional downstream components including the formin SepA, which polymerizes actin cables for targeted cargo delivery.Citation60) Deletion of cell-end markers results in highly curved or zigzagged instead of straight hyphae.Citation58) Structurally straight MTs growing to hyphal tips transmit positional information to cell-end markers at hyphal tips. This mechanism is important for the maintenance of polarity sites at hyphal tips and growth direction.Citation5) In addition, a functional connection between TeaA and a MT polymerase AlpA is required for the proper regulation of MT growth at hyphal tips and focusing of polarity.Citation11,61)
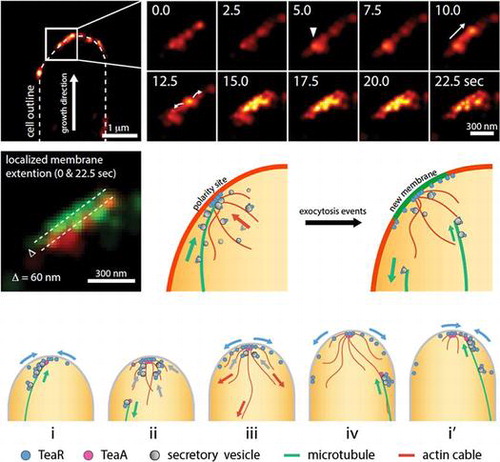
Fig. 3. Transient polarity model. (adapted from [63]).
![Fig. 3. Transient polarity model. (adapted from [63]).](/cms/asset/96adbc82-ffdb-4639-bed1-a0d78845129d/tbbb_a_1179092_f0003_oc.gif)
In A. nidulans, hyphal tips extend with speeds of 0.3–1.0 μm/min, requiring a high frequency of exocytosis events (400–2000 vesicles/min). Hyphal elongation in A. nidulans is approximately 10–30 times faster than bud formation in budding yeast, indicating more active vesicle transport and exocytosis.Citation29,54,62) Although thousands of exocytosis events take place at the apex every minute, which may displace membrane-associated polarity markers from the growing tip (Fig. (B)), filamentous fungi are able to maintain their polarity sites. How can filamentous fungi maintain the accumulation of polarity markers in the presence of rapid insertions of membranes via highly active exocytosis?
V. Transient polarity assembly in active exocytosis
Our recent study shows that polarity sites visualized by the membrane-associated cell-end marker TeaR are highly dynamic at growing hyphal tips.Citation63) A MT grows towards the hyphal tip, pauses in close contact with the apical membrane, and then undergoes a catastrophe resulting in retraction. The TeaR signal accumulates at the hyphal tip membrane, whenever a MT plus-end touches the tip membrane. The TeaR fluorescence decreases immediately after the MT starts to shrink. Colocalization studies further support the notion that TeaR clusters represent zones of exocytosis and extension of the apical membrane.
The localization of TeaR cluster at hyphal tips has been analyzed by super-resolution microscopy PALM (photoactivation localization microscopy).Citation63) The resolution of conventional light microscopy techniques is limited around 250 nm due to light diffraction. Super-resolution microscopy techniques, such as STED, SIM, STORM, PALM, etc., have overcome the diffraction limit, resulting in lateral image resolution as high as 20 nm and providing a powerful tool for the investigation of protein localization in high detail.Citation64,65) The PALM imaging clearly shows TeaR clusters near the apex of the cell and also along the plasma membrane (Fig. (B)). The average size of TeaR clusters is approximately 120 nm. Quantified analysis using the PALM data suggests an estimated 20 TeaR proteins per cluster on average. The timelapse PALM reveals many abrupt appearances and disappearances of clusters near the plasma membrane. In some instances, a cluster appears, moves along the plasma membrane, mostly towards the apex, spreads along the membrane and then disappears (Fig. (B)). Overlay of the first and the last frame shows a small membrane growth. These images are most likely to indicate a fusion-like morphological change of TeaR clusters followed by membrane extension, where a small patch of membrane is added via exocytosis, and the TeaR cluster gets quickly dispersed over the membrane.
