Abstract
Ascorbate and glutathione are indispensable cellular redox buffers and allow plants to acclimate stressful conditions. Arabidopsis contains three functional dehydroascorbate reductases (DHAR1-3), which catalyzes the conversion of dehydroascorbate into its reduced form using glutathione as a reductant. We herein attempted to elucidate the physiological role in DHAR1 and DHAR2 in stress responses. The total DHAR activities in DHAR knockout Arabidopsis plants, dhar1 and dhar2, were 22 and 92%, respectively, that in wild-type leaves. Under high light (HL), the levels of total ascorbate and dehydroascorbate were only reduced and increased, respectively, in dhar1. The oxidation of glutathione under HL was significantly inhibited in both dhar1 and dhar2, while glutathione contents were only enhanced in dhar1. The dhar1 showed stronger visible symptoms than the dhar2 under photooxidative stress conditions. Our results demonstrated a pivotal role of DHAR1 in the modulation of cellular redox states under photooxidative stress.
Light is one of the most important environmental cues for plant growth and development. Illumination is required for photosynthesis, the sole energy source for autotrophic plants, and provides signals that modulate photomorphogenesis and gene expression.Citation1–3) However, the production of reactive oxygen species (ROS) in photosynthesis and photorespiration occurs under illumination.Citation4) High light (HL) results in the over-reduction of the photosynthetic electron transport chain, leading to the photo-reduction of oxygen, which produces ROS within chloroplasts (photooxidative stress). ROS play dual roles: as harmful toxic compounds and signals that modulate gene expression for plant acclimation to stress.Citation5–8) Therefore, the balance between ROS production and scavenging must be regulated tightly in plant cells under photooxidative stress.
Ascorbate and glutathione are major low-molecular-weight antioxidants in plant cells and, thus, act as essential redox buffers.Citation9) They comprise the ascorbate-glutathione cycle, in which ascorbate peroxidase (APX) reduces hydrogen peroxide (H2O2) to water using reduced ascorbate (ascorbic acid, AsA) as an electron donor, leading to the production of monodehydroascorbate (MDHA). MDHA is reduced back to AsA by MDHA reductase (MDAR) or ferredoxin, otherwise it is spontaneously disproportionated into AsA and dehydroascorbate (DHA). DHA reductase (DHAR) reduces DHA to AsA using reduced glutathione (GSH). Oxidized glutathione (GSSG), a product of the DHAR reaction, is then recycled by glutathione reductase (GR). NAD(P)H serves as an electron donor for MDAR and GR activities. This cycle is distributed to the cytosol, chloroplasts, mitochondria, and peroxisomes and contributes to the modulation of cellular redox states.Citation10–14) In addition to its role in this cycle, AsA serves as an electron donor for the regeneration of oxidized tocopherol, which is the most abundant lipophilic antioxidant in plants,Citation15,16) and acts as co-factor for various enzymes such as violaxanthin de-epoxidase, which is required for the dissipation of light energy as heat under HL.Citation17–19) Furthermore, the role of AsA as an electron donor in photosystem I (PSI) and PSII has been demonstrated.Citation20,21) Thus, ascorbate recycling via DHAR and MDAR is important for the modulation of the redox states of cellular components, photosynthesis, and stress responses in plants.
The physiological role of DHAR has been well characterized using a reverse genetic approach in tobacco.Citation13) The overexpression of a wheat cytosolic DHAR markedly enhances ascorbate levels and its redox states (AsA/AsA + DHA) in tobacco plants.Citation22) In contrast, the knockdown of cytosolic DHAR (co-suppression) through the introduction of DHAR cDNA under the control of the 35S promoter has the opposite effectCitation23); however, it should be noted that this method also suppresses the expression of chloroplastic DHAR as well as cytosolic DHAR proteins.Citation24) Thus, ascorbate recycling via cytosolic DHAR is one of the rate-limiting steps regulating the ascorbate pool size and its redox states in tobacco. Furthermore, using these transgenic tobacco plants, it has been also demonstrated that DHAR is required for photosynthesis, growth and development, and oxidative stress tolerance.Citation23–25) Interestingly, DHAR has a negative effect on stomata closure by modulating H2O2 levels in the guard cells of tobacco, and, thus, its knockdown enhances drought tolerance in tobacco.Citation25) These findings demonstrate the physiological importance of DHAR under abiotic stresses in tobacco plants. The positive effects of the overexpression of DHAR on the levels and redox states of ascorbate and on tolerance to various types of stresses have also been reported in many plant species.Citation13) However, only a few studies have examined the prominent physiological role(s), such as the contribution to ascorbate recycling and stress response, of DHAR isoforms in other plant species including Arabidopsis using a reverse genetic approach.
Arabidopsis plants contain three functional genes that encode DHAR: DHAR1 (At1g19570), DHAR2 (At1g75270), and DHAR3 (At5g16710). Although there are two more DHAR-like genes (At5g36270 and At1g19950), they have been suggested to be pseudogenes.Citation26)
Our previous report showed that DHAR3 had an N-terminal extension sequence similar to chloroplast-targeting signal and was localized in chloroplasts in Arabidopsis leaves.Citation27) DHAR2 appeared to be distributed in the cytosol.Citation28) Recent proteomic and bio-imaging analyses indicated that DHAR1 was localized to peroxisomes without a known peroxisome-targeting signal.Citation29) In contrast, DHAR1 fused with the EosFP, a fluorescent protein, was found to be localized in the cytosol.Citation30) Thus, DHAR1 is found in multiple subcellular organelles.
