Abstract
Linalool is an important compound that contributes to the floral aroma in wines. This study showed the effect of light exposure on linalool accumulation in berries. The grape bunches were covered with films that block the full light spectrum (Shade) and the UV spectrum (UV-block), and a transparent film (Control). The linalool content was significantly higher in juice from Control-covered berries than in juice from Shade- and UV-block-covered berries, and the expression levels of the representative genes in linalool biosynthesis in Shade- and UV-block-covered berries were markedly lower than in Control-covered berries. These findings suggest that exposing berries to light is essential for linalool biosynthesis. To reflect sunlight onto grape clusters, reflective sheets were placed on the ground of a vineyard. The linalool content in berries exposed to sunlight reflected from the reflective sheets was higher than those in the control.
Graphical abstract
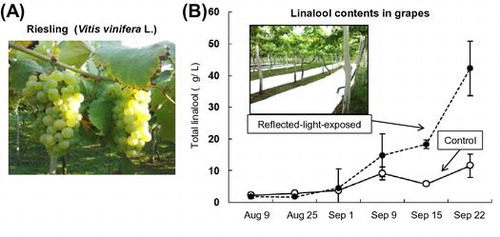
Effect of reflective sheets on linalool accumulation. (A) The grapes of Riesling (Vitis vinifera L.), (B) Linalool contents in grapes.
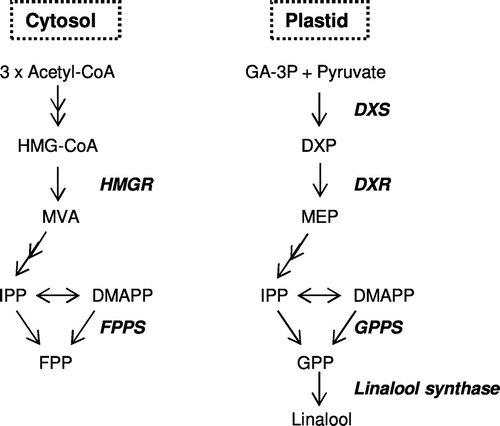
Monoterpenols have a large variety of chemical structures and physiological functions, such as the defense against pathogens and herbivores, the attraction of pollinators to floral scents, and the attraction of seed-disseminating animals to the flavors of ripening fruits. They are derived from geranyl pyrophosphate (GPP), which is biosynthesized by the condensation of isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) (Fig. ). IPP and DMAPP are produced via two pathways: the mevalonate (MVA) pathway in the cytosol and the methylerythritol phosphate (MEP) pathway in plastids. A possible crosstalk between the MVA and MEP pathways across the plastid membrane has been suggested at the compound level in plantsCitation1,2); however, the MEP pathway has also been reported to be the major route for monoterpenol biosynthesis in grapevine.Citation3) It has been demonstrated that 1-deoxy-D-xylulose 5-phosphate synthase (DXS) and 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR) catalyze the rate-limiting steps in the MEP pathway; in particular, DXS has also been suggested as a positional candidate gene for the concentration of several monoterpenols in grape berries.Citation4)
Fig. 1. Representative genes in MEP and MVA pathway.
The numerous secondary metabolites derived from grapes, such as volatile phenols, terpenoids, and sulfur compounds, are reported to contribute to wine flavor and aroma. Monoterpenols are among the most important wine aromatic compounds associated with floral scents and are reported to be present in grapes in the free or glycosylated form.Citation5,6) Some of the conjugated volatile compounds including glycosides can be converted to free volatiles by enzymes or acid hydrolysis in alcohol fermentation,Citation7) and they have been identified in many grapevines as important flavor precursors.Citation8,9) The contents and compositions of these secondary metabolites in grapevines are affected by the genotype, growth stage, and environmental conditions, such as air temperature, sunlight exposure, and soil characteristics.Citation10–12) In grapevine cultivation, the manipulation of environmental conditions has been carried out using various viticultural techniques such as leaf removal and irrigation,Citation10,13,14) which control the synthesis of secondary metabolites. It has also been shown that the expression of DXS and DXR, the important genes in the MEP pathway, is induced by light in Arabidopsis plants.Citation15,16) In this study, we investigated the effect of light exposure on the biosynthesis and content of linalool, one of the most important monoterpenols in grapevines, using two approaches: reducing light exposure using films and enhancement of light exposure using reflective sheets.
