Abstract
Bovine angiogenin is a major component of the bone resorption inhibitory activity of milk basic protein (MBP). The intestinal absorption of bovine angiogenin was investigated in a rat model, where it was detected in an intact form in the peripheral blood after the oral administration of MBP. This finding demonstrates that orally administered bovine angiogenin is absorbed without being degraded.
Milk is widely consumed as an excellent source of calcium for bone health, but it also contains other components that affect bone health. In previous in vitro and in vivo studies, we have shown that milk whey protein, particularly its basic protein fraction (milk basic protein; MBP), improves bone metabolism. We demonstrated in vitro that MBP stimulates the proliferation and differentiation of osteoblastic cellsCitation1) and suppresses osteoclast-mediated bone resorption and osteoclast cell formation.Citation2) We also demonstrated in vivo that MBP increased the bone mineral density and bone strength of ovariectomized rats.Citation3,4) In human studies, we showed that MBP increased bone mineral density and reduced the urine levels of two bone resorption biomarkers, cross-linked N-telopeptides of type I collagen, and deoxypyridinoline.Citation5–7) These findings suggest that the principal effect of MBP is to suppress osteoclast-mediated bone resorption. Angiogenin (ANG), known as an angiogenic factor and a member of the ribonuclease family,Citation8) was identified as a major component of the bone resorption inhibitory activity of MBP that acts directly on osteoclasts.Citation9) It is possible that bovine ANG exerts its bone resorption inhibitory activity via absorption into the body through the gastrointestinal tract because when an everted gut sac was incubated in MBP solution, the solution within the sac showed bone resorption inhibitory activity.Citation2) Moreover, purified intact bovine ANG administered intravenously increased the bone mineral content and bone mineral density of mice.Citation9) However, it is unclear whether bovine ANG is absorbed into the body. In this study, we investigated whether bovine ANG was absorbed into the blood circulation of rats orally administered MBP. Our results indicate that bovine ANG is absorbed in an intact form not only into the portal blood, but also into the peripheral blood, in rats.
All animal experiments were approved by the Animal Care and Use Committee of the Milk Science Research Institute of Megmilk Snow Brand Co., Ltd, whose guidelines are based on those of the Science Council of Japan. MBP was prepared from defatted bovine milk, as described previously,Citation4) and bovine ANG content was about 6.2% in MBP (w/w) in this experiments. Bovine ANG was purified from MBP with the procedure reported by Morita et al.Citation9) Seven-week-old male Sprague Dawley rats (Japan SLC, Shizuoka, Japan) were fed a commercial diet (CE-2; Clea Japan, Tokyo, Japan) for a week before the experiments, and then fasted for at least 15 h before the absorption experiments. In the first experiment, the rats (211.7–241.7 g) were intragastrically administered MBP (0.4 g) dissolved in saline (1.6 mL, Otsuka Pharmaceutical Factory, Tokushima, Japan) or with the same volume of saline as a control. Blood samples (100 μL) were collected from the portal vein of identical individuals before the administration of MBP, and 15, 30, 45, and 60 min after its administration with a heparin-coated syringe connected to indwelling catheter via laparotomy under isoflurane anesthesia. Peripheral blood (100 μL) was also collected from the jugular vein 60 min after the administration of MBP. In the second experiment, the rats were intragastrically administered MBP (0.4 g) dissolved in saline (1.6 mL), and peripheral blood (100 μL) was collected from the tail veins before the administration of MBP, and 0.5, 1, 2, 4, and 8 h after its administration. All blood samples were placed on ice immediately after collection and then centrifuged at 1000 × g for 30 min at 4 °C. The plasma was stored at −80 °C until analysis.
Mouse hybridoma clones 4G4 and 4H1, which secrete bovine-ANG-specific monoclonal antibodies (4G4 antibody and 4H1 antibody, respectively), were produced by Japan Lamb Inc. (Hiroshima, Japan). The 4H1 antibody was biotinylated by ITM Co. Ltd (Nagano, Japan). A rabbit anti-bovine-ANG polyclonal antibody was prepared as described previously.Citation9) To detect bovine ANG in the rat plasma, enzyme-linked immunosorbent assay (ELISA) plates (Fluoro-Nunc™ 96-well plates; Thermo Fisher Scientific, Waltham, MA, USA) were coated with 5 μg/mL of 4G4 antibody in 100 mM Na2HPO4 solution at 4 °C overnight. The plates were blocked with 1% bovine serum albumin (BSA) in Tris-buffered saline containing 0.05% Tween 20 (TBST) at room temperature for 1 h. Standard bovine ANG was diluted to 0–3 ng/mL with 1% BSA in TBST. The standard solutions and diluted plasma samples (1:10–40) were applied to the antibody-coated plates, and the plates were incubated at 4 °C overnight. The plates were washed with TBST and incubated at room temperature for 1 h with 2.5 μg/mL biotinylated 4H1 antibody diluted with 1% BSA in TBST. After the plates were washed with TBST, they were incubated with horseradish peroxidase (HRP)-conjugated streptavidin (Thermo Fisher Scientific) diluted with 1% BSA in TBST at room temperature for 1 h. Finally, the HRP activity was determined with QuantaBlu™ fluorogenic peroxidase substrate (Thermo Fisher Scientific).