In Arabidopsis, overexpression of DHAR1 increased ascorbate content and the redox state, and enhanced tolerance against heat and methylviologen (MV), a ROS generator.Citation31) Consistent with the report, ascorbate recycling via DHAR enzyme also contributes to stress tolerance in Arabidopsis, likely in tobacco plants. In fact, we very recently found that the lack of DHAR3 led to dysregulation of levels and redox states of ascorbate and glutathione, and enhanced photooxidative stress susceptibility.Citation27) Previously, Yoshida et al.Citation28) reported that disruption of DHAR2 affected ascorbate redox state but not pool size, and increased ozone sensitivity. In contrast to these DHAR isoforms, although the lack of DHAR1 in Arabidopsis inhibits promotion of growth and seed production by the presence of Piriformospora indica, an endophytic fungus of Sebacinales,Citation32) the levels and redox states of antioxidants, including ascorbate and glutathione, in DHAR1 null Arabidopsis mutants are still unknown. Thus, further studies are warranted in order to determine that DHAR1 contributes to ascorbate recycling-mediated responses to stress. We herein attempted to elucidate the expression and physiological role in DHAR1 and DHAR2. Our results identified DHAR1 as a major DHAR in Arabidopsis cells and one of main regulators of both ascorbate and glutathione redox states under photooxidative stress.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana ecotype Columbia (Col-0) was used as the wild-type plant. T-DNA insertion lines of DHAR1 (dhar1-1: SALK_029966, and dhar1-2: SALK_008446) and DHAR2 (dhar2-1: SALK_026089) were obtained from the Arabidopsis Biological Resource Center. A double mutant lacking DHAR1 and DHAR2 (dhar1-1 dhar2-1) was generated by crossing dhar1-1 and dhar2-1 single mutants. The surface-sterilized seeds of the wild type and mutants were cold-treated at 4 °C for 2–3 days before germination. Plants were grown on Murashige & Skoog (MS) medium containing 3% sucrose under 23 °C during 16 h of light (100 μmol photons m−2 s−1) and at 20 °C during 8 h of darkness. Two-week-old plants were exposed to excess light (1000 or 1500 μmol photons m−2 s−1) or treated with 50 μM methylviologen, a ROS producing agent, solution containing 0.1% (v/v) Tween-20 under growth light. For growth on soil, 10-day-old seedlings were potted in soil and grown in the same growth chamber. The collection of control (non-stressed) samples and application of stress were performed 4 h after illumination.
Quantitative real-time PCR analysis
Total RNA was isolated from the leaves of Arabidopsis according to our previous report.Citation27) First strand cDNA was synthesized using ReverTra Ace reverse transcriptase (TOYOBO, Osaka, Japan) with an oligo dT primer. Gene-specific primer pairs for the quantitative Real-Time PCR analysis (q-PCR) were designed using PRIMER EXPRESS software (Applied Biosystems, California, USA). Primer sequences were as follows; DHAR1-qF (5′-CGGCGACTGTCCGTTCAG-3′), DHAR1-qR (5′-TCAGATGGATTTTGTAGGTAAGACTCTTC-3′), DHAR2-qF (5′-GTTTGAGAACACCAAGGCTAAGAAAG-3′), DHAR2-qR (5′-TCACGCATTCACCTTCGATTC-3′), DHAR3-qF (5′-AGGGTTGGCCGGTTTGTTAC-3′), DHAR3-qR (5′-ATAGAAGCTTTAACGCAGATTTCAAGAG-3′), Actin2-qF (5′-GGTGGTTCCATTCTTGCTTCCC-3′), and Actin2-qR (5′-TCATACTCGGCCTTGGAGATCC-3′). q-PCR was performed with a LightCycler® 96 System using the FastStart Universal SYBR Green Master (ROX) (Roche, Basel, Switzerland). Actin2 mRNA was used as an internal standard in all experiments. q-PCR data were obtained from more than three biological replicates (more than 10 plants and 3 experimental replicates were used for one biological replicate).
Enzyme assay
DHAR activity was measured according to Shigeoka et al.Citation33) with minor modifications. Arabidopsis leaves (0.2 g) were homogenized with 400 μL of 50 mM potassium phosphate (pH 7.5) containing 1 mM EDTA and 10% (w/v) D-sorbitol. After centrifugation (20,000×g) for 20 min at 4 °C, the supernatant was used to analyze enzymatic activity. The extract (20 μL) was added to 1 mL of reaction mixture containing 50 mM potassium phosphate (pH 7.5), 2 mM GSH, and 1 mM DHA, 0.2 mM NADPH, and 1 unit GR. GSH-dependent DHA reduction was assayed spectrophotometrically at 340 nm. MDAR activity was measured according to Eltayeb et al.Citation34) with minor modifications. Arabidopsis leaves (0.2 g) were homogenized with 400 μL of 50 mM potassium phosphate (pH 7.5) containing 0.2 mM EDTA, 10 mM 2-mercaptoethanol, and 1% D-sorbitol. After centrifugation (20,000×g) for 20 min at 4 °C, the supernatant was used to analyze enzymatic activity. The extract (20 μL) was added to 1 mL of reaction mixture containing 100 mM potassium phosphate (pH 7.5), 1 mM AsA, and 0.2 mM NADH. The reaction was started by the addition of 0.2 unit AsA oxidase after a pre-incubation for 2 min. The decrease in absorbance at 340 nm was monitored and the activity was calculated using absorbance coefficient of 6.2 mM−1 cm−1. GR activity was measured according to Foyer and HalliwellCitation35).