These results propose a “transient polarity model” (Fig. (C)).Citation63) In this model, the interaction between the MT plus-end, where TeaA is located, and TeaR at the apical membrane initiates the recruitment of other polarity markers, resulting in the assembly of TeaR polarity site (Fig. (C, i)). The accumulated cell-end markers induce actin polymerization followed by active exocytosis (ii). Newly synthesized TeaR is delivered to the tip membrane on secretory vesicles through MT and actin cables. The plasma membrane extends locally at the site of vesicle fusion (iii) and, subsequently, the TeaR polarity site is dispersed or displaced along the membrane (iv). Once the polarity site is disassembled, however, next MT comes to the tip and gathers TeaR floating in the membrane through the interaction with TeaA at the MT plus-end and the cycle starts over (i′). This model is qualitatively reproduced by computer simulation that includes the plasma membrane, exocytic and endocytic vesicles, MTs, actin, and TeaR.Citation63) Moreover, simultaneous visualization of actin cables and MTs suggests the temporally and spatially coordinated polymerization and depolymerization between the two cytoskeletons, as shown in the model.Citation22)
This transient polarity model provides an explanation for maintenance of the polarized growth in filamentous fungi. In contrast to the static polarity site picture, the polarity site is transiently established by MTs that transport cell-end marker proteins to the apical membrane. The polarity sites are localized near the apex of the hyphae because MT plus-ends tend to reach there before the catastrophe.Citation61) When MT plus-ends come in contact with the apex, downstream reactions are triggered locally. The transfer of membrane via exocytosis depletes active binding proteins for secretory vesicles, dilutes the material at the site, and leads to disassembly of polarity. Using the residual TeaR as a guide, the polarity site is rapidly reassembled by the arrival of a new MT carrying the next batch of cell-end marker proteins at a slightly displaced location. In line with this model, recent work on another filamentous fungus, Neurospora crassa, reports “bursts of exocytosis” events at different positions within the apical membrane rather than a persistent exocytosis site.Citation66) The subtle change in TeaR accumulation sites can result in a change in the overall growth direction. This mechanism of short bursts of cell extension also provides more opportunities for the cell to adjust growth directions and allows quicker responses to environmental signals, such as chemotropism, or to avoid obstacles.
VI. Coordinated cycles of cell growth
The transient polarity model suggests a coordinated process by several steps and cycles (or oscillations without a fixed period). Coordinated cycles or oscillations of exocytosis associated with cell growth may be a conserved phenomenon shared among various organisms, including filamentous fungi,Citation67) mammalian cells,Citation68) fission yeast,Citation69) and plant root hairsCitation70) and pollen tubes.Citation71) The intracellular Ca2+ level is known to regulate actin assembly and vesicle fusion.Citation72,73) Plant cells show an oscillation in the Ca2+ concentrations at the cell tip, which is correlated with the change in growth rate. In mammalian cells, oscillations of cortical actin and Ca2+ concentrations correlate with oscillations of vesicle secretion.Citation68) Ca2+ oscillations have also been observed at hyphal tips in filamentous fungi, suggesting that the oscillations are related to the growth rate.Citation74) The Ca2+ oscillation is likely to play an important role in regulating actin polymerization and triggering the synchronized fusion of accumulated vesicles within a local region in the tip growth of filamentous fungi as well.
These cycles of cell growth suggest a transient waiting time of secretory vesicles before exocytosis. Polarity markers, exocyst complex, and SNARE proteins, which are required for exocytosis, could be diluted locally on the plasma membrane after the exocytosis. The transient waiting time until an upcoming exocytosis event may allow these components to rearrange at the next polarity sites. Traffic lights indicating “go” and “stop” for secretory vesicles may be necessary to guarantee robust polarized growth. In addition, the stepwise growth allows cells to respond more quickly and often to internal and external cues such as chemical or mechanical environmental signals.
Disclosure statement
No potential conflict of interest was reported by the author.