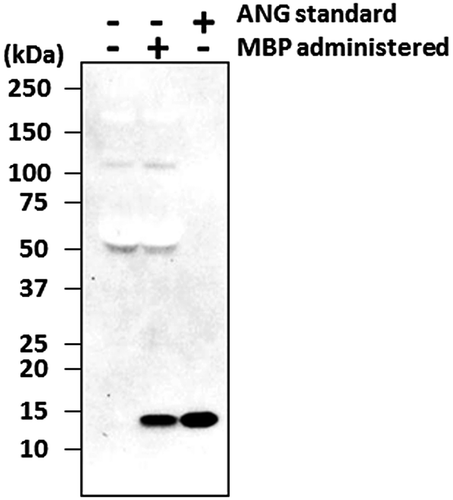
Bovine ANG was detected in the portal vein plasma collected 15 min after the administration of MBP into the stomachs of the rats (Fig. (A)). The bovine ANG content in the plasma increased gradually until 60 min after the administration of MBP. In contrast, no bovine ANG was detected in the plasma collected either before the administration of MBP or after the administration of saline without MBP. Bovine ANG was also detected in the peripheral vein plasma, 60 min after the administration of MBP into the stomach of the rats and two rats exhibited higher level than the others, but not after the administration of saline (Fig. (B)). The time course of the appearance and disappearance of bovine ANG in the peripheral blood was monitored. As shown in Fig. , bovine ANG was detected in the peripheral plasma collected 0.5 h after the administration of MBP into the stomachs of the rats, reached its maximum level 1 h after its administration, and then gradually decreased, with an apparent half-life of about 4 h. Bovine ANG was still observed 8 h after its administration. These results indicate that bovine ANG is absorbed through the gastrointestinal tract and circulates in the body after the oral administration of MBP.
Fig. 1. ELISA of bovine ANG in rat portal and peripheral vein plasma after the oral administration of MBP.
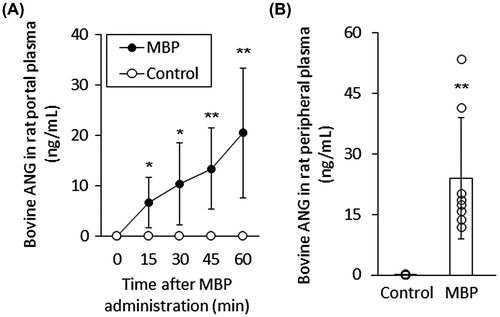
Fig. 2. Time course of bovine ANG absorption into rat peripheral blood after the oral administration of MBP.
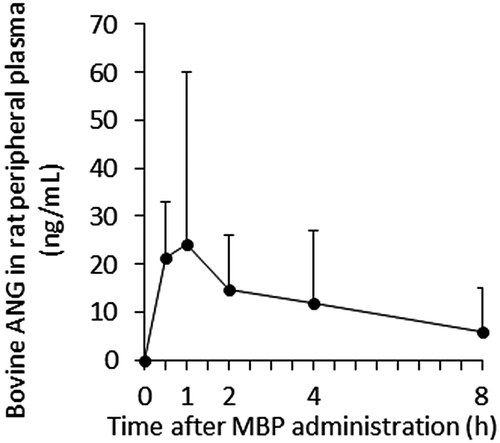
Fig. 3. Immunoblotting analysis of bovine ANG in rat peripheral blood plasma before and after the administration of MBP.
To determine whether intragastrically administered bovine ANG remains intact, retaining its molecular size, in the rat peripheral plasma, rat peripheral plasma was mixed with SDS-PAGE sample buffer containing 2% β-mercaptoethanol, and boiled for 5 min. The proteins in the plasma sample (0.3 μL/well) were separated with SDS-PAGE in a 4–20% gradient gel (Bio-Rad, Hercules, CA, USA) under reducing conditions and electrophoretically blotted onto a polyvinylidene difluoride membrane (Bio-Rad). After the membrane was blocked with 1% BSA at room temperature for 1 h, it was incubated with rabbit anti-bovine-ANG polyclonal antibody at 4 °C overnight, and then with HRP-conjugated secondary anti-rabbit-IgG antibody (Cell Signaling, Danvers, MA, USA) for 1 h at room temperature. The protein bands were visualized with ECL Prime Western Blotting Detection Reagent (GE Healthcare, Buckinghamshire, England) and the ChemiDoc™ MP Imaging System (Bio-Rad). A 14-kDa band corresponding to purified intact bovine ANG was clearly detected as the major band in the plasma samples from rats orally administered MBP (Fig. ). In contrast, this 14-kDa band was not detected in the preadministration plasma. These results indicate that bovine ANG was absorbed into the body in its intact form through the gastrointestinal tract.