Materials and methods
Reducing light exposure of grape clusters using various films
The vineyards cultivating Sauvignon blanc grapevines were located in Yamanashi Prefecture, Japan (latitude, 35°39′33″N; longitude, 138°43′25″E; elevation, 392 m), and these grapevines were approximately 10 years old and trained in a vertical shoot position (Guyot Double type). The bunches of Sauvignon blanc grape were covered with films that block the full light spectrum (Shade) and the UV (<390 nm) spectrum (UV-block), and a transparent film as a control (Control) on July 31 2012. The Shade, UV-block, and Control films were as follows: Hakuryoku, Cutace (Mitsubishi Plastics Agri Dream Co., Tokyo, Japan), and Ziploc (Asahi Kasei Home Products Co., Tokyo, Japan), respectively. Approximately 100 grape berries were randomly sampled from grape clusters. Sampling was conducted five times from August 1 to September 11 in the 2012 growing season. Li-190 SA (Li-COR Inc., Lincoln, NE, USA) sensor for Photosynthetically active radiation (PAR) level, Li-201SA sensor (Li-COR Inc.) for full-light spectral illuminance, and the UD-360 sensor (range: 310–400 nm, UVR-300, Topcon Technohouse Co. Tokyo, Japan) and the UV-6.0 sensor (range: 280–315 nm, MK Scientific, Inc. Kanagawa, Japan) for UV light illuminance were used, respectively.
Placement of reflective sheets on the ground of a Riesling vineyard
Reflective sheets (Pearl light; Futamura Chemical Co., Nagoya, Japan) were placed on the ground of a Riesling vineyard located in Akita Prefecture, Japan (latitude, 39°20′15″N; longitude, 140°23′33″E; elevation, 52 m) on August 9 2012. Illuminance was measured using a Digital Lux meter (LX-1332D, Custom Co.) at 12:00 am on this same day, which had fair weather conditions. These grapevines were approximately 30 years old and grown using overhead trellis. Grape berries were randomly sampled six times from August 9 to September 22 in the 2012 growing season, and the repeatability of measurements was confirmed in 2011 and 2013 vintages. In 2012, the start of flowering was June 18; full bloom occurred on June 28; start of veraison was August 21.
Extraction of total amounts of linalool from grape tissue
The tissue of grape berries was frozen and then pulverized in liquid nitrogen using a mixer mill (MM 400, Retsch, Haan, Germany). Samples were extracted according to a previous report with slight modifications.Citation17) The powder (1.0 g, fresh weight) was mixed with 80% methanol (2.5 mL) for 16 h at 4 °C and the resulting extract was diluted with water (7.5 mL). The diluted extract was centrifuged and the resulting supernatant was subjected to stir-bar-sorptive extraction (SBSE) as described below.
Preparation and analysis of grape juice sample
Juice was prepared by pressing whole berries to 60% of the total berry weight using a micropress (Quick juicer, Chibakogyosyo Co., Chiba, Japan). The contents of total soluble solids in the juice were measured with a refractometer (Pocket PaL-1, Atago Co. Ltd., Tokyo, Japan) and expressed as Brix. Titratable acidity was determined by adding 10 mL of distilled water to 10 mL of juice and the diluted juice was subjected to neutralization titration using 0.1 N NaOH. Titratable acidity was expressed as g tartaric acid/100 mL. Linalool glycosides were liberated by acid hydrolysis according to a previous report with slight modifications,Citation17) and then the free and glycoconjugated forms of linalool were quantitatively analyzed as total amounts of linalool by SBSE-GC-MS as described below.
Stir-bar-sorptive extraction (SBSE)
A stir bar coated with 24 μL of polydimethylsiloxane (PDMS, Twister, GERSTEL GmbH, Mülheim an der Ruhr, Germany) was conditioned for 60 min at 300 °C in a flow of nitrogen. The juice was transferred to 10 mL vials, and the vials were sealed with a screw cap after the addition of the stir bar. SBSE was performed at 100 °C for 60 min by stirring at 1500 rpm. The stir bar was washed with Milli-Q water, dried with lint-free paper, and introduced to a glass thermal desorption liner. The glass liner was placed in a thermal desorption unit.