Measurement of oxidants and antioxidants
The measurement of ascorbate was carried out as described previously.Citation36) AsA and DHA levels were determined spectrophotometrically using ascorbate oxidase. Arabidopsis leaves (0.2 g) frozen in liquid N2 were ground using a mortar and pestle with 2.5 mL of 6% (v/v) HClO4 and centrifuged at 20,000×g for 10 min at 4 °C. In order to measure AsA, a 100-μL aliquot of the obtained leaf extract was added directly to 900 μL of a 200 mM succinate buffer (pH 12.7, adjusted with NaOH) in the spectrophotometer. The final pH was very near 6.0. AsA was determined by a change in absorbance at 265 nm following the addition of 5 units of AsA oxidase. In order to determine total ascorbate, 700 μL of the leaf extract was adjusted to pH 6.0 with K2CO3 and this was followed by the addition of dithiothreitol, dissolved in 50 mM HEPES-KOH buffer (pH 7.5), to a final concentration of 10 mM. The resulting solution was incubated in darkness at 25 °C for 30 min. After centrifugation for 15 min at 20,000×g (4 °C), the supernatant was assayed as described above. The amount of DHA was determined as the difference between these two assays. The measurement of glutathione was according to Shigeoka et al.Citation37)
The thiobarbituric acid test, which determines the amount of malondialdehyde as an end-product of lipid peroxidation, was used to analyze lipid oxidation.Citation38) Arabidopsis leaves (0.2 g) were frozen in liquid nitrogen, ground in 1 mL of 1% (v/v) trichloroacetic acid, and homogenized with an equal volume of 0.34% (w/v) thiobarbituric acid in 50% (v/v) acetate. The mixtures were heated in a water bath at 95 °C for 60 min and centrifuged at 20,000×g for 10 min at room temperature. The supernatants were mixed with the double equivalent of n-butanol and centrifuged at 4000×g for 10 min at room temperature. The content of malondialdehyde was estimated by measuring the absorbance of the n-butanol layers from 532 to 600 nm. Absorbance values were converted into n moles using an extinction coefficient of 1.56 × 105 M−1 cm−1.Citation39)
H2O2 accumulation was quantified using Amplex red hydrogen peroxide/peroxidase assay kit (Molecular Probes; Eugene; Oregon; USA) according to our previous procedure.Citation40)
Measurement of chlorophyll fluorescence
Chlorophyll fluorescence was measured according to Maruta et al.Citation41) Fv/Fm in Arabidopsis leaves was determined after dark adaptation for 20 min. Chlorophyll fluorescence in Arabidopsis leaves was measured at 23 °C with a Closed FluorCam 800MF (Photon Systems Instruments, Brno, Czech Republic).
Data analysis
The statistical analysis of data was based on Student’s t-tests. Calculations were performed on more than three independent biological replicates. In all experiments, except for the measurement of chlorophyll fluorescence, leaves (from >20 seedlings) were pooled and used for 1 biological replicate.
Results
Transcript levels of DHARs increased under photooxidative stress conditions
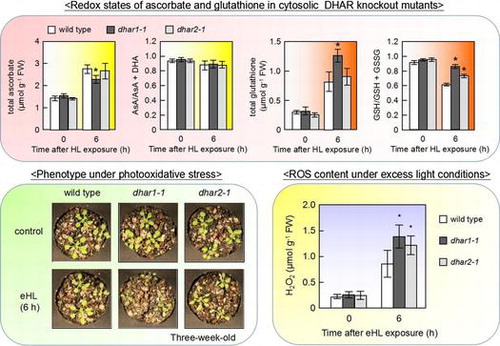
We analyzed the expression of DHAR isoforms under photooxidative stress in Arabidopsis leaves. Two-week-old wild-type plants were exposed to HL at 1000 μmol photons m−2 s−1 or treated with 50 μM MV for 6 h. The transcript levels of all DHARs were increased by these photooxidative stress treatments (Fig. ). The transcript levels of DHAR1 were markedly higher than those of other DHARs under both before and after the treatments. Previously, Vadassery et al.Citation32) indicated that the transcript level of DHAR1 was higher than that of other DHAR isoforms in Arabidopsis roots on the basis of previous microarray data. Additionally, Yoshida et al.Citation28) reported that the transcript level of DHAR2 was relatively lower than that of the other DHAR genes. These data suggest that DHAR1 may act as a major DHAR in plant cells under normal and stress conditions, including photooxidative stress.
Fig. 1. Expression of DHAR isoforms under normal and HL.
Levels and redox states of ascorbate and glutathione in dhar1 mutants
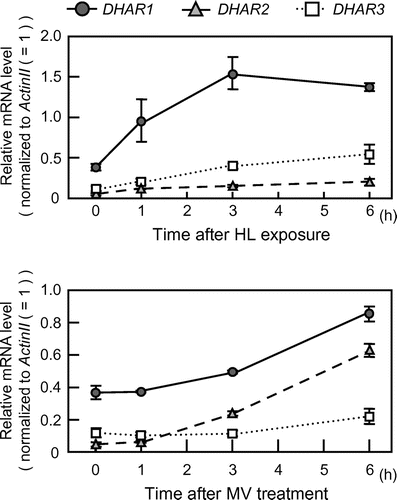
We obtained two T-DNA insertion lines, dhar1-1 (SALK_029966) and dhar1-2 (SALK_008446). A T-DNA insertion occurred in the third exon of the DHAR1 gene in the dhar1-1 mutant, and just before the transcription start site in the dhar1-2 mutant (Fig. (A)). The sites of T-DNA insertion for these mutants were confirmed by sequencing of genomic DNA extracted from the mutants (data not shown). qRT-PCR analysis revealed that these mutants had null or little expression of DHAR1 (Fig. (B)). Total DHAR activity in two-week-old dhar1-1 and dhar1-2 seedlings grown under normal conditions was 22.4 and 43.2% that of the wild-type plants, respectively, demonstrating that DHAR1 is the predominant enzyme in Arabidopsis plants (Fig. (C)). Total MDAR activity was slightly but significantly increased only in dhar1-1 probably to compensate for the null mutation (Fig. (D)).
Fig. 2. Characterization of T-DNA insertion lines lacking DHAR1.