Funding
The work was supported by the DFG [TA819/2-1]; JSPS KAKENHI [grant number 15K18663].
References
- Wu CF, Lew DJ. Beyond symmetry-breaking: competition and negative feedback in GTPase regulation. Trends Cell Biol. 2013;23:476–483.10.1016/j.tcb.2013.05.003
- Goehring NW, Grill SW. Cell polarity: mechanochemical patterning. Trends Cell Biol. 2013;23:72–80.10.1016/j.tcb.2012.10.009
- Riquelme M, Yarden O, Bartnicki-Garcia S, et al. Architecture and development of the Neurospora crassa hypha – a model cell for polarized growth. Fungal Biol. 2011;115:446–474.10.1016/j.funbio.2011.02.008
- Steinberg G. Motors in fungal morphogenesis: cooperation versus competition. Curr. Opin. Microbiol. 2011;14: 660–667.
- Fischer R, Zekert N, Takeshita N. Polarized growth in fungi–interplay between the cytoskeleton, positional markers and membrane domains. Mol. Microbiol. 2008;68:813–826.10.1111/j.1365-2958.2008.06193.x
- Garcia-Vidal C, Viasus D, Carratalà J. Pathogenesis of invasive fungal infections. Curr. Opin. Infect. Dis. 2013;26:270–276.10.1097/QCO.0b013e32835fb920
- Punt PJ, van Biezen N, Conesa A, et al. Filamentous fungi as cell factories for heterologous protein production. Trends Biotechnol. 2002;20:200–206.10.1016/S0167-7799(02)01933-9
- Kobayashi T, Abe K, Asai K, et al. Genomics of Aspergillus oryzae. Biosci. Biotech. Biochem. 2007;71:646–670.10.1271/bbb.60550
- Chang F, Peter M. Yeasts make their mark. Nature Cell Biol. 2003;5:294–299.10.1038/ncb0403-294
- Harris SD, Momany M. Polarity in filamentous fungi: moving beyond the yeast paradigm. Fungal Genet. Biol. 2004;41:391–400.10.1016/j.fgb.2003.11.007
- Takeshita N, Manck R, Grün N, et al. Interdependence of the actin and the microtubule cytoskeleton during fungal growth. Curr. Opin. Microbiol. 2014;20:34–41.10.1016/j.mib.2014.04.005
- Berepiki A, Lichius A, Read ND. Actin organization and dynamics in filamentous fungi. Nat. Rev. Microbiol. 2011;9:876–887.10.1038/nrmicro2666
- Torralba S, Raudaskoski M, Pedregosa AM, et al. Effect of cytochalasin A on apical growth, actin cytoskeleton organization and enzyme secretion in Aspergillus nidulans. Microbiology. 1998;144(Pt 1):45–53.10.1099/00221287-144-1-45
- Taheri-Talesh N, Xiong Y, Oakley BR. The functions of myosin II and myosin V homologs in tip growth and septation in Aspergillus nidulans. PLoS ONE. 2012;7:e31218.10.1371/journal.pone.0031218
- Delgado-Álvarez DL, Callejas-Negrete OA, Gómez N, et al. Visualization of F-actin localization and dynamics with live cell markers in Neurospora crassa. Fungal Genet. Biol. 2010;47:573–586.10.1016/j.fgb.2010.03.004
- Araujo-Bazán L, Peñalva MA, Espeso EA. Preferential localization of the endocytic internalization machinery to hyphal tips underlies polarization of the actin cytoskeleton in Aspergillus nidulans. Mol. Microbiol. 2008;67:891–905.10.1111/mmi.2008.67.issue-4
- Peñalva MA. Endocytosis in filamentous fungi: Cinderella gets her reward. Curr. Opin. Microbiol. 2010;13:684–692.10.1016/j.mib.2010.09.005
- Shaw BD, Chung DW, Wang CL, et al. A role for endocytic recycling in hyphal growth. Fungal Biol. 2011;115:541–546.10.1016/j.funbio.2011.02.010
- Brent Heath I, Bonham M, Akram A, et al. The interrelationships of actin and hyphal tip growth in the ascomycete Geotrichum candidum. Fungal Genet. Biol. 2003;38:85–97.10.1016/S1087-1845(02)00511-X
- Taheri-Talesh N, Horio T, Araujo-Bazan L, et al. The tip growth apparatus of Aspergillus nidulans. Mol. Biol. Cell. 2008;19:1439–1449.10.1091/mbc.E07-05-0464
- Berepiki A, Lichius A, Shoji JY, et al. F-actin dynamics in Neurospora crassa. Eukaryot. Cell. 2010;9:547–557.10.1128/EC.00253-09
- Bergs A, Ishitsuka Y, Evangelinos M, et al. Dynamics of actin cables in polarized growth of the filamentous fungus Aspergillus nidulans. Front Microbiol. in press.
