Abstract
Adiponectin, an adipokine with insulin-sensitizing effect, is secreted from adipocytes into circulation as high, medium, and low molecular weight forms (HMW, MMW, and LMW). The HMW adiponectin oligomers possess the most potent insulin-sensitizing activity. WSF-P-1(N-methyl-1,2,3,4,5,6-hexahydro-1,1,5,5-tetramethyl-7H-2,4α-methanonaphthalen-7-amine) is derived from natural sesquiterpene longifolene by chemical modifications. We found that WSF-P-1 activates AMPK in both 3T3-L1 adipocytes and 293T cells in this study. Activation of AMPK by WSF-P-1 promotes the assembly of HMW adiponectin and increases the HMW/total ratio of adiponectin in 3T3-L1 adipocytes. We demonstrated that the Ca2+-dependent CaMKK signaling pathway is involved in WSF-P-1-induced AMPK activation and adiponectin multimerization. WSF-P-1 also activates GLUT1-mediated glucose uptake in 3T3-L1 adipocytes, making it a potential drug candidate for the treatment of type 2 diabetes, obesity, and other obesity-related metabolic diseases.
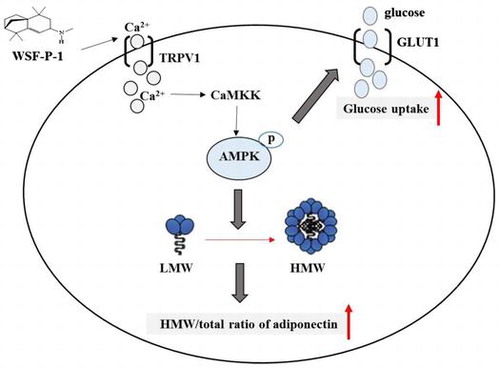
Graphical abstract
WSF-P-1 activates AMPK via the Ca2+-CaMKK signaling pathway and promotes adiponectin multimerization and glucose uptake in 3T3-L1 adipocytes.
Adiponectin is the most abundant adipokine produced and secreted by the adipose tissue.Citation1) The serum level of adiponectin inversely correlates with obesity and directly correlates with insulin sensitivity.Citation2,3) Treatment with PPARγ agonists, such as thiazolidinediones (TZDs), weight loss or caloric restriction leads to increased level of adiponectin in serum.Citation4–6) Therefore, adiponectin is a potential drug candidate for the treatment of obesity-related metabolic diseases.Citation7)
Adiponectin is secreted from adipocytes into the circulation as three oligomeric forms: low molecular weight (LMW) trimers, medium molecular weight (MMW) hexamers, and high molecular weight (HMW) 18–36 oligomers.Citation8,9) Each type of adiponectin oligomers exerts distinct biological functions, with the HMW oligomers possessing the most potent insulin-sensitizing activity.Citation10) Proportion of the oligomers changes according to metabolic status and disease states. The serum level of HMW adiponectin decreases in patients with obesity, type 2 diabetes, metabolic syndrome, and cardiovascular diseases.Citation11) TZDs, which are widely used to treat type 2 diabetes, increase the ratio of HMW adiponectin to total adiponectin and improve the insulin sensitivity of the patients.Citation10) However, treatment with TZDs was associated with various undesirable side effects, including edema, obesity, congestive heart failure, and bone loss.Citation12,13) Since adiponectin mRNA is expressed at high levels in adipocytes, optimizing the assembly and secretory pathway should be the most effective way to increase the level of HMW adiponectin in the serum.Citation14,15)
The AMP-activated protein kinase (AMPK), a serine/threonine kinase, regulates energy homeostasis in response to internal and external stress.Citation16,17) Activation of AMPK has previously been found to promote adiponectin multimerization.Citation18–20) Therefore, the AMPK signaling pathway plays an important role in regulating the assembly of HMW adiponectin, providing more evidence that AMPK is a prime therapeutic candidate for obesity-related metabolic disorders.