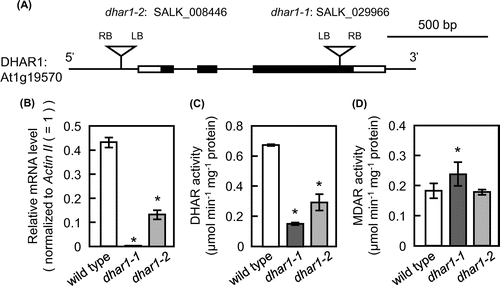
To determine the physiological role of DHAR1 in the regulation of ascorbate and glutathione redox states, two-week-old wild-type and mutant plants were exposed to HL. When subjected to this stress for 9 h, although all of them had slightly wilting leaves, the phenotypes of the dhar1 mutant plants were similar to those of the wild-type plants (Supplementary Fig. S1). Before and 1 h after HL, the ascorbate redox states (AsA/AsA + DHA) in dhar1 mutants were significantly lower than those in the wild type (Fig. (B)). This provides genetic evidence for the role of DHAR1 in ascorbate recycling. However, no significant difference was observed in ascorbate redox states at more than 3 h in the wild type and mutants. Importantly, although the levels of ascorbate were enhanced in the wild type at 6 and 9 h after HL, this increase was significantly inhibited in dhar1-1 and dhar1-2 mutants (Fig. (A)), suggesting that DHAR1 is essential for maintenance of ascorbate pool size under HL.
Fig. 3. Levels and redox states of ascorbate and glutathione in dhar1 mutants under HL.
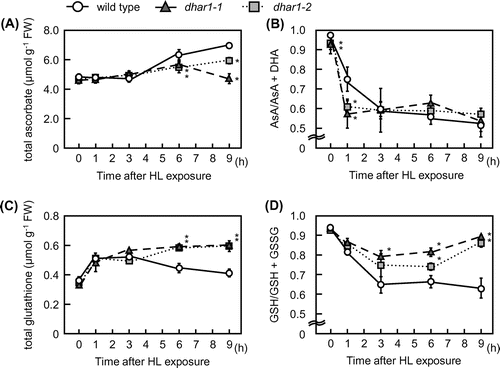
Similar to ascorbate levels, total glutathione levels were increased by HL in all genotypes. This increase was peaked at 3 h and then decreased in wild type, but was maintained for more than 6 h after HL in dhar1 mutants (Fig. (C)). In wild-type plants, the glutathione redox states (GSH/GSH + GSSG) were decreased in response to HL (Fig. (D)). However, this decrease in the glutathione redox states under HL was significantly suppressed in dhar1 mutants, especially in dhar1-1. Furthermore, there was no difference in GR activity among the wild type and dhar1 mutants both before and after HL exposure (Supplementary Fig. S2). These findings indicate that DHAR1 contributes to oxidize GSH under HL.
Photooxidative stress sensitivity of dhar1 mutants
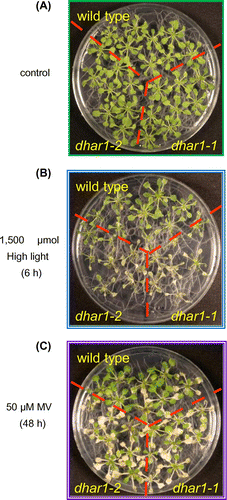
To examine the effects of the dhar1 mutations on cellular oxidative damage, we investigated maximum quantum yield of photosystem II (Fv/Fm) by measuring chlorophyll fluorescence in the wild type and dhar1 mutants under HL. After HL exposure, Fv/Fm decreased in all genotypes; however, this decrease was significantly enhanced in the dhar1-1 (Fig. (A)). Similar effect seemed to be observed in the knockdown dhar1-2, though it lacked statistical significance. In addition, malondialdehyde levels in the wild type were not altered by HL. However, they were increased significantly in dhar1 mutants (Fig. (B)). Therefore, we analyzed intracellular levels of H2O2 in the leaves of wild-type and dhar1 mutant plants under photooxidative stress using Amplex Red reagent, a highly sensitive and stable probe for H2O2. As shown in Fig. (C), dhar1-1 mutant plants significantly accumulated more H2O2 than those of wild type at 6 h after exposure to HL. These results suggest that DHAR1 is important for protecting cells from photooxidative damage. Actually, when exposed to extreme HL (eHL) at 1500 μmol photons m−2 s−1 with high temperature (40 °C) for 6 h, both dhar1 mutants showed stronger visible symptoms than wild-type plants (Fig. ). The sensitivity of dhar1 mutants to such extreme light was not due to a decrease in thermotolerance, because neither mutant was sensitive to heat stress only (Supplementary Fig. S3). Moreover, dhar1 mutants were highly sensitive to the treatment with 50 μM MV under growth light. These results demonstrate that DHAR1 plays a pivotal role in photooxidative stress tolerance through cellular redox regulation in Arabidopsis.
Fig. 4. Oxidative damage in dhar1 mutants under HL.
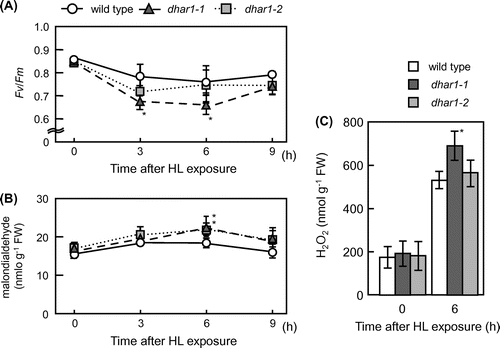
Fig. 5. Sensitivity of dhar1 mutants to photooxidative stresses.
Levels and redox states of ascorbate and glutathione in cytosolic dhar mutants grown on soil
As described above, recently we had reported that DHAR3 is required for photooxidative stress tolerance in Arabidopsis leaves.Citation27) To elucidate the importance of Arabidopsis cytosolic DHAR isoforms in photooxidative stress response in more detail, we obtained and used a T-DNA insertion Arabidopsis mutant of DHAR2. Total DHAR activity in the mutant (dhar2-1: SALK_026089) was approximately 92% that in wild-type leaves, although this mutant had little expression level of DHAR2 (Supplementary Fig. S4). Similar to dhar1-1 mutant, total MDAR activity in dhar2-1 mutant was significantly increased (Supplementary Fig. S4).