GC-MS conditions
GC-MS was performed using a GC system (7890N, Agilent Technologies Japan Ltd., Tokyo, Japan) and an MS detector system (5975C inert mass selective detector, Agilent Technologies). The GC system was equipped with a thermal desorption unit (TDU, GERSTEL GmbH) and a programmed temperature vaporizing injector (CIS4, GERSTEL GmbH). For thermal desorption, the TDU was programmed from 40 °C (held for 0.5 min) to 250 °C (held for 3 min) at 720 °C/min and 50 mL/min desorption flow. The transfer capillary temperature was fixed at 300 °C. Desorbed compounds were trapped at 10 °C on a Tenax TA packed liner in CIS4. After desorption, CIS4 was programmed from 10 °C to 250 °C (held for 1 min) at 720 °C/min to inject trapped compounds onto the column in the splitless mode with the splitless time of 2 min. A fused silica capillary column (BP-20, 50 m × 0.22 mm, 0.25 μm film thickness, SGE Analytical Science, Melbourne, Australia) was used and the temperature program had a cycle of 60 °C (3 min), 3 °C/min to 200 °C, and 10 °C/min to 245 °C (10 min). The ions monitored in single-ion-mode runs corresponded to m/z 71, 93, and 121 for linalool, and m/z 83, 101, and 73 for the internal standard (4-nonanol). Each compound was identified by direct comparison with the standard specimen.
Quantitative RT-PCR
Total RNA was extracted from whole berries including the skin, flesh, and seeds by the CTAB method and purified using an RNeasy Plant Mini kit (Qiagen, Hilden, Germany). DNase treatment was conducted prior to column purification of RNA according to the manufacturer’s instructions (Qiagen). Reverse transcription was performed using SuperScript III RNaseH- transcriptase (Thermo Fisher Scientific Inc., MA, USA) with 2.0 μg of total RNA as a template. Polymerase chain reaction was carried out using an ABI PRISM 7300 real-time PCR system (Thermo Fisher Scientific Inc.) with SYBR Premix Ex Taq II according to the manufacturer’s instructions. The primer pairs were as follows: DXS (Fw, 5′-GCTGGCTTAGGGATGGCAGTTG-3′; Rv, 5′-GAGGAGCAGGGCCATCAACAGT-3′), DXR (Fw, 5′-TGCTGGGGGTCCTTTTGTCCTTCC-3′; Rv, 5′-TCAACGGGCCAATCCCTGAATGC-3′), GPP synthase (GPPS; accession number, AY351862; Fw, 5′-TGTGTGGCGCTTGCCTCTCTGA-3′; Rv, 5′-TCCGCAGTTTGCCCTGCAAGAA-3′), linalool synthase (accession number, JQ062931; Fw, 5′-GCATGTGCCAAAGGCCCACGAGTA-3′; Rv, 5′-TGACCCGTCGTGGCCATCTTGA-3′), HMG-CoA reductase (HMGR; accession numbers, XM_002275791.3 and XM_002283147.3; Fw, 5′-TGAAGGGCGTGGTAAGTCTGTGG-3′; Rv, 5′-AACATTTTGGGCCGGATCTTGG-3′), farnesyl pyrophosphate synthase (FPPS; accession number, AY966012; Fw, 5′-TTGCCTCTTCACCGCCGCATTG-3′; Rv, 5′-TGGGGGTCACCGAAACAATCCAGA-3′).
For normalization, 18S rRNA was used as an external standard (accession number, AF207053; Fw, CGAAAGCATTTGCCAAGGAT; Rv, CCTGGTCGGCATCGTTTATG). The experiment was carried out in triplicate.
Results and discussion
Organ-specific accumulation of linalool and genes involved in linalool biosynthesis
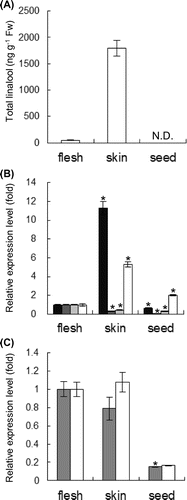
Linalool is one of monoterpenols responsible for the specific aroma found in grapes and wines. It is biosynthesized by linalool synthases from GPP via the MEP pathway, and DXS and DXR are the important enzymes of this pathway. In Riesling berries, total linalool (sum of free and glycosylated forms) accumulated mainly in the skin (80% per berry) and was not detected in the seed (Fig. (A)).Citation6) Gene expression analysis showed that DXS and linalool synthase were mainly expressed in the skin, consistent with the accumulation patterns of total linalool (Fig. (B)). However, DXR and GPPS were mainly expressed in the flesh, suggesting that DXS and linalool synthase were more important genes in monoterpenol biosynthesis in Vitis vinifera berries. This finding coincides with a previous report that the functional effect of DXS polymorphism may in turn affect the terpenoid content in V. vinifera.Citation4) The expression levels of the representative genes in the MVA pathway, HMGR and FPPS, were also measured. These genes were expressed mainly in both of the skin and flesh; this expression pattern differed from that of MEP pathway genes (Fig. (C)). These findings demonstrated that the total linalool content in the skin of Riesling berries was mainly regulated by the expression levels of DXS and monoterpene synthases such as linalool synthase.