- Sudbery P. Fluorescent proteins illuminate the structure and function of the hyphal tip apparatus. Fungal Genet Biol. 2011;48:849–857.10.1016/j.fgb.2011.02.004
- Grove SN, Bracker CE. Protoplasmic organization of hyphal tips among fungi: vesicles and Spitzenkörper. J. Bacteriol. 1970;104:989–1009.
- Harris SD, Read ND, Roberson RW, et al. Polarisome meets spitzenkorper: microscopy, genetics, and genomics converge. Eukaryot. Cell. 2005;4:225–229.10.1128/EC.4.2.225-229.2005
- Riquelme M, Reynaga-Peña CG, Gierz G, et al. What determines growth direction in fungal hyphae? Fungal Genet. Biol. 1998;24:101–109.10.1006/fgbi.1998.1074
- Bartnicki-Garcia S, Bartnicki DD, Gierz G, et al. Evidence that Spitzenkörper behavior determines the shape of a fungal hypha: a test of the hyphoid model. Exp. Mycol. 1995;19:153–159.10.1006/emyc.1995.1017
- Egan MJ, Tan K, Reck-Peterson SL. Lis1 is an initiation factor for dynein-driven organelle transport. J. Cell Biol. 2012;197:971–982.10.1083/jcb.201112101
- Horio T, Oakley BR. The role of microtubules in rapid hyphal tip growth of Aspergillus nidulans. Mol Biol Cell. 2005;16:918–926.
- Xiang X, Fischer R. Nuclear migration and positioning in filamentous fungi. Fungal Genet. Biol. 2004;41:411–419.10.1016/j.fgb.2003.11.010
- Oakley BR, Oakley CE, Yoon Y, et al. γ-tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans. Cell. 1990;61:1289–1301.10.1016/0092-8674(90)90693-9
- Oakley CE, Oakley BR. Identification of γ-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature. 1989;338:662–664.10.1038/338662a0
- Veith D, Scherr N, Efimov VP, et al. Role of the spindle-pole-body protein ApsB and the cortex protein ApsA in microtubule organization and nuclear migration in Aspergillus nidulans. J. Cell Sci. 2005;118:3705–3716.10.1242/jcs.02501
- Xiong Y, Oakley BR. In vivo analysis of the functions of gamma-tubulin-complex proteins. J. Cell Sci. 2009;122:4218–4227.10.1242/jcs.059196
- Zekert N, Fischer R. The Aspergillus nidulans kinesin-3 UncA motor moves vesicles along a subpopulation of microtubules. Mol. Biol. Cell. 2009;20:673–684.
- Konzack S, Rischitor PE, Enke C, et al. The role of the kinesin motor KipA in microtubule organization and polarized growth of Aspergillus nidulans. Mol. Biol. Cell. 2005;16:497–506.