AMPK is a heterotrimer composed of a catalytic α subunit and a regulatory β and γ subunits. Activation of AMPK occurs when AMP binds to the nucleotide binding site on the γ subunit, causing a conformational change in the α subunits which allows the upstream kinases, such as LKB1 and CaMKKβ, to phosphorylate AMPK at Τhr-172.Citation21) AMPK activators identified so far activate AMPK either directly or indirectly. A-769662, a potent AMPK activator, activates the kinase directly by binding to the AMPK complex at a site different from the AMP-binding site.Citation22) Other activators, such as berberine and metformin, activate AMPK indirectly by inhibiting the respiratory chain and increasing the AMP/ATP ratio.Citation23–25) As a result, these indirect AMPK activators inhibit the mitochondrial function.Citation23) Therefore, it is urgent to identify more specific and potent AMPK activators, which should have few side effects as anti-diabetic drugs.
WSF-P-1, N-methyl-1,2,3,4,5,6-hexahydro-1,1,5,5-tetramethyl-7H-2,4α-methanonaphthalen-7-amine, is derived from natural sesquiterpene longifolene. In the present study, we found that WSF-P-1 is a novel AMPK activator, which activates AMPK via the Ca2+-dependent CaMKK signaling pathway. We found that activation of AMPK by WSF-P-1 promotes adiponectin multimerization and glucose uptake in 3T3-L1 adipocytes.
Materials and methods
Reagents and antibodies
All chemicals and antibody against actin were purchased from Sigma. Antibodies against phospho-AMPK, AMPK, AMPKα1, or GLUT1 were purchased from Cell Signaling. siRNA oligonucleotide targeting mouse AMPKα1 (the sequence: ACAUAUGCUGCAGGUGGA) was synthesized at GenePharma (Shanghai, China).
Chemical synthesis of WSF-P-1
0.025 mol of isolongifolenone, 0.15 mol of 30% methamine ethanol solution, 0.15 mol of BF3⋅(C2H5)2O, and 30 mL of ethanol were separately added into the 100 mL three-necked flask equipped with stirrer, reflux condenser, and thermometer, and the mixture was then heated at 30 °C for 24 h with protection of N2. The reaction was monitored with GC until the content of isolongifolenylimine reached 85%, and 0.072 mol of NaBH4 was then added to the mixture at 0 °C. After finishing adding NaBH4, the reduction reaction was continued for 12 h at room temperature. The reaction was stopped by adding 20 mL of distilled water, and extracted with EtOAc for three times. The combined organic layer was washed with water until neutrality, followed by dry with anhydrous Na2SO4. After recovering the solvent, the residue was dissolved in EtOAc and added dropwise into 30% HCl-ethanol solution under ice bath condition. The resulting white precipitate was washed with EtOAc, and the residual EtOAc was removed. The refined salt was dissolved in distilled water again, and added NaOH solution to reach weak basic. The solution was extracted with EtOAc (3 × 20 mL), and the combined EtOAc layer was washed with water (3 × 20 mL) and saturated NaCl solution (50 mL) successively, and then dried over anhydrous sodium sulfate. 2.72 g of refined compound WSF-P-1 was obtained with 46.7% yield and 99.2% purity.
WSF-P-1 was characterized by IR, mass spectrometry, 1H-NMR, and 13C-NMR (Supplementary materials). White crystals; IR (KBr, ν cm−1): 2957, 2868, 2786, 1461, 1381, 1362, 1124; 1H NMR (CDCl3, 300 MHz) δ: 0.8967 (t, J = 6.75 Hz, 3H), 0.9502 (s, 3H), 0.9769 (s, 3H), 1.0070 (s, 3H), 1.0734 (s, 3H), 1.2374 (d, J = 9.6 Hz, 1H), 1.2732–1.3990 (m, 8H), 1.4133–1.5591 (m, 5H), 1.6093–1.7410 (m, 2H), 1.8101 (d, J = 3.87 Hz, 1H), 2.5728–2.7419 (m, 2H), 3.1014–3.1413(m, 1H), 5.3649 (d, J = 3.81 Hz); 13C NMR (CDCl3, 75 MHz) δ: 157.43, 113.10, 54.59, 46.84, 46.67, 41.49, 39.18, 37.13, 36.61, 34.41, 33.09, 29.00, 26.77, 25.55, 24.93, 24.70; MS (70 eV) m/z (%): 233 (M+,57) 56 (25), 73 (4), 77 (25), 91 (42), 108 (14), 115 (22), 119 (48), 131 (77), 159 (65), 176 (12), 190 (43), 204 (100), 218 (73).