It has been reported that some dietary proteins are absorbed into the body through the gastrointestinal tract in their intact forms, although most dietary proteins are digested and absorbed as amino acids. This intact transfer is thought to have physiological significance, for example, bovine milk lactoferrin is transported across the intestinal lumen in its intact form and may display immunoregulatory properties.Citation10) In this study, we have demonstrated that bovine ANG, a major component of the bone resorption inhibitory activity of MBP, is absorbed into the peripheral blood and circulates in the bodies of rats. Moreover, immunoblotting analysis revealed that the bovine ANG absorbed into the blood corresponds to purified intact bovine ANG, which increased the bone mineral density and bone mineral content of mice after its intravenous injection.Citation9) In this study, rats with an average body weight of 224 g were administered 0.4 g MBP, i.e. MBP (1786 mg/kg of body weight) was administered, and about 23.9 ng/mL bovine ANG was detected in rat peripheral plasma. On the other hand, in a previous human study,Citation7) subjects with an average body weight of 54 kg had consumed MBP (0.04 g per day) for six months, i.e. MBP (0.74 mg/kg of body weight per day) was administered, and their bone metabolism was improved. Therefore, in a previous human study, intake of MBP per body weight was about 2400-fold lower than that of the present study. If there is no difference in the absorption efficiency of bovine ANG between rats and humans, it is assumed that bovine ANG in human peripheral plasma reached about 10 pg/mL, its maximum level, after intake of 0.04 g MBP. Taken together, it is suggested that, even if the bovine ANG content in human peripheral plasma is the pg/mL level, the presence of bovine ANG in the peripheral blood for a certain period may contribute to the improvement of bone metabolism by MBP intake. It is still unclear how bovine ANG is absorbed through the gastrointestinal tract, and further research is required to clarify the transport mechanism of bovine ANG across the intestinal epithelium.
Author contributions
Y. I., T. Y., and K. K. designed the study. Y. I., T. Y., and H. M. performed the experiments and analyzed the results. Y. I., T. Y., and K. K. prepared the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgment
We thank Dr. Yoshikazu Morita (Megmilk Snow Brand Co., Ltd.) for advice.
Notes
Abbreviations: MBP, milk basic protein; ANG, angiogenin; BSA, bovine serum albumin; TBST, Tris-buffered saline containing Tween 20; HRP, horseradish peroxidase.
References
- Takada Y, Aoe S, Kumegawa M. Whey protein stimulated the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun. 1996;223:445–449.10.1006/bbrc.1996.0913
- Takada Y, Kobayashi N, Matsuyama H, et al. Whey protein suppresses the osteoclast-mediated bone resorption and osteoclast cell formation. Int Dairy J. 1997;7:821–825.10.1016/S0958-6946(97)00073-3
- Kato K, Toba Y, Matsuyama H, et al. Milk basic protein enhances the bone strength in ovariectomized rats. J Food Biochem. 2000;24:467–476.10.1111/jfbc.2000.24.issue-6
- Toba Y, Takada Y, Yamamura J, et al. Milk basic protein: a novel protective function of milk against osteoporosis. Bone. 2000;27:403–408.10.1016/S8756-3282(00)00332-X
- Aoe S, Toba Y, Yamamura J, et al. Controlled trial of the effects of milk basic protein (MBP) supplementation on bone metabolism in healthy adult women. Biosci Biotechnol Biochem. 2001;65:913–918.10.1271/bbb.65.913
- Aoe S, Koyama T, Toba Y, et al. A controlled trial of the effect of milk basic protein (MBP) supplementation on bone metabolism in healthy menopausal women. Osteoporos Int. 2005;16:2123–2128.10.1007/s00198-005-2012-3
- Uenishi K, Ishida H, Toba Y, et al. Milk basic protein increases bone mineral density and improves bone metabolism in healthy young women. Osteoporos Int. 2007;18:385–390.10.1007/s00198-006-0228-5
- Komolova GS, Fedorova TV. Milk angiogenin (Review). Prikl Biokhim Mikrobiol. 2002;38:229–236.
- Morita Y, Matsuyama H, Serizawa A, et al. Identification of angiogenin as the osteoclastic bone resorption-inhibitory factor in bovine milk. Bone. 2008;42:380–387.10.1016/j.bone.2007.10.012
- Fischer R, Debbabi H, Blais A, et al. Uptake of ingested bovine lactoferrin and its accumulation in adult mouse tissues. Int Immunopharmacol. 2007;7:1387–1393.10.1016/j.intimp.2007.05.019