- Schoch CL, Aist JR, Yoder OC, et al. A complete inventory of fungal kinesins in representative filamentous ascomycetes. Fungal Genet. Biol. 2003;39:1–15.10.1016/S1087-1845(03)00022-7
- Egan MJ, McClintock MA, Reck-Peterson SL. Microtubule-based transport in filamentous fungi. Curr. Opin. Microbiol. 2012;15:637–645.10.1016/j.mib.2012.10.003
- Seidel C, Moreno-Velasquez SD, Riquelme M, et al. Neurospora crassa NKIN2, a kinesin-3 motor, transports early endosomes and is required for polarized growth. Eukaryot. Cell. 2013;12:1020–1032.10.1128/EC.00081-13
- Higuchi Y, Ashwin P, Roger Y, et al. Early endosome motility spatially organizes polysome distribution. J. Cell Biol. 2014;204:343–357.10.1083/jcb.201307164
- Seiler S, Nargang FE, Steinberg G, et al. Kinesin is essential for cell morphogenesis and polarized secretion in Neurospora crassa. EMBO J. 1997;16:3025–3034.10.1093/emboj/16.11.3025
- Seiler S, Plamann M, Schliwa M. Kinesin and dynein mutants provide novel insights into the roles of vesicle traffic during cell morphogenesis in Neurospora. Curr. Biol. 1999;9:779–785.10.1016/S0960-9822(99)80360-1
- Requena N, Alberti-Segui C, Winzenburg E, et al. Genetic evidence for a microtubule-destabilizing effect of conventional kinesin and analysis of its consequences for the control of nuclear distribution in Aspergillus nidulans. Mol. Microbiol. 2001;42:121–132.
- Lehmler C, Steinberg G, Snetselaar KM, et al. Identification of a motor protein required for filamentous growth in Ustilago maydis. EMBO J. 1997;16:3464–3473.10.1093/emboj/16.12.3464
- Schuster M, Treitschke S, Kilaru S, et al. Myosin-5, kinesin-1 and myosin-17 cooperate in secretion of fungal chitin synthase. EMBO J. 2012;31: 214–227.
- Takeshita N, Wernet V, Tsuizaki M, et al. Transportation of Aspergillus nidulans class III and V chitin synthases to the hyphal tips depends on conventional kinesin. PLoS ONE. 2015;10:e0125937.10.1371/journal.pone.0125937
- Markina-Iñarrairaegui A, Pantazopoulou A, Espeso EA, et al. The Aspergillus nidulans peripheral ER: disorganization by ER stress and persistence during mitosis. PLoS ONE. 2013;8:e67154.10.1371/journal.pone.0067154
- Pinar M, Pantazopoulou A, Arst HN Jr, et al. Acute inactivation of the Aspergillus nidulans Golgi membrane fusion machinery: correlation of apical extension arrest and tip swelling with cisternal disorganization. Mol. Microbiol. 2013;89:228–248.10.1111/mmi.12280
- Zhang J, Tan K, Wu X, et al. Aspergillus myosin-V supports polarized growth in the absence of microtubule-based transport. PLoS ONE. 2011;6:e28575.10.1371/journal.pone.0028575
- Pantazopoulou A, Pinar M, Xiang X, et al. Maturation of late Golgi cisternae into RabERAB11 exocytic post-Golgi carriers visualized in vivo. Mol. Biol. Cell. 2014;25:2428–2443.10.1091/mbc.E14-02-0710
- Etienne-Manneville S. Cdc42–the centre of polarity. J. Cell Sci. 2004;117:1291–1300.10.1242/jcs.01115
- Johnson JM, Jin M, Lew DJ. Symmetry breaking and the establishment of cell polarity in budding yeast. Curr. Opin. Genet. Dev. 2011;21:740–746.10.1016/j.gde.2011.09.007
- Wedlich-Soldner R, Altschuler S, Wu L, et al. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science. 2003;299:1231–1235.10.1126/science.1080944
- Layton AT, Savage NS, Howell AS, et al. Modeling vesicle traffic reveals unexpected consequences for Cdc42p-mediated polarity establishment. Curr. Biol. 2011;21:184–194.10.1016/j.cub.2011.01.012
- Savage NS, Layton AT, Lew DJ. Mechanistic mathematical model of polarity in yeast. Mol. Biol. Cell. 2012;23:1998–2013.10.1091/mbc.E11-10-0837
- Dyer JM, Savage NS, Jin M, et al. Tracking shallow chemical gradients by actin-driven wandering of the polarization site. Curr. Biol. 2013;23:32–41.10.1016/j.cub.2012.11.014
- Virag A, Lee MP, Si H, et al. Regulation of hyphal morphogenesis by cdc42 and rac1 homologues in Aspergillus nidulans. Mol Microbiol. 2007;66:1579–1596.