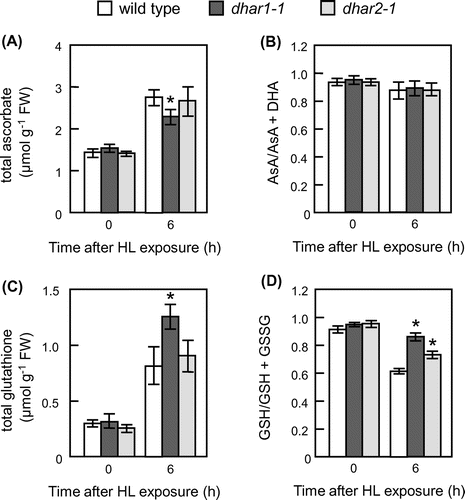
To clarify the physiological roles of cytosolic DHAR isoforms in modulation of ascorbate and glutathione under photooxidative stress conditions, dhar1-1 and dhar2-1 mutant plants were grown on soil. No significant difference was observed in growth between wild type and these DHAR knockout mutants, dhar1-1 and dhar2-1. We confirmed that these dhar mutants had lower DHAR activity even under HL (Supplementary Fig. S5). The HL irradiation decreased the DHAR activity of dhar1 mutants, whereas that of wild-type plants was increased under the HL. Furthermore, the lack of DHAR2 significantly inhibited the enhancement of DHAR activity by HL irradiation. These results indicate that both Arabidopsis cytosolic DHAR isoforms, especially DHAR1, are required for the HL response. At 6 h after exposure to HL, the levels of total ascorbate increased in wild type and these dhar mutants, but levels were much lower in dhar1-1 than wild type and dhar2-1 (Fig. (A)). Moreover, the lack of DHAR1 and DHAR2 had no detectable effect on the ascorbate redox states under the stress conditions (Fig. (B)). As shown in Fig. (C), HL exposure caused the total glutathione levels to increase in all genotypes, but levels were also much higher in dhar1-1 than the others. In addition, glutathione redox states were significantly higher in the dhar1-1 and dhar2-1 mutant plants than that of the wild-type plants under the HL (Fig. (D)). These results revealed that not only DHAR1 but also DHAR2 are required for glutathione oxidation under photooxidative stress conditions. Thus, it seems likely that both DHAR1 and DHAR2 have a contribution to response to excess light.
Fig. 6. Levels and redox states of ascorbate and glutathione in dhar1 and dhar2 mutants grown on soil.
Sensitivity to photooxidative stresses in cytosolic dhar mutants grown on soil
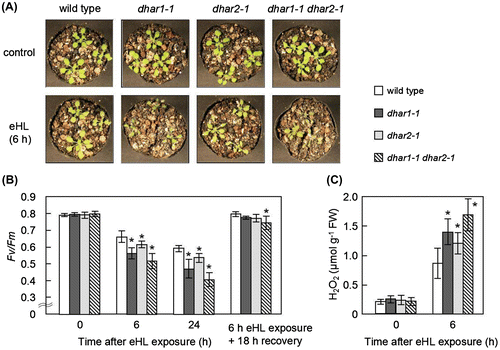
To check the sensitivity of these mutants to photooxidative stress under the condition, three-week-old wild-type, dhar1-1, and dhar2-1 plants were subjected to eHL. At 6 h after eHL exposure, dhar1-1 mutant plants exhibited a more sensitive phenotype than that of the wild-type plants (Fig. (A)). In addition, Fv/Fm decreased significantly in the dhar1-1 mutant plants compared with the wild-type plants under eHL (Fig. (B)). The decrease in the Fv/Fm by the eHL exposure was also prominent in dhar2-1 than that of the wild-type plants, whereas phenotypes of these mutants were similar to each other under the condition. To clarify the contribution of cytosolic DHAR to photooxidtive stress tolerance, we generated a double mutant lacking both DHAR1 and DHAR2, dhar1-1 dhar2-1. This mutant contained no transcript of the respective genes (data not shown). Total DHAR activity in three-week-old this mutant grown on soil was 33% that of the wild-type plants, while the activity of dhar1-1 and dhar2-1 mutants was approximately 37 and 70% that of the wild type, respectively (Supplementary Table S1). Similar to dhar1-1 mutant plants, a high sensitivity of the dhar1-1 dhr2-1 mutant plants to eHL exposure was visibly observed (Fig. (A)). When eHL irradiated plants were shifted to normal light condition, Fv/Fm of them was recovered to normal value at 18 h after the treatment; however, this recovery of Fv/Fm after eHL irradiation was not sufficient in dhar1-1 dhar2-1 (Fig. (B)). Thus, this double mutant is more sensitive than the parental single-mutant strains to eHL exposure. In addition, H2O2 accumulation under eHL was more increased in dhar mutants than in wild-type plants (Fig. (C)). These results indicate that both DHAR1 and DHAR2 are required for tolerance to light-induced oxidative stress.
Fig. 7. Photooxidative stress sensitivity of dhar mutants grown on soil.