Treatment of 293T cells
293T cells (ATCC) were grown in DMEM (Invitrogen) supplemented with 10% FBS (Hyclone) at 37 °C in 5% CO2. To investigate whether WSF-P-1 activates AMPK, 70–80% confluent 293T cells were pretreated with Compound C for one hour followed by treatment with WSF-P-1 for another hour. All chemicals used in the treatment were dissolved in DMSO. There are matched vehicle controls for each treatment so that the final concentration of DMSO is identical for each experiment.
Cell culture, cell differentiation, and treatment
3T3-L1 preadipocytes (ATCC) were grown and differentiated into mature adipocytes as described previously.Citation18–20) To examine the effect on differentiation, 3T3-L1 preadipocytes were induced to differentiate in the presence of WSF-P-1 or berberine. At Day 8 post-differentiation, the lipid droplets in the cells were stained and quantified as previously described.Citation26) To investigate the mechanism by which WSF-P-1 activates AMPK, 3T3-L1 adipocytes were pretreated with capsazepine, STO-609 or Compound C for one hour followed by treatment with WSF-P-1 for another hour. To examine the effect on adiponectin multimerization, 3T3-L1 adipocytes were treated with WSF-P-1 for 48 h. 3T3-L1 adipocytes were transfected with mouse AMPKα1 siRNA or control siRNA for 48 h followed by treatment with WSF-P-1.
SDS-PAGE and western blot analysis
Cell lysates of 3T3-L1 adipocytes were subjected to 2–15% gradient gel electrophoresis under non-reducing and non-heat-denaturing conditions.Citation8) Adiponectin oligomers and the total amount of adiponectin were detected as described.Citation18–20) AMPK was detected using antibodies specific for phospho-AMPK or AMPK. The relative abundance of adiponectin oligomers or monomer was quantificated by analyzing the western blots using the NIH ImageJ software. All experiments were performed at least three times and representative results were presented.
Measurement of intracellular calcium
After treatment with WSF-P-1 or evodiamine, 293T cells were harvested and incubated with 500 nM Fluo-3 at 37 °C for 30 min and then were immediately analyzed on a flow cytometer (530 nm) using FL-1 as a detector, as described previously.Citation20)
Glucose uptake assay
3T3-L1 adipocytes were treated with WSF-P-1 or berberine alone or pre-treated with Compound C for 1 h followed by treatment with WSF-P-1 or berberine. After 24 h treatment, the cells were incubated in serum-free and glucose-free DMEM containing 1 mM insulin for 30 min. Glucose uptake was measured by a bioluminescent assay,Citation27) using the Glucose Uptake-GloTM Assay Kit (Promega).
Membrane isolation
Total membranes were isolated from 3T3-L1 adipocytes using Membrane Protein Extraction Kit (Beyotime, Shanghai, China).
Statistical analysis
All data are expressed as the means ± SD. Student’s t-test was used to compare two samples. Statistical comparisons among three samples of the same treatment group were performed using one-way ANOVA with Dunnett’s test as post hoc test. In all cases, P value < 0.05 was considered to be statistically significant.
Results
WSF-P-1 activates AMPK in 293T cells
WSF-P-1 is derived from natural sesquiterpene longifolene,Citation28) with structure shown in Fig. (A). To examine whether WSF-P-1 activates AMPK, 293T cells were treated with WSF-P-1 and the level of phosphorylated AMPK (pAMPK) was examined by western blot using AMPK phosphorylation-specific antibodies. WSF-P-1 increased the level of pAMPK. Compound C (CC), a specific AMPK inhibitor, abolished the effect of WSF-P-1 on AMPK activation (Fig. (B) and (C)). Therefore, WSF-P-1 activates AMPK in 293T cells.
