Abstract
The present study was designed to evaluate the hepatoprotective potential of α-lactalbumin (αLA) against dimethylnitrosamine (DMN)-induced toxic insults in the rat liver. The liver damage was induced in rats by the repeated administration of DMN (10 mg/kg, i.p.) on three consecutive days per week for three weeks. The rats were maintained on either a standard AIN-93 M or αLA-enriched diet starting one week before the DMN injection until the termination of the experiment. The DMN treatment produced a progressive increase in the plasma markers (aspartate aminotransferase, alanine aminotransferase, total bililbin, hyarulonic acid, and matrix metalloproteinase-2) in 28 days after the first DMN injection. Dietary treatment with αLA significantly reduced the DMN-induced damage toward normalcy. NG-nitro-L-arginine methyl ester, a nitric oxide synthase inhibitor, significantly attenuated the hepatoprotective effect of αLA. These findings show that αLA has a marked suppressive effect on hepetic fibrosis through a nitric oxide-mediated mechanism.
Bovine milk-derived α-lactalbumin has a suppressive effect on dimethylnitrosamine-induced hepatic fibrosis by via nitric oxide pathway in rats.
Liver fibrosis is the final stage of various liver diseases such as viral hepatitis, alcoholic hepatitis, autoimmune hepatitis, and nonalcoholic fatty liver disease.Citation1,2) The progression of liver fibrosis leads to cirrhosis and eventually to hepatocellular carcinoma (HCC). Regardless of the cause, hepatic fibrosis involves the accumulation of extracellular matrix protein components. Hepatic fibrogenesis is accompanied by hepatocellular necrosis and inflammation. In the development of hepatic fibrosis, hepatic stellate cells undergo activation and produce the amounts of extracellular matrix, especially collagen.Citation3,4) Hepatic stellate cells are involved in not only the collagen deposition and the hepatic fibrosis development but also the regulation of intrahepatic blood flow by sinusoid contraction.Citation3,5) The contraction of hepatic stellate cells is counterbalanced by vasorelaxing substances such as nitric oxide.Citation6) Therefore, induction of nitric oxide is thought to lead to the suppression of hepatic stellate cells activation and hepatic fibrosis.
Whey protein accounts for 20% of milk protein. Its principal components are β-lactoglobulin, α-lactalbumin (αLA), immunoglobulin, bovine serum albumin (BSA), and lactoferrin.Citation7) Whey protein has been reported to have various activities such as antioxidant,Citation8,9) antibacterial,Citation10) antiviral,Citation11) anticancer,Citation12) antihypertensive,Citation13) and immnomodulatory effects.Citation14) Whey protein and peptides were found to have hepatoprotective effects in a galactosamine-induced hepatitis rat model.Citation15) A diet which was enriched with whey hydrolyzed peptides prevented the progression of concanavalin A-induced hepatitisCitation16) and dimethylnitrosamine (DMN)-induced liver fibrosis.Citation17)
αLA, which accounts for 20% of whey protein, shows anti-inflammatory,Citation18) anticancer,Citation19,20) antidiabetic,Citation21) and gastroprotective effects.Citation22) We reported that αLA suppressed pro-inflammatory release after intestinal ischemia/reperfusion via nitric oxide-mediated mechanism.Citation23) However, it has not been clarified whether liver fibrosis can be suppressed via nitric oxide-mediated mechanism by αLA.
In the present study, we investigated whether αLA prevents the progression of DMN-induced liver fibrosis via nitric oxide-mediated mechanism.
Materials and methods
Reagents
αLA (BioPURE AlphalactalbuminTM) was obtained from Davisco Foods International Inc.(Eden Prairie, MN, USA). DMN was obtained from Wako (Osaka, Japan). NG -nitro-L-arginine methyl ester (L-NAME)was obtained from Sigma (St. Louis, MO, USA). Pentobarbital sodium solution (Somnopentyl) was obtained from Kyoritsu Seiyaku (Tokyo, Japan).
A commercially available diet, AIN-93 M, was purchased from Funabashi Farm (Chiba, Japan). The αLA-enriched diet was also prepared by Funabashi Farm Co., Ltd.