Discussion
We here investigated the physiological roles of Arabidopsis DHAR1 using its knockout and knockdown mutants. Total DHAR activity in the leaves of the null mutants of DHAR1 was reduced to approximately 25 and 37% of the control values when growing on MS medium and soil, respectively (Fig. (C) and Supplementary Table S1), demonstrating that DHAR1 is dominant among the three ascorbate recycling enzymes in Arabidopsis leaves. Consistent with this result, we recently reported that the DHAR3 knockout Arabidopsis mutant, dhar3, contained one-fourth of wild-type total DHAR activity.Citation27) Moreover, the DHAR activity of dhar2 mutants grown on MS medium was approximately 90% that of the wild type (Supplementary Fig. S4). In contrast, Yoshida et al.Citation28) showed that an Arabidopsis null mutant lacking DHAR2 had approximately 30% the DHAR activity of the control plants. This discrepancy may have been due to a difference in growth conditions; plants were grown on MS medium in this study, but on rock wool in the previous study.Citation28) In this study, the DHAR activity of dhar2 mutants grown on soil was approximately 70% that of the wild type (Supplementary Table S1). The results provide a possibility that the ratio of DHAR activity of each DHAR isoform from Arabidopsis to the total activity was depended on nutrients and cultivation conditions, and the ratio of DHAR2 activity might be increased under low-nutrient conditions. Under our experimental conditions, the transcript levels of DHAR1 might be higher than those of other isoforms both before and after stress application in leaves (Fig. ). Likewise, Yoshida et al.Citation28) showed that the expression level of DHAR2 was relatively lower than that of the other DHARs. Furthermore, a comparative enzymological analysis using recombinant proteins revealed that the DHA reduction activity of DHAR1 is approximately eightfold higher than that of DHAR2.Citation42) These findings supported our conclusion that DHAR1 accounts for the majority of DHAR activity in Arabidopsis leaves at least under our experimental conditions.
The lack of DHAR1 negatively affected the redox states of ascorbate before and at 1 h after HL (Fig. (B)). In addition, at more than 6 h after HL, the level of total ascorbate in dhar1 was lower than that in wild-type plants (Fig. (A)). This was consistent with previous findings that were obtained using DHAR-suppressed tobacco plants.Citation24) Therefore, DHAR1 plays a role in regulating the ascorbate pool size through its recycling. However, at more than 3 h after HL, no significant difference was observed in ascorbate redox states between the wild type and dhar1 mutants. Since total MDAR activity was actually increased in dhar1-1 mutant (Fig. (D)), this may have been due to a degree of compensation by MDAR and/or residual DHAR isoform(s). Although GSH can chemically reduce DHA to AsA,Citation43) this reaction would not have been involved in ascorbate recycling in the dhar1 mutants because the accumulation of GSSG was inhibited in the mutants (Fig. (D)). Importantly, enhanced MDAR activity was insufficient to maintain the ascorbate pool size in dhar1-1 mutant under HL (Fig. (A)), suggesting that high ascorbate redox states at the early stage of photooxidative stress by the DHAR1 action is important for maintaining its pool size at the late stage.
An increase in the total levels and oxidation of glutathione are typical responses observed in plants exposed to oxidative stress. For example, mutants lacking catalase 2 (cat2), a predominant catalase in Arabidopsis, accumulate glutathione, especially in its oxidized form.Citation44) Increases in the levels and oxidation of glutathione are also strongly enhanced in Arabidopsis mutants lacking stromal and thylakoid membrane-associated APXs (chlAPXs).Citation41) Recent findings have revealed that the levels and redox states of glutathione play significant roles in the redox-based regulation of stress acclimation, hormonal signaling, and growth and development.Citation45–47) Similar to these mutants, dhar1 accumulated higher levels of glutathione under excess light conditions (Figs. (C) and (C)). Therefore, this was attributed to an increase in cellular oxidative damage in the mutants. In fact, high-light-induced H2O2 accumulation was increased in dhar1 mutant plants (Figs. (C) and (C)). Interestingly, the accumulation of GSSG was significantly suppressed in dhar1 mutants without GR activation (Fig. (D) and Supplementary Fig. S2), unlike cat2 and chlapx mutants.Citation41,48) These results indicate that the reduced accumulation of GSSG in dhar1 mutants was due to a decrease in the glutathione oxidation reaction. In this study, the dhar2 mutation also inhibited glutathione oxidation under excess light and slightly increased photooxidative stress sensitivity (Figs. (D) and ). In addition, recently we reported that DHAR3 regulates glutathione redox states to acclimate to excess light.Citation27) Based on these findings and results, we consider that DHAR isoforms play a crucial role in glutathione oxidation under photooxidative stress conditions. This was supported by transgenic tobacco plants that overexpress DHAR accumulating higher amounts of GSSG than control plants.Citation49,50) As described above, in ascorbate-glutathione cycle, APX reduces H2O2 to water using AsA as an electron donor. Among the APX isoforms, the lack of cytosolic APX1 is known to inhibit the accumulation of glutathione as well as cell death in cat2.Citation51) Therefore, it is conceivable that APX1 has an effect on the GSH oxidation to provide DHA for DHAR1. In contrast, Arabidopsis plants overexpressing DHAR1 accumulated higher levels of GSH, but not GSSG, than control plants.Citation31) Thus, more detailed analyses are required for our conclusion.
As described above, DHAR1 and DHAR2 are located in the cytosol, while DHAR3 is located in chloroplasts. In this study, the lack of DHAR1 slightly but significantly enhanced oxidation of ascorbate under normal conditions when grown on MS medium (Fig. (B)). On the other hand, our previous report showed that the lack of DHAR3 affected the oxidative level of glutathione, but not that of ascorbate, under normal conditions.Citation27) These findings suggest that cytosolic form of DHAR enzymes, DHAR1 and DHAR2, may participate more than the chloroplastic form, DHAR3, in reduction of ascorbate. Consistent with this speculation, MDAR activity increased in dhar1 and dhar2 mutants, but not in dhar3 plant, under normal conditions (Fig. (D) and Supplementary Fig. S4). Thus, it is implied that the maintenance of ascorbate redox pool by DHAR in cytosol could be compensated by MDAR enzymes in Arabidopsis plants. Recently, the lack of MDAR6 located in both chloroplasts and mitochondria had no effect on the levels of ascorbate but increased the levels of glutathione in root.Citation52) This findings and our previous report suggest that ascorbate-glutathione cycle related enzymes in chloroplasts have little effect on ascorbate regulation in response to photooxidative stress. In fact, the lack of chloroplastic APX enzymes, thylakoid-bound and stromal APX, led to the perturbation of the levels and redox state of glutathione but not those of ascorbate under photooxidative stress.Citation41) These findings indicated that the component enzymes of ascorbate-glutathione cycle in chloroplasts have an important role in modulation of glutathione redox pool, implying that responses to photooxidative stress may be triggered by the collapse of chloroplastic glutathione redox homeostasis. However, as mentioned above, growth conditions may be affected to the ratio of DHAR activity of each DHAR isoform from Arabidopsis to the total activity. Additionally, even in this study, differences in growth conditions have non-negligible effects on the contents and redox states of ascorbate and glutathione before and/or after photooxidtive stress treatments. In future studies, the comprehensive analysis of DHAR isoforms under unifying conditions is required for understanding the physiological importance of ascorbate-glutathione cycle in each cellular compartment individually.