Fig. 1. WSF-P-1 activates AMPK by increasing intracellular calcium concentrations in 293T cells.
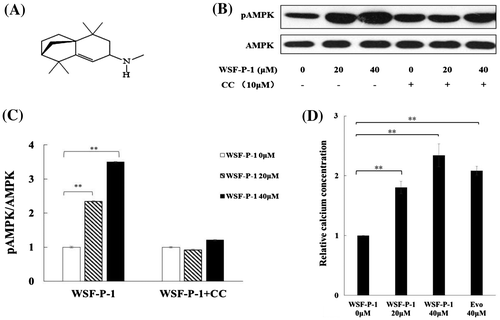
AMPK can be activated by an elevated intracellular Ca2+ level.Citation16,20,29) Evodiamine was reported to activate AMPK by inducing extracellular Ca2+ flux.Citation20) WSF-P-1 also increased the intracellular calcium concentration in a dose-dependent way, similar to evodiamine (Fig. (D)). This result suggested that Ca2+ flux might be involved in WSF-P-1-induced activation of AMPK.
WSF-P-1 activates AMPK via the Ca2+-dependent CaMKK signaling pathway in 3T3-L1 adipocytes
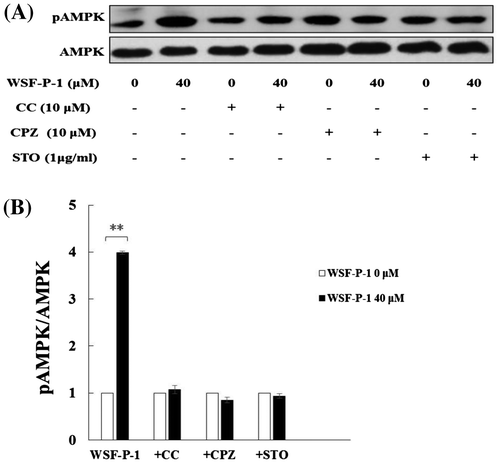
We also found that WSF-P-1 activates AMPK in 3T3-L1 adipocytes (Fig. (A) and (B)). Evodiamine was reported to function as an agonist for the vanilloid receptor TRPV1 and activate AMPK by increasing intracellular Ca2+ concentration. The activation of AMPK by evodiamine was inhibited by capsazepine (CPZ), a specific TRPV1 antagonist.Citation20,30) The activation of AMPK by WSF-P-1 was also inhibited by CPZ (Fig. (A) and (B)). The result demonstrated that Ca2+ flux is involved in WSF-P-1-induced activation of AMPK.
Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) has been demonstrated to act upstream of AMPK.Citation31) To determine whether CaMKK is responsible for WSF-P-1-induced AMPK activation, we pretreated 3T3-L1 adipocytes with STO-609 (STO), an inhibitor of CaMKK. We found that STO abrogated WSF-P-1-induced increase in AMPK phosphorylation (Fig. (A) and (B)). Therefore, CaMKK is the upstream kinase that mediates the effect of WSF-P-1 on AMPK activation.
Taken together, our results demonstrate that WSF-P-1 activates AMPK via the Ca2+/CaMKK-mediated signaling pathway.