Animal experiments
Male Sprague-Dawley rats at 7 weeks of age were obtained from SLC Japan, Inc. (Shizuoka, Japan) and used in the following experiments after 1 week of acclimation in a room maintained at 22 ± 1 °C with a humidity of 55 ± 15% and 12 h light/dark cycle, with the light period running from 7:00 to 19:00. All animal experiments were performed in accordance with the guidelines of the Meiji Co., Ltd. for the care and use of laboratory animals.
In experiment 1, the rats were divided into three groups (n = 9); (1) the normal group; (2) the DMN-treated group (DMN); and (3) the DMN and αLA-treated group (DMN + αLA). Rats were fed a standard AIN-93 M diet or an αLA-enriched diet (Table ). One week after starting the diets, rats were intraperitoneally given DMN (1% dissolved in saline; 1 mL/kg) for three consecutive days each week for three weeks, whereas rats in the normal group were injected with saline. Blood samples were obtained from the saphenous vein at 6, 13, and 20 days after the first DMN injection. The rats were sacrificed under pentobarbital anesthesia at 28 days after the first DMN injection. At sacrifice, blood samples were collected from the abdominal aorta and centrifuged for 15 min at 1500 × g. Plasma was then collected and frozen at –80 °C for biochemical analysis. Livers and spleens were excised and weighed. The liver specimens were immediately fixed in 10% neutral buffered formalin for histochemical studies.
Table 1 Diet composition.
In experiment 2, the rats were divided into four groups (n = 9); (1) the DMN-treated group; (2) the DMN and αLA-treated group; (3) the DMN, αLA and L-NAME-treated group; (4) the DMN and L-NAME-treated group. The rats were fed a standard AIN-93 M diet or an αLA-enriched diet (Table ). One week after starting the diets, the rats were intraperitoneally given DMN (1% dissolved in saline; 1 mL/kg) for 3 consecutive days each week for 3 weeks, whereas the rats in the control group were injected with saline. The L-NAME-treated groups were given 0.02% L-NAME in their drinking water, starting 1 week before the DMN injection, until the termination of the experiment. Blood samples were obtained from the saphenous vein at 3, 10, and 17 days after the first DMN injection. The rats were sacrificed under pentobarbital anesthesia at 20 days after the first DMN injection. Blood samples were collected from the abdominal aorta and centrifuged for 15 min at 1500 × g at sacrifice. Plasma was then collected and frozen at – 80 °C for biochemical analysis. The rats’ livers and spleens were excised and weighed.
Biochemical analysis
The plasma levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and total bilirubin were measured by colorimetric slides using Fuji Dri-Chem NX500 (Fujifilm, Tokyo, Japan). The plasma levels of hyaluronic acid were measured using an enzyme-linked immunosorbent assay kit (Biotech Trading Partners, USA) following the manufacturer’s protocol. The plasma levels of matrix metalloprotease-2 (MMP-2) were measured using a fluorimetric kit (Anaspec, USA).
Liver histopathology
The liver specimens were fixed and embedded in paraffin. The liver sections were deparaffinized and processed routinely for hematoxylin-eosin, Masson trichrome staining. Fibrosis was scored by calculating of the grade (grade 0, not detected; grade 1,rare; grade 2, slight; grade 3, moderate; grade 4, marked).Citation24)
Statistical analysis
Data were expressed as means ± SEM. Ordinal variables were compared using a cumulative χ2 test. Continuous variables were analyzed using Dunnett’s test (parametric test) or Steel’s test (non-parametric test). P-value < 0.05 was considered statistically significant.
Results
Effect of αLA on blood ALT and AST levels
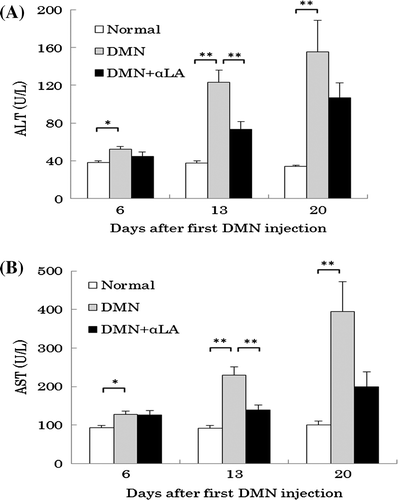
The plasma levels of ALT and AST were markedly elevated by DMN treatment (Fig. ). The plasma ALT and AST levels in DMN + αLA-treated rats at Day 13 (73.6 ± 12.6 IU/L and 138.8 ± 13.9 IU/L) were significantly lower than those in the DMN group (123.2 ± 12.6 IU/L and 230.0 ± 21.5 IU/L).