In conclusion, we identified DHAR1 as a crucial regulator of cellular redox states under photooxidative stress. This redox regulation is required for photooxidative stress tolerance in Arabidopsis. The lack of DHAR1 more strongly affected the redox states of glutathione than those of ascorbate, at least at the late stage of HL, which implied that its function was associated with glutathione oxidation rather than ascorbate recycling. To address this in more detail, we are now starting to analyze double mutants of dhar1 and cat2 as well as double and triple mutants of DHAR isoforms. Future analyses will clarify the physiological significance of DHAR1 from the point of view of glutathione oxidase in planta.
Author contributions
M.N., H.Y., and R.H., carried out the experiments. M.N. drafted the manuscript. N.T. and M.T. edited the manuscript. N.T., M.T., and S.S. conceived of the study and participated in its design and coordination.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Grants-in-Aid for Scientific Research (A) Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan [grant number S.S.: 22248042]; Strategic Project to Support the Formation of Research Bases at Private University: Matching Fund from MEXT, 2011–2015 [grant number S1101035].
Supplemental material
Supplemental material for this article can be accessed at http://dx.doi.org/10.1080/09168451.2016.1256759
TBBB_1256759_Supplemental_Material.ppt
Download MS Power Point (1.4 MB)Acknowledgments
We are grateful to Dr. Takanori Maruta (Shimane University) for helpful discussions. We thank Mr. Tsukasa Shimazaki (Kindai University) for technical assistance.
Notes
Abbreviations: APX, ascorbate peroxidase; AsA, reduced ascorbic acid; DHA, dehydroascorbate; DHAR, dehydroascorbate reductase; eHL, extreme high light; GSH, reduced glutathione; GSSG, oxidized glutathione; GR, glutathione reductase; H2O2, hydrogen peroxide; HL, high light; MDHA, monodehydroascorbate; MDAR, monodehydroascorbate reductase; MV, methylviologen; ROS, reactive oxygen species.
References
- Chen M, Chory J, Fankhauser C. Light signal transduction in higher plants. Annu Rev Genet. 2004;38:87–117.10.1146/annurev.genet.38.072902.092259
- Jiao Y, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants. Nat Rev Genet. 2007;8:217–230.10.1038/nrg2049
- Eberhard S, Finazzi G, Wollman FA. The dynamics of photosynthesis. Annu Rev Genet. 2008;42:463–515.10.1146/annurev.genet.42.110807.091452
- Foyer CH, Noctor G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant. 2003;119:355–364.10.1034/j.1399-3054.2003.00223.x
- Mittler R, Vanderauwera S, Gollery M, et al. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498.10.1016/j.tplants.2004.08.009
- Gadjev I, Vanderauwera S, Gechev TS, et al. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol. 2006;141:436–445.10.1104/pp.106.078717
- Foyer CH, Shigeoka S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011;155:93–100.10.1104/pp.110.166181
- Schmidt R, Schippers JH. ROS-mediated redox signaling during cell differentiation in plants. Biochem Biophys Acta. 2014;1850:1497–1508.
- Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 2011;155:2–18.10.1104/pp.110.167569
- Asada K. The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639.10.1146/annurev.arplant.50.1.601
- Shigeoka S, Ishikawa T, Tamoi M, et al. Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot. 2002;53:1305–1319.10.1093/jexbot/53.372.1305
- Chew O, Whelan J, Millar AH. Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J Biol Chem. 2003;278:46869–46877.10.1074/jbc.M307525200
- Gallie DR. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J Exp Bot. 2013;64:433–443.10.1093/jxb/ers330
- Shigeoka S, Maruta T. Cellular redox regulation, signaling, and stress response in plants. Biosci Biotechnol Biochem. 2014;78:1457–1470.10.1080/09168451.2014.942254
- Munné-Bosch S, Alegre L. Interplay between ascorbic acid and lipophilic antioxidant defences in chloroplasts of water-stressed Arabidopsis plants. FEBS Lett. 2002;524:145–148.10.1016/S0014-5793(02)03041-7
- Szarka A, Tomasskovics B, Bánhegyi G. The ascorbate-glutathione-α-tocopherol triad in abiotic stress response. Int J Mol Sci. 2012;13:4458–4483.10.3390/ijms13044458
- Niyogi KK. Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Mol Biol. 1999;50:333–359.10.1146/annurev.arplant.50.1.333
- Smirnoff N, Wheeler GL. Ascorbic acid in plants: biosynthesis and function. Crit Rev Biochem Mol Biol. 2000;35:291–314.10.1080/10409230008984166
- Muller-Moule P, Conklin PL, Niyogi KK. Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol. 2002;128:970–977.10.1104/pp.010924
- Mano J, Hideg E, Asada K. Ascorbate in thylakoid lumen functions as an alternative electron donor to photosystem II and photosystem I. Arch Biochem Biophys. 2004;429:71–80.10.1016/j.abb.2004.05.022
- Tóth SZ, Schansker G, Garab G. The physiological roles and metabolism of ascorbate in chloroplasts. Physiol Plant. 2013;148:161–175.10.1111/ppl.2013.148.issue-2
- Chen Z, Young TE, Ling J, et al. Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA. 2003;100:3525–3530.10.1073/pnas.0635176100
- Chen Z, Gallie DR. Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol. 2006;142:775–787.10.1104/pp.106.085506
- Chen Z, Gallie DR. Dehydroascorbate reductase affects non-photochemical quenching and photosynthetic performance. J Biol Chem. 2008;283:21347–21361.10.1074/jbc.M802601200
- Chen Z, Gallie DR. The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell. 2004;16:1143–1162.10.1105/tpc.021584
- Dixon DP, Edwards R. Glutathione transferases. Arabidopsis Book. 2010;8:e0131.10.1199/tab.0131
- Noshi M, Hatanaka R, Tanabe N, et al. Redox regulation of ascorbate and glutathione by a chloroplastic dehydroascorbate reductase is required for high-light stress tolerance in Arabidopsis. Biosci Biotechnol Biochem. 2016;80:870–877.10.1080/09168451.2015.1135042
- Yoshida S, Tamaoki M, Shikano T, et al. Cytosolic dehydroascorbate reductase is important for ozone tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2006;47:304–308.