WSF-P-1 promotes adiponectin multimerization in 3T3-L1 adipocytes
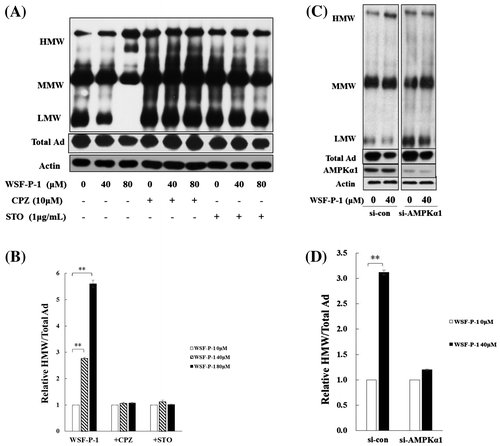
It was previously reported that activation of AMPK by berberine, emodin, or evodiamine promotes adiponectin multimerization in mature 3T3-L1 adipocytes.Citation18–20) To investigate the effect of WSF-P-1 on adiponectin multimerization, 3T3-L1 adipocytes were treated with WSF-P-1 for 48 h. The level of total adiponectin (Total Ad) was reduced after WSF-P-1 treatment (Fig. (A)). Therefore, WSF-P-1 inhibits the expression of adiponectin in mature adipocytes. The level of HMW adiponectin was increased, whereas the level of LMW adiponectin was decreased by WSF-P-1 (Fig. (A), top panel). The HMW/total ratio was increased by WSF-P-1 in a dose-dependent way (Fig. (B)). Suppression of AMPKα1 expression by siRNA completely abolished the effect of WSF-P-1 on adiponectin multimerization (Fig. (C) and (D)). These results demonstrated that WSF-P-1 promotes adiponectin multimerization by activating AMPK.
Fig. 2. WSF-P-1 activates AMPK via the Ca2+-dependent CaMKK signaling pathway.
To further investigate the mechanism by which WSF-P-1 promotes the multimerization of adiponectin, 3T3-L1 adipocytes were pretreated with CPZ or STO followed by treatment with WSF-P-1 for 48 h. Adiponectin multimerization promoted by WSF-P-1 was inhibited by CPZ or STO (Fig. (A) and (B)). Therefore, these results demonstrated that WSF-P-1 promotes adiponectin via the Ca2+-mediated AMPK signaling pathway.
WSF-P-1 inhibits the differentiation of 3T3-L1 preadipocytes
PPARγ is a dominant positive regulator of preadipocyte differentiation. Activation of AMPK leads to phosphorylation of PPARγ and inhibits its transcriptional activity, leading to inhibition of differentiation of 3T3-L1 preadipocytes.Citation32–34) To determine the effect of WSF-P-1 on differentiation, 3T3-L1 preadipocytes were induced to differentiate in the presence of WSF-P-1 or berberine. We found that differentiation of 3T3-L1 preadipocytes was inhibited by WSF-P-1 dose-dependently, similar to berberine (Fig. (A) and (B)). This result demonstrated that WSF-P-1 inhibits the differentiation of 3T3-L1 preadipocytes. The inhibitory effect of WSF-P-1 on differentiation is the result of reduced PPARγ activity resulting from AMPK activation.
Fig. 3. WSF-P-1 promotes adiponectin multimerization in 3T3-L1 adipocytes.
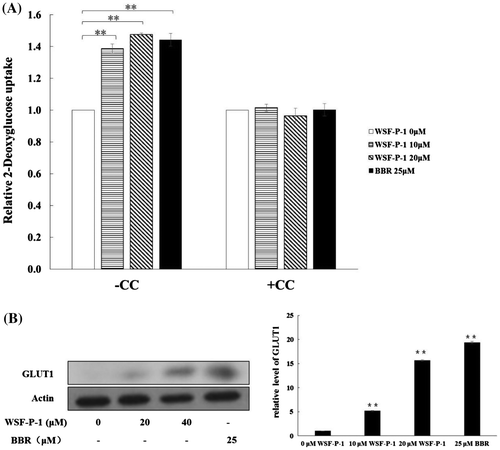
WSF-P-1 promotes glucose uptake in 3T3-L1 adipocytes
Activation of AMPK by berberine promotes glucose uptake.Citation35) To examine the effect of WSF-P-1 on glucose uptake, 3T3-L1 adipocytes were treated with WSF-P-1 and glucose transport was measured using a bioluminescent assay. We found that glucose uptake was increased by WSF-P-1, and that the effect of WSF-P-1 was completely abolished in the presence of CC (Fig. (A)). The level of GLUT1 on the membrane was also increased after WSF-P-1 treatment (Fig. (B)). Therefore, WSF-P-1 promotes GLUT1-mediated glucose uptake by activating AMPK.