Fig. 1. Effect of αLA on ALT (A) and AST (B) levels in plasma of DMN-treated rats.
Effect of αLA on the weights of the liver and spleen
The absolute organ weight and relative organ weight of the liver were decreased by the DMN treatment (Table ). The absolute organ weight and relative organ weight of the spleen were significantly increased by the DMN treatment. The absolute organ weight of the liver in DMN + αLA-treated rats was significantly higher than that in the DMN group. The absolute organ weight and relative organ weight of the spleen in DMN + αLA-treated rats were significantly lower than those in the DMN group.
Table 2. Effect of αLA on the weights of the liver and spleen in rats treated with DMN.
Effect of αLA on the plasma parameters
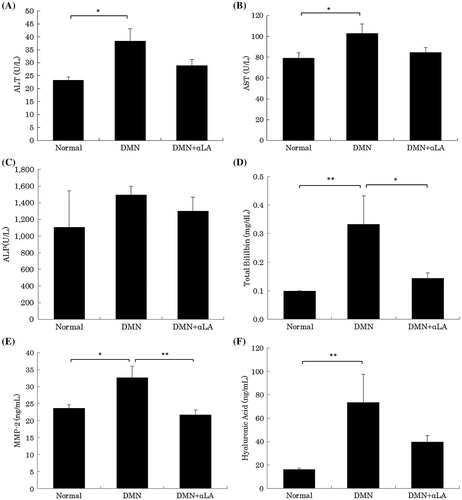
The plasma ALT, AST, total bililbin, hyaluronic acid and MMP-2 levels were significantly elevated by the DMN treatment (Fig. ). The plasma total bililbin and MMP-2 levels in DMN + αLA-treated rats at sacrifice (0.14 ± 0.02 and 21.7 ± 1.3 ng/mL) were significantly lower than those in the DMN group (0.33 ± 0.10 and 32.6 ± 3.4 mg/dL).
Fig. 2. Effect of αLA on ALT (A), AST (B), ALP (C), total bililbin (D), MMP-2 (E), and hyaluronic acid (F) levels in plasma of DMN-treated rats.
Effect of αLA on hepatic fibrosis
In normal liver stained with Masson trichrome, collagen was observed only around small central venous walls (Fig. (A)). In contrast to normal rat liver, the treatment of DMN caused the deposition of collagen which stretched from the portal area to lobular areas (Fig. (B)). These alterations were remarkably reduced in the DMN + αLA-treated rat liver (Fig. (C)). The presence of hepatic fibrosis was graded as +++: “marked” in three of nine rats in the DMN group at 28 days after the first DMN injection (Fig. ). Dietary treatment of αLA decreased the fibrosis grades (Table ).
Fig. 3. Histological analysis of liver sections.
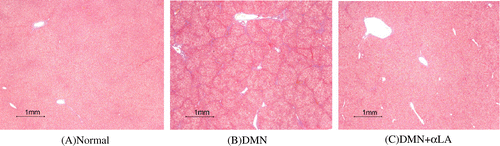
Table 3. Effect of αLA on the fibrosis grade in Masson trichrome-stained liver sections prepared from rats at 28 days after the first DMN injection.
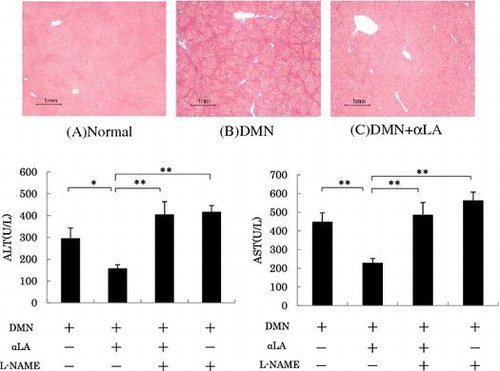
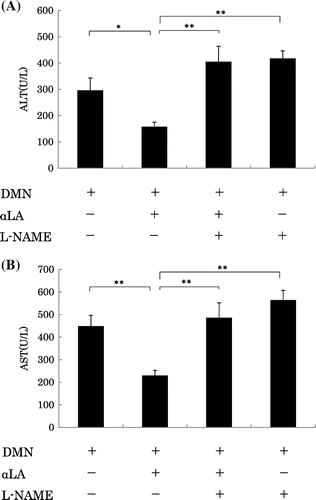
Effect of the NO inhibitor on the suppression of ALT and AST levels by αLA
The suppression of plasma ALT and AST levels was partially but significantly attenuated by L-NAME (Fig. ). L-NAME alone did not affect the increase of in the plasma in the ALT and AST levels induced by DMN.