Fig. 2. Organ-specific accumulation of linalool and genes involved in terpenoid biosynthesis.
Effects of light exposure of fruit surface
Light is one of the important factors for the biosynthesis of secondary metabolites including monoterpene; therefore, the effect of light exposure on the total linalool content in the skin was examined. The bunches of grapes were covered with the films that block the full light spectrum (Shade) and UV spectrum (UV-block), and the transparent film (Control) (Fig. (A)). The PAR level and UV light and full-light spectral illuminances for each film are shown in Table , confirming that PAR and UV light and full-light spectral illuminances were cut down by the Shade film, and UV light illuminance was decreased by 50% by the UV-block film but PAR level and full-light spectral illuminance were not affected by the UV-block film. The films covering grape bunches were unclosed at the bottom, so the UV-block film might block only 50% of UV light illuminance. The inside temperature of three films is not significantly different from air temperature. Brix values in juice of the UV-block- and Shade-covered grapes were lower than that of Control-covered grapes, however, these values in all groups are shown to increase during the maturation (Fig. (B)). On the other hand, total linalool in the grapes covered with the Shade- and UV-block films did not accumulate compared with that in the grapes covered with the Control film (Fig. (C)). Linalool is metabolized to various aroma compounds such as cis-/trans-furanoid linalooloxides, cis-/trans-pyranoid linalooloxides.Citation18,19) The total contents of cis-/trans-furanoid linalooloxides in the grapes covered with the Control film at the harvest time (September 21) was 6.1 μg/L. In contrast, cis-/trans-furanoid linalooloxides were not detected in the UV-block- and Shade-covered grapes. cis-/trans-Pyranoid linalooloxides were not detected in the juice of all groups. The gene expression levels in the skin of the Control-, UV-block-, Shade-covered grapes on September 1 were also determined by real-time PCR analysis. The expression levels of DXS and linalool synthase in both the UV-block- and Shade-covered berries, which were expressed mainly in the skin, were 80% lower than those in Control-covered berries (Fig. (D)). On the other hand, the expression levels of DXR and GPPS involved in the MEP pathway, HMGR and FPPS in the MVA pathway in both the UV-block- and Shade-covered berries were only 20–40% lower than those in Control-covered berries (Fig. (E)). These results demonstrated that UV light was essential for the biosynthesis of linalool and its metabolites in grape berries.
Fig. 3. Effects of light reduction on linalool accumulation and expression levels of genes involved in terpenoid biosynthesis.
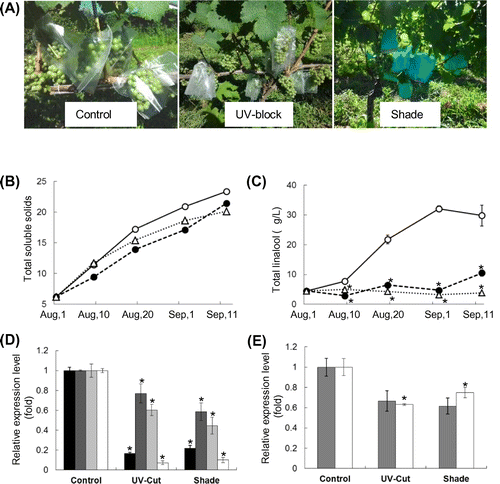
Table 1. Light exposure condition of Sauvignon blanc clusters covered with films.