- Reumann S, Quan S, Aung K, et al. In-depth proteome analysis of arabidopsis leaf peroxisomes combined with in vivo subcellular targeting verification indicates novel metabolic and regulatory functions of peroxisomes. Plant Physiol. 2009;150:125–143.10.1104/pp.109.137703
- Grefen C, Donald N, Hashimoto K, et al. A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. Plant J. 2010;64:355–365.10.1111/tpj.2010.64.issue-2
- Wang Z, Xiao Y, Chen W, et al. Increased vitamin C content accompanied by an enhanced recycling pathway confers oxidative stress tolerance in Arabidopsis. J Integr Plant Biol. 2010;52:400–409.10.1111/jipb.2010.52.issue-4
- Vadassery J, Tripathi S, Prasad R, et al. Monodehydroascorbate reductase 2 and dehydroascorbate reductase 5 are crucial for a mutualistic interaction between Piriformospora indica and Arabidopsis. J Plant Physiol. 2009;166:1263–1274.10.1016/j.jplph.2008.12.016
- Shigeoka S, Nakano Y, Kitaoka S. Metabolism of hydrogen peroxide in Euglena gracilis z by L-ascorbic acid peroxidase. Biochem J. 1980;186:377–380.10.1042/bj1860377
- Eltayeb AE, Kawano N, Badawi GH, et al. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta. 2007;225:1255–1264.10.1007/s00425-006-0417-7
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25.10.1007/BF00386001
- Maruta T, Noshi M, Tanouchi A, et al. H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J Biol Chem. 2012;287:11717–11729.10.1074/jbc.M111.292847
- Shigeoka S, Onishi T, Nakano Y, et al. Characterization and physiological function of glutathione reductase in Euglena gracilis z. Biochem J. 1987;242:511–515.10.1042/bj2420511
- Maruta T, Noshi M, Nakamura M, et al. Ferulic acid 5-hydroxylase 1 is essential for expression of anthocyanin biosynthesis-associated genes and anthocyanin accumulation under photooxidative stress in Arabidopsis. Plant Sci. 2014;219–220:61–68.10.1016/j.plantsci.2014.01.003
- Gueta-Dahan Y, Yaniv Z, Zilinskas BA, et al. Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in Citrus. Planta. 1997;203:460–469.10.1007/s004250050215
- Noshi M, Mori D, Tanabe N, et al. Arabidopsis clade IV TGA transcription factors, TGA10 and TGA9, are involved in ROS-mediated responses to bacterial PAMP flg22. Plant Sci. 2016;252:12–21.10.1016/j.plantsci.2016.06.019
- Maruta T, Tanouchi A, Tamoi M, et al. Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant Cell Physiol. 2010;51:190–200.10.1093/pcp/pcp177
- Dixon DP, Davis BG, Edwards R. Functional divergence in the glutathione transferase superfamily in plants: identification of two classes with putative functions in redox homeostasis in Arabidopsis thaliana. J Biol Chem. 2002;277:30859–30869.10.1074/jbc.M202919200
- Foyer CH, Mullineaux PM. The presence of dehydroascorbate and dehydroascorbate reductase in plant tissues. FEBS Lett. 1998;425:528–529.10.1016/S0014-5793(98)00281-6
- Mhamdi A, Queval G, Chaouch S, et al. Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot. 2010;61:4197–4220.10.1093/jxb/erq282
- Noctor G, Queval G, Mhamdi A, et al. Glutathione. Arabidopsis Book. 2011;9:e0142.
- Noctor G, Mhamdi A, Chaouch S, et al. Glutathione in plants: an integrated overview. Plant Cell Environ. 2012;35:454–484.10.1111/j.1365-3040.2011.02400.x
- Garcia-Gimenez JL, Markovic J, Dasi F, et al. Nuclear glutathione. Biochem Biophys Acta. 1830;2013:3304–3316.
- Queval G, Issakidis-Bourguet E, Hoeberichts FA, et al. Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J. 2007;52:640–657.10.1111/j.1365-313X.2007.03263.x
- Kwon SY, Choi SM, Ahn YO, et al. Enhanced stress-tolerance of transgenic tobacco plants expressing a human dehydroascorbate reductase gene. J Plant Physiol. 2003;160:347–353.10.1078/0176-1617-00926
- Le Martret B, Poage M, Shiel K, et al. Tobacco chloroplast transformants expressing genes encoding dehydroascorbate reductase, glutathione reductase, and glutathione-S-transferase, exhibit altered anti-oxidant metabolism and improved abiotic stress tolerance. Plant Biotechnol J. 2011;9:661–673.10.1111/pbi.2011.9.issue-6
- Vanderauwera S, Suzuki N, Miller G, et al. Extranuclear protection of chromosomal DNA from oxidative stress. Proc Natl Acad Sci USA. 2011;108:1711–1716.10.1073/pnas.1018359108
- Johnston EJ, Rylott EL, Beynon E, et al. Monodehydroascorbate reductase mediates TNT toxicity in plants. Science. 2015;349:1072–1075.10.1126/science.aab3472