Fig. 4. Effect of αLA on activity of ALT (A) and AST (B) levels in plasma of DMN-treated rats.
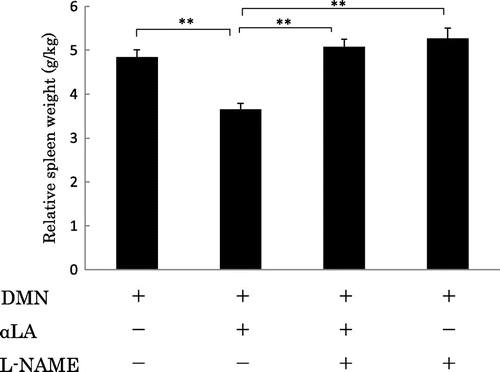
Effect of the NO inhibitor on the suppression of the spleen weight increase by αLA
The suppression of spleen weight increase was partially, but significantly attenuated by L-NAME (Fig. ). L-NAME alone did not affect the increase of the weight of the spleen induced by DMN.
Fig. 5. Effect of αLA on relative spleen weight in rats treated with DMN.
Discussion
In experiment 1, the plasma ALT and AST levels were elevated after DMN treatment. The liver was contracted and the spleen was enlarged at sacrifice. Moreover, the total bililbin, hyaluronic acid, and MMP-2 levels were significantly elevated. These results indicated that DMN induced hepatic fibrosis. αLA treatment suppressed the DMN-induced contraction of the liver, enlargement of the spleen, and hepatic fibrosis seen in the histological analysis. αLA treatment suppressed DMN-induced increase of ALT, AST, total bililbin and MMP-2.
DMN is a potent hepatotoxin, carcinogen, and mutagen.Citation25) The DMN-induced liver fibrosis in rats reflects changes that occur in human hepatic fibrosis. This model exhibits many of the same features, such as portal hypertension, ascites, and histopathological and biochemical abnormalities.Citation26) This model is an appropriate animal model for studying the mechanisms of hepatic fibrosis. Therefore, we used DMN model to evaluate the effect of αLA.
The DMN-induced liver fibrosis model induces shrinking of the liver, enlargement of the spleen,Citation27) and the alteration of biochemical parameters in the plasma.Citation17,28) DMN induces the increase of ALT, AST, ALP, total bililbin, and hyaluronic acid. MMP-2 is secreted from activated hepatic stellate cells and increased in the presence of type I collagen. It was reported that the expression of MMP-2 is increased in the DMN-induced liver fibrosis.Citation29,30) Our data are consistent with the previous results, so the MMP-2 levels in plasma were induced by DMN. αLA suppressed the increase of MMP-2 levels.
In experiment 2, the suppression of increased plasma ALT and AST levels was significantly attenuated by L-NAME. The suppression of the spleen weight increase was partially but significantly attenuated by L-NAME. These data indicate that the induction of nitric oxide production is involved in the mechanism of hepatic fibrosis suppression by αLA.
Several studies of the relationship between nitric oxide production and the development of liver cirrhosis with DMN-induced hepatic injury in rats have been reported.Citation31–Citation33) The inhibition of nitric oxide production by L-NAME, an inhibitor of nitric oxide synthase, accelerates intravascular coagulation and hepatic injury.Citation31) Treatment with NaNO2, the donor of nitric oxide, improves the liver morphology and serum marker enzyme activities.Citation32) Nitric oxide synthase consists of isoforms. One of them, endothelial nitric oxide synthase (eNOS), attenuates hepatic vascular resistance, whereas other isoform, inducible nitric oxide synthase (iNOS), involves in hepatic injury.Citation34) DMN-induced liver injury results in the activation of hepatic stellate cells and induces the depression of eNOS expression and the enhancement of iNOS expression.Citation33) Our present study suggested that αLA prevents hepatotoxicity induced by DMN via the nitric oxide pathway. It may be possible that the induction of eNOS expression leads to the attenuation of hepatic vascular resistance. Futher study is necessary to clarify the cell types of producing nitric oxide in the hepatic fibrosis.