- Takeshita N, Higashitsuji Y, Konzack S, et al. Apical sterol-rich membranes are essential for localizing cell end markers that determine growth directionality in the filamentous fungus Aspergillus nidulans. Mol. Biol. Cell. 2008;19:339–351.10.1091/mbc.E07-06-0523
- Takeshita N, Fischer R. On the role of microtubules, cell end markers, and septal microtubule organizing centres on site selection for polar growth in Aspergillus nidulans. Fungal Biol. 2011;115:506–517.10.1016/j.funbio.2011.02.009
- Higashitsuji Y, Herrero S, Takeshita N, et al. The cell end marker protein TeaC is involved in growth directionality and septation in Aspergillus nidulans. Eukaryot. Cell. 2009;8:957–967.10.1128/EC.00251-08
- Takeshita N, Mania D, Herrero S, et al. The cell-end marker TeaA and the microtubule polymerase AlpA contribute to microtubule guidance at the hyphal tip cortex of Aspergillus nidulans to provide polarity maintenance. J. Cell Sci. 2013;126:5400–5411.10.1242/jcs.129841
- Marco E, Wedlich-Soldner R, Li R, et al. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell. 2007;129:411–422.10.1016/j.cell.2007.02.043
- Ishitsuka Y, Savage N, Li Y, et al. Superresolution microscopy reveals a dynamic picture of cell polarity maintenance during directional growth. Science Adv. 2015;1:e1500947.10.1126/sciadv.1500947
- Betzig E, Patterson GH, Sougrat R, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645.10.1126/science.1127344
- Sahl SJ, Moerner WE. Super-resolution fluorescence imaging with single molecules. Curr. Opin. Struct. Biol. 2013;23:778–787.10.1016/j.sbi.2013.07.010
- Riquelme M, Bredeweg EL, Callejas-Negrete O, et al. The Neurospora crassa exocyst complex tethers Spitzenkorper vesicles to the apical plasma membrane during polarized growth. Mol. Biol. Cell. 2014;25:1312–1326.10.1091/mbc.E13-06-0299
- Lopez-Franco R, Bartnicki-Garcia S, Bracker CE. Pulsed growth of fungal hyphal tips. Proc. Natl. Acad. Sci. 1994;91:12228–12232.10.1073/pnas.91.25.12228
- Wollman R, Meyer T. Coordinated oscillations in cortical actin and Ca2+ correlate with cycles of vesicle secretion. Nat. Cell Biol. 2012;14:1261–1269.10.1038/ncb2614
- Das M, Drake T, Wiley DJ, et al. Oscillatory dynamics of Cdc42 GTPase in the control of polarized growth. Science. 2012;337:239–243.10.1126/science.1218377
- Monshausen GB, Messerli MA, Gilroy S. Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol. 2008;147:1690–1698.10.1104/pp.108.123638
- Holdaway-Clarke TL, Feijo JA, Hackett GR, et al. Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell. 1997;9:1999–2010.10.1105/tpc.9.11.1999
- Janmey PA. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu. Rev. Physiol. 1994;56:169–191.10.1146/annurev.ph.56.030194.001125
- Schneggenburger R, Neher E. Presynaptic calcium and control of vesicle fusion. Curr. Opin. Neurobiol. 2005;15:266–274.10.1016/j.conb.2005.05.006
- Kim HS, Czymmek KJ, Patel A, et al. Expression of the Cameleon calcium biosensor in fungi reveals distinct Ca2+ signatures associated with polarized growth, development, and pathogenesis. Fungal GenetBiol. 2012;49:589–601.10.1016/j.fgb.2012.05.011