Fig. 4. WSF-P-1 inhibits preadipocyte differentiation.
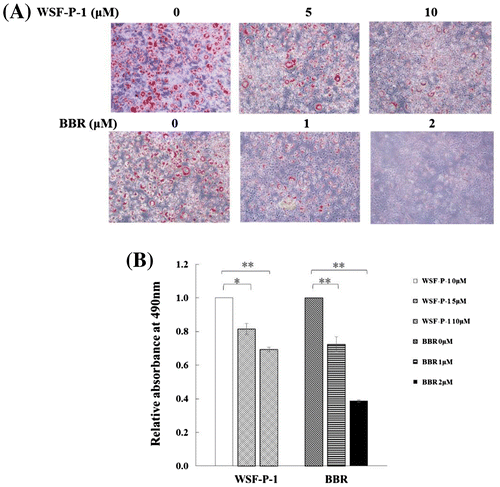
Fig. 5. WSF-P-1 promotes glucose uptake.
Discussion
It is well established that adiponectin enhances insulin sensitivity in both skeletal muscle and liver tissues. Adiponectin circulates in serum as LMW trimers, MMW hexamers, and HMW oligomers. The HMW adiponectin oligomers possess the most potent insulin-sensitizing activity. It has been reported that the three forms of adiponectin oligomers are very stable and do not convert into each other once secreted into the circulation.Citation10) Therefore, adiponectin multimerization and secretion is tightly regulated by the adipocytes. In this study, we demonstrated that the synthetic WSF-P-1 is a novel AMPK activator; it activates AMPK in both 293T cells and 3T3-L1 adipocytes (Figs. and ). We have previously reported that several natural products from traditional Chinese medicine promote adiponectin multimerization by activating AMPK.Citation18–20) WSF-P-1 is the first synthetic AMPK activator we have identified in our screen. Activation of AMPK by WSF-P-1 also promotes the assembly of HMW adiponectin in adipocytes (Fig. ). Therefore, the present study provides further evidence that the AMPK signaling pathway positively regulates adiponectin multimerization.
Metformin and TZDs,the two major classes of anti-diabetic drugs, activate AMPK by suppressing Complex Ι of the respiratory chain.Citation24) Berberine activates AMPK by a mechanism similar to that of metformin,Citation23) whereas A-769662 activates AMPK directly by binding to it.Citation22) WSF-P-1 was found to activate AMPK by increasing intracellular calcium concentration. Blocking TRPV1-mediated Ca2+ flux or inhibiting CaMKK signaling abolished the effect of WSF-P-1 (Figs. and ). Therefore, WSF-P-1 activates AMPK via the Ca2+-dependent CaMKK signaling pathway. Ca2+-dependent PI3 K/Akt/CaMKII signaling pathway was involved in evodiamine-induced AMPK activation.Citation20) Therefore, the Ca2+-mediated AMPK signaling pathways promote the multimerization of adiponectin. However, WSF-P-1 does not induce Akt phosphorylation and inhibition of PI3 K/Akt signaling has no effect on AMPK activation induced by WSF-P-1 (data not shown), suggesting that WSF-P-1 activates AMPK via a different Ca2+-mediated signaling pathway.
The ratio of HMW to total adiponectin directly correlates with TZD-mediated improvement in insulin sensitivity.Citation10) Activation of AMPK by WSF-P-1 promotes adiponectin multimerization by increasing the HMW/Total ratio of adiponectin (Fig. ). By activating AMPK, WSF-P-1 might lead to the phosphorylation of downstream target proteins which can promote adiponectin oligomerization. We have identified several candidate proteins, some of which are AMPK substrates. Further investigation of the molecular mechanism is currently underway.