We have confirmed that αLA suppresses IL-6 by increasing nitric oxide production after intestinal ischemia/reperfusion and this suppression by αLA is inhibited by the pretreatment with L-NAME.Citation23) Nitric oxide appears to inhibit inflammatory cytokines such as TNF-α, IL-8, IL-1b, IL-2, and IL-6 by inhibiting NF-κB-dependent transcription.Citation35,36) These findings suggest that αLA may have inhibited DMN-induced hepatotoxicity by inhibiting NF-κB transcriptional activity via NO production in our present study.
DMN leads to the formation of reactive oxygen species, and DMN-treated rats exhibited increased levels of malondialdehyde (MDA), the end product of lipid peroxidation, and decreased levels of superoxide dismutase (SOD), antioxidative enzyme.Citation37,38) Whey-hydrolyzed peptide-enriched diet inhibits the increase levels of MDA in DMN-induced hepatic injury rats.Citation17) Enzymatic hydrolysates from αLA have an antioxidant activity because these hydrolysates include a high amount of tryptophan and tyrosine which belong to phenolic and indolic groups to serve as hydrogen donors.Citation39) We may guess that αLA exhibits the antioxidant activity due to peptides and amino acids which are decomposed from αLA in the process of ingestion and absorption.
In conclusion, it was suggested that αLA suppressed DMN-induced liver fibrosis and that nitric oxide production may be a part of its mechanism. Long-term intake of αLA may be effective for the prevention of liver damage.
Author contributions
A. Fukawa, M. Yamaguchi, and A. Hosono designed the experiments. A. Fukawa and O. Kobayashi performed the experiments. A. Fukawa performed statistical analysis. A. Fukawa and M. Uchida analyzed histopathological data. A. Fukawa wrote the manuscript. M. Yamaguchi and M. Uchida edited the manuscript. A. Hosono played an important role in interpreting the results. All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
The authors would like to thank Dr. Hisae Kume for her useful suggestions and valuable discussions. We also thank Dr. Taketo Yamaji and Dr. Hiroyuki Itoh for their constant encouragement and advice.
References
- Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669.10.1053/j.gastro.2008.03.003
- Wallace K, Burt AD, Wright MC. Liver fibrosis. Biochem J. 2008;411:1–18.10.1042/BJ20071570
- Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Eng J Med. 1993;328:1828–1835.
- Friedman SL. Molecular mechanisms of hepatic fibrosis and principles of therapy. J Gastroenterol. 1997;32:424–430.10.1007/BF02934504
- Rockey DC, Housset CN, Friedman SL. Activation-dependent contractility of rat hepatic lipocytes in culture and in vivo. J Clin Invest. 1993;92:1795–1804.10.1172/JCI116769
- Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344–351.10.1016/S0016-5085(98)70487-1
- Walzem RL, Dillard CJ, German JB. Whey components: millennia of evolution create functionalities for mammalian nutrition: what we know and what we may be overlooking. Crit Rev Food Sci Nutr. 2002;42:353–375.10.1080/10408690290825574
- Gad AS, Khadrawy YA, El-Nekeety AA, et al. Antioxidant activity and hepatoprotective effects of whey protein and Spirulina in rats. Nutrition. 2011;27:582–589.10.1016/j.nut.2010.04.002
- Xu R, Liu N, Xu X, et al. Antioxidative effects of whey protein on peroxide-induced cytotoxicity. J Dairy Sci. 2011;94:3739–3746.10.3168/jds.2010-3891
- Iwamori T, Nukumi N, Itoh K, et al. Bacteriostatic activity of whey acidic protein (WAP). J Vet Med Sci. 2010;72:621–625.10.1292/jvms.08-0331
- Elattar G, Saleh Z, EL-Shebini S, et al. The use of whey protein concentrate in management of chronic hepatitis C virus—a pilot study. Arch Med Sci. 2010;5:748–755.10.5114/aoms.2010.17091
- Hakkak R, Korourian S, Ronis MJ, et al. Dietary whey protein protects against azoxymethane-induced colon tumors in male rats. Cancer Epidemiol Biomarkers Prev. 2001;10:555–558.