Manipulation of linalool biosynthesis using reflective sheets
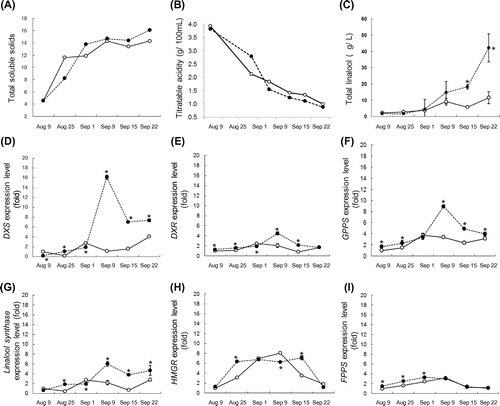
Light was shown to be essential for the linalool biosynthesis in the grape skin; therefore, the usefulness of reflective sheets for linalool biosynthesis in grape berries was examined. To reflect sunlight onto grape clusters, reflective sheets were placed on the ground of a Riesling vineyard during the 2011–2013 growing season (Supplementary Data). The illuminance from reflective sheets on grape clusters was higher than that on control grape clusters (control: 2500–3500 klux, reflected sheets: 7000–14,000 klux). Brix and titratable acidity were not much different between the grapes exposed to light reflected from reflective sheets (reflected-light-exposed grapes) and the control grapes (Fig. (A) and (B)). These results coincide with a previous report that reflective mulches do not affect the must composition such as Brix, titratable acidity, total phenolic content.Citation20) On the other hand, the total linalool content from the reflected-light-exposed grapes was significantly higher than that from the control grapes from the middle of September (Fig. (C)). The data of 2011 and 2013 vintages showed the same pattern as that of 2012 vintage (Supplementary Fig. 1). The total contents of cis-/trans-furanoid linalooloxides in the reflected-light-exposed grapes (8.0 μg/L) at the harvest time (September 22) were higher than that from the control grapes (5.8 μg/L). On the other hand, the total contents of cis-/trans-pyranoid linalooloxides in the reflected-light-exposed grapes were 1.8 μg/L, and those in the control grapes were 1.7 μg/L. The gene expression levels during the 2012 growing season were determined by real-time PCR analysis. The expression levels of the MEP pathway genes and linalool synthase in the reflected-light-exposed grapes were highest on September 9 and significantly higher than those in the control grapes on the same day (Fig. (D)–(G)). On the other hand, the expression levels of the MVA pathway genes HMGR and FPPS were not significantly different between the reflected-light-exposed grapes and the control grapes (Fig. (H) and (I)). The difference in total linalool content between the reflective-light-exposed grapes and the control grapes on and after September 15 appeared to be associated with the higher expression levels of the MEP pathway genes in the reflected-light-exposed grapes on September 9.
Fig. 4. Effects of reflective sheets on total linalool accumulation and expression levels of genes involved in terpenoid biosynthesis during 2012 growing season.
Conclusions
The biosynthesis of secondary metabolites including monoterpenols has been reported to be induced by various environmental factors such as the infection by pathogens, the attacks by insects, and stresses from heat, cold, and drought among others. In some plants, it has also been shown that monoterpenols are biosynthesized and emitted in accordance with the circadian rhythm, resulting from the up-regulation of MEP pathway gene expressions induced by light. This study demonstrated that exposing grape berries to light is essential for the biosynthesis and accumulation of linalool. It also suggests that the control of light exposure such as the use of reflective sheets could regulate the contents of monoterpenols such as linalool in the berries. It is hoped that the increase in the contents of monoterpenols by manipulating light condition can contribute to the improvement of wine quality.
Author contributions
Kanako Sasaki, Hideki Takase, Shuhei Matsuyama and Hironori Kobayashi conceived and designed the experiments. Kanako Sasaki, Hideki Takase, Shuhei Matsuyama and Hironori Kobayashi performed the experiments. Kanako Sasaki and Hideki Takase analyzed data. Hironori Matsuo, Gen Ikoma, and Ryoji Takata contributed reagents/materials/analysis tools. Kanako Sasaki wrote the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental materials
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2016.1217148.
TBBB_1217148_Supplementary_Material.pptx
Download MS Power Point (243.2 KB)Acknowledgment
We are grateful to Dr. Kazufumi Yazaki (Kyoto University) for valuable discussion.