Activation of AMPK increased phosphorylation of PPARγ, which inhibits its transcriptional activity and inhibits the expression of adiponectin.Citation18,32) We have also demonstrated that activation of AMPK promotes adiponectin multimerization.Citation18,20) Therefore, our study suggested that AMPK signaling pathway plays a dual role in regulating adiponectin biosynthesis. On one hand, it inhibits the transcription of adiponectin by inhibiting the transcriptional activity of PPARγ resulting in low expression of adiponectin. On the other hand, it promotes the assembly of LMW adiponectin into HMW adiponectin, leading to an increased ratio of HWM to total adiponectin. Our results with siRNA support this model; both effects of WSF-P-1 on adiponectin were abolished when the expression of endogenous AMPKα1 was knocked down (Fig. (C)). Therefore, this study further demonstrated the role of the AMPK signaling pathway in the biosynthesis of adiponectin.
The derivatives of isolongifolenone, a naturally occurring sesquiterpene, have been used extensively as ingredients in the cosmetics industry. In addition, they are also reported to repel ticks and mosquitoes effectively.Citation36) Isolongifolenone can be easily synthesized from inexpensive turpentine oil feedstock. WSF-P-1 is derived from natural sesquiterpene longifolene by chemical modifications. The fact that WSF-P-1 increases the HMW/total ratio of adiponectin and activates glucose uptake makes it a potential drug candidate for the treatment of type 2 diabetes, obesity, and other obesity-related metabolic diseases.
Author contributions
YY Wang, H Peng, and J Rui participated in the chemical synthesis of WSF-P-1. Y Wang and YD Zhang carried out all the other experiments, analyzed the results, and wrote the manuscript. ZJ Zhang, SF Wang, and Z Li designed and supervised the study. Z Li edited the manuscript. All the authors have approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) [grant number 31570760 to Z. L.], [grant number 31470592 to S.W.]
Supplemental materials
The supplemental material for this paper is available at https://doi.org/10.1080/09168451.2017.1336923.
TBBB_1336923Supplementary_materials.pptx
Download MS Power Point (120.1 KB)Notes
Abbreviations: AMPK, AMP-activated protein kinase; PPARγ, peroxisome proliferators activated receptor γ; CaMKK, Calcium/calmodulin-dependent protein kinase kinase; Total Ad, total adiponectin; HMW, high molecular weight;MMW, medium molecular weight; LMW, low molecular weight; CC, Compound C; CPZ, capsazepine; STO, STO-609; GLUT1, glucose transporter 1.
References
- Rajala MW, Scherer PE. Minireview: the adipocyte – at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773.10.1210/en.2003-0580
- Shetty S, Kusminski CM, Scherer PE. Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci. 2009;30:234–239.10.1016/j.tips.2009.02.004
- Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in Type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599.10.1161/01.ATV.20.6.1595
- Bobbert T, Rochlitz H, Wegewitz U, et al. Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes. 2005;54:2712–2719.10.2337/diabetes.54.9.2712
- Combs TP, Berg AH, Rajala MW, et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–276.10.2337/diabetes.52.2.268
- Oowatari Y, Ogawa T, Katsube T, et al. Wasabi leaf extracts attenuate adipocyte hypertrophy through PPARγ and AMPK. Biosci Biotechnol Biochem. 2016;80:1594–1601.10.1080/09168451.2016.1179093
- Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326.10.1007/s00125-012-2598-x
- Waki H, Yamauchi T, Kamon J, et al. Impaired multimerization of human adiponectin mutants associated with diabetes: molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363.10.1074/jbc.M300365200
- Pajvani UB, Du, X, Combs, TP, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085.10.1074/jbc.M207198200
- Pajvani UB, Hawkins M, Combs TP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162.10.1074/jbc.M311113200
- Basu R, Pajvani UB, Rizza RA, et al. Selective downregulation of the high molecular weight form of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from nondiabetic subjects. Diabetes. 2007;56:2174–2177.10.2337/db07-0185
- Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795.10.2337/db09-9028
- Silva JC, César FA, de Oliveira EM, et al. New PPARγ partial agonist improves obesity-induced metabolic alterations and atherosclerosis in LDLr(-/-) mice. Pharmacol Res. 2016;104:49–60.10.1016/j.phrs.2015.12.010
- Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. Biochem J. 2010;425:41–52.10.1042/BJ20091045
- Liu M, Liu F. Regulation of adiponectin multimerization, signaling and function. Best Pract Res Clin Endocrinol Metab. 2014;28:25–31.10.1016/j.beem.2013.06.003
- Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–341.10.1161/01.RES.0000256090.42690.05
- Hardie DG, Schaffer BE, Brunet A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016;26:190–201.10.1016/j.tcb.2015.10.013
- Li Y, Wang P, Zhuang Y, et al. Activation of AMPK by berberine promotes adiponectin multimerization in 3T3-L1 adipocytes. FEBS Lett. 2011;585:1735–1740.10.1016/j.febslet.2011.04.051
- Chen Z, Zhang L, Yi J, et al. Promotion of adiponectin multimerization by emodin: a novel AMPK activator with PPARγ-agonist activity. J Cell Biochem. 2012;113:3547–3558.10.1002/jcb.24232
- Liu LH, Xie JY, Guo WW, et al. Evodiamine activates AMPK and promotes adiponectin multimerization in 3T3-L1 adipocytes. J Asian Nat Prod Res. 2014;16:1074–1083.10.1080/10286020.2014.939071
- Carling D, Sanders MJ, Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obesity. 2008;32(Suppl 4):S55–S59.10.1038/ijo.2008.124
- Sanders MJ, Ali ZS, Hegarty BD, et al. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J Biol Chem. 2007;282:32539–32548.10.1074/jbc.M706543200
- Hawley SA, Ross FA, Chevtzoff C, et al. Use of cells expressing γ subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565.10.1016/j.cmet.2010.04.001
- Brunmair B, Staniek K, Gras F, et al. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 2004;53:1052–1059.10.2337/diabetes.53.4.1052
- Turner N, Li JY, Gosby A, et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57:1414–1418.10.2337/db07-1552
- Wu G, Yi J, Liu L, et al. Pseudoginsenoside F11, a novel partial PPARγ agonist, promotes adiponectin oligomerization and secretion in 3T3-L1 adipocytes. PPAR Res. 2013;2013:701017.
- Valley MP, Karassina N, Aoyama N, et al. A bioluminescent assay for measuring glucose uptake. Anal Biochem. 2016;505:43–50.10.1016/j.ab.2016.04.010
- Zhang AJ, Wang SF. Facile and efficient synthesis of isolongifolenone. Org Prep Proced Int. 2008;40:405–410.
- Hinkley JM, Ferey JL, Brault JJ, et al. Constitutively active CaMKKα stimulates skeletal muscle glucose uptake in insulin-resistant mice in vivo. Diabetes. 2014;63:142–151.10.2337/db13-0452
- Pearce LV, Petukhov PA, Szabo T, et al. Evodiamine functions as an agonist for the vanilloid receptor TRPV1. Org Biomol Chem. 2004;2:2281–2286.10.1039/b404506h
- Woods A, Dickerson K, Heath R, et al. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metabo. 2005;2:21–33.10.1016/j.cmet.2005.06.005
- Lee YS, Kim WS, Kim KH, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2264.10.2337/db06-0006
- Ito-Nagahata T, Kurihara C, Hasebe M, et al. Stilbene analogs of resveratrol improve insulin resistance through activation of AMPK. Biosci Biotechnol Biochem. 2013;77:1229–1235.10.1271/bbb.121000
- Han YH, Li Z, Um JY, et al. Anti-adipogenic effect of glycoside St-E2 and glycoside St-C1 isolated from the leaves of Acanthopanax henryi (Oliv.) Harms in 3T3-L1 cells. Biosci Biotechnol Biochem. 2016;80:2391–2400.10.1080/09168451.2016.1217150
- Kim SH, Shin EJ, Kim ED, et al. Berberine activates GLUT1-mediated glucose uptake in 3T3-L1 adipocytes. Biol Pharm Bull. 2007;30:2120–2125.10.1248/bpb.30.2120
- Zhang A, Klun JA, Wang S, et al. Isolongifolenone: a novel sesquiterpene repellent of ticks and mosquitoes. J Med Entomol. 2009;46:100–106.10.1603/033.046.0113