References
- Hemmerlin A, Hoeffler JF, Meyer O, et al. Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco bright yellow-2 cells. J. Biol. Chem. 2003;278:26666–26676.10.1074/jbc.M302526200
- Hampel D, Mosandl A, Wüst M. Biosynthesis of mono- and sesquiterpenes in strawberry fruits and foliage: 2H labeling studies. J. Agric. Food Chem. 2006;54:1473–1478.10.1021/jf0523972
- Luan F, Wust M. Differential incorporation of 1-deoxy-D-xylulose into (3S)-linalool and geraniol in grape berry exocarp and mesocarp. Phytochemistry. 2002;60:451–459.10.1016/S0031-9422(02)00147-4
- Duchêne E, Butterlin G, Claudel P, et al. A grapevine (Vitis vinifera L.) deoxy-d-xylulose synthase gene colocates with a major quantitative trait loci for terpenol content. Theor. Appl. Genet. 2009;118:541–552.10.1007/s00122-008-0919-8
- Williams PJ, Strauss CR, Wilson BWRA. Use of C18 reversed-phase liquid chromatography for the isolation of monoterpene glycosides and nor-isoprenoid precursors from grape juice and wines. J. Chromatogr. A. 1982;235:471–480.10.1016/S0021-9673(00)85911-7
- Gunata YZ, Bayonove CL, Baumes RL, et al. The aroma of grapes. Localisation and evolution of free and bound fractions of some grape aroma components c.v. Muscat during first development and maturation. J. Sci. Food Agric. 1985;36:857–862.10.1002/(ISSN)1097-0010
- Arévalo Villenaa M, Díez Pérezb J, Úbedaa JF, et al. A rapid method for quantifying aroma precursors: application to grape extract, musts and wines made from several varieties. Food Chem. 2006;99:183–190.10.1016/j.foodchem.2005.07.039
- Strauss CR, Gooley PR, Wilson B, et al. Application of droplet countercurrent chromatography to the analysis of conjugated forms of terpenoids, phenols, and other constituents of grape juice. J. Agric. Food Chem. 1987;35:519–524.10.1021/jf00076a020
- Noble AC, Strauss CR, Williams PJ, et al. Contribution of terpene glycosides to bitterness in Muscat wines. Am. J. Enol. Vitic. 1988;39:129–131.
- Bindon KA, Dry PR, Loveys BR. Influence of plant water status on the production of C13-norisoprenoid precursors in Vitis vinifera L. cv. Cabernet Sauvignon grape berries. J. Agric. Food Chem. 2007;55:4493–4500.10.1021/jf063331p
- Scacco A, Verzera A, Lanza CM, et al. Influence of soil salinity on sensory characteristics and volatile aroma compounds of Nero d’Avola wine. Am. J. Enol. Vitic. 2010;61:498–505.10.5344/ajev.2010.10003
- Vilanova M, Genisheva Z, Bescansa L, et al. Changes in free and bound fractions of aroma compounds of four Vitis vinifera cultivars at the last ripening stages. Phytochemistry. 2012;74:196–205.10.1016/j.phytochem.2011.10.004
- Zoecklein BW, Wolf TK, Duncan SE, et al. Effect of fruit zone leaf removal on total glycoconjugates and conjugate fraction concentration of Riesling and Chardonnay (Vitis vinifera L.) grapes. Am. J. Enol. Vitic. 1998;49:259–265.
- Matsuyama S, Tanzawa F, Kobayashi H, et al. Leaf removal accelerated accumulation of delphinidin-based anthocyanins in ‘Muscat Bailey A’ [Vitis × labruscana (Bailey) and Vitis vinifera (Muscat Hamburg)] grape skin. J. Japan. Soc. Hort. Sci. 2014;83:17–22.10.2503/jjshs1.CH-062
- Carretero-Paulet L, Ahumada I, Cunillera N, et al. Expression and molecular analysis of the Arabidopsis DXR gene encoding 1-deoxy-D-xylulose 5-phosphate reductoisomerase, the first committed enzyme of the 2-C-methyl-D-erythritol 4-phosphate pathway. Plant Physiol. 2002;129:1581–1591.10.1104/pp.003798
- Hsieh MH, Goodman HM. The Arabidopsis IspH homolog is involved in the plastid nonmevalonate pathway of isoprenoid biosynthesis. Plant Physiol. 2005;138:641–653.10.1104/pp.104.058735
- Maicas S, Mateo JJ. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: a review. Appl. Microbiol. Biotechnol. 2005;67:322–335.10.1007/s00253-004-1806-0
- Zhu BQ, Cai J, Wang ZQ, et al. Identification of a plastid-localized bifunctional nerolidol/linalool synthase in relation to linalool biosynthesis in young grape berries. Int. J. Mol. Sci. 2014;15:21992–22010.10.3390/ijms151221992
- Schwab W, Wüst M. Understanding the constitutive and induced biosynthesis of mono- and sesquiterpenes in grapes (Vitis vinifera): a key to unlocking the biochemical secrets of unique grape aroma profiles. J. Agric. Food Chem. 2015;63:10591–10603.10.1021/acs.jafc.5b04398
- Sandler HA, Brock PE II, Vanden Heuvel JE. Effects of three reflective mulches on yield and fruit composition of coastal New England winegrapes. Am. J. Enol. Vitic. 2009;60:332–338.