- Pal S, Ellis V. The chronic effects of whey proteins on blood pressure, vascular function, and inflammatory markers in overweight individuals. Obesity. 2010;18:1354–1359.10.1038/oby.2009.397
- Kloek J, Mortaz E, Van Ark I, et al. A whey-based glutathione-enhancing diet decreases allergen-induced airway contraction in a guinea-pig model of asthma. Br J Nutr. 2011;105:1465–1470.10.1017/S0007114510005337
- Kume H, Okazaki K, Sasaki H. Hepatoprotective effects of whey protein on d-galactosamine-induced hepatitis and liver fibrosis in rats. Biosci Biotechnol Biochem. 2006;70:1281–1285.10.1271/bbb.70.1281
- Kume H, Okazaki K, Yamaji T, et al. A newly designed enteral formula containing whey peptides and fermented milk product protects mice against concanavalin A-induced hepatitis by suppressing overproduction of inflammatory cytokines. Clin Nutr. 2012;31:283–289.10.1016/j.clnu.2011.10.012
- Jobara K, Kaido T, Hori T, et al. Whey-hydrolyzed peptide-enriched immunomodulating diet prevents progression of liver cirrhosis in rats. Nutrition. 2014;30:1195–1207.10.1016/j.nut.2014.02.005
- Yamaguchi M, Yoshida K, Uchida M. Novel functions of bovine milk-derived α-lactalbumin: anti-nociceptive and anti-inflammatory activity caused by inhibiting cyclooxygenase-2 and phospholipase A2. Biol Pharm Bull. 2009;32:366–371.10.1248/bpb.32.366
- Yamaguchi M, Takai S, Hosono A, et al. Bovine milk-derived α-lactalbumin inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sodium sulfate-treated mice. Biosci Biotechnol Biochem. 2014;78:672–679.10.1080/09168451.2014.890034
- Roy SS, Mukherjee S, Ballard BR, Das SK. Protection against dimethylbenz[a] anthracene-induced breast cancer in female rats by α-lactalbumin. Int J Cancer Oncol. 2016;3:1–6.
- Yamaguchi M, Takai S. Chronic administration of bovine milk-derived α-lactalbumin improves glucose tolerance via enhancement of adiponectin in Goto-Kakizaki rats with type 2 diabetes. Biol Pharm Bull. 2014;37:404–408.10.1248/bpb.b13-00762
- Ushida Y, Shimokawa Y, Toida T, et al. Bovine α-lactalbumin stimulates mucus metabolism in gastric mucosa. J Dairy Sci. 2007;90:541–546.10.3168/jds.S0022-0302(07)71537-0
- Yamaguchi M, Uchida M. α-Lactalbumin suppresses interleukin-6 release after intestinal ischemia/reperfusion via nitric oxide in rats. Inflammopharmacology. 2007;15:43–47.10.1007/s10787-006-1558-9
- Nakano S, Nagasawa T, Ijiro T, et al. Bezafibrate prevents hepatic stellate cell activation and fibrogenesis in a murine steatohepatitis model, and suppresses fibrogenic response induced by transforming growth factor-β1 in a cultured stellate cell line. Hepatol Res. 2008;38:1026–1039.10.1111/hep.2008.38.issue-10
- Haggerty HG, Holsapple MP. Role of metabolism in dimethylnitrosamine-induced immunosuppression: a review. Toxicology. 1990;63:1–23.10.1016/0300-483X(90)90064-N
- George J, Rao KR, Stern R, et al. Dimethylnitrosamine-induced liver injury in rats: the early deposition of collagen. Toxicology. 2001;156:129–138.10.1016/S0300-483X(00)00352-8
- Tung YT, Tang TY, Chen HL, et al. Lactoferrin protects against chemical-induced rat liver fibrosis by inhibiting stellate cell activation. J Dairy Sci. 2014;97:3281–3291.10.3168/jds.2013-7505
- Shin MO, Moon JO. Effect of dietary supplementation of grape skin and seeds on liver fibrosis induced by dimethylnitrosamine in rats. Nutr Res Pract. 2010;4:369–374.10.4162/nrp.2010.4.5.369
- Shin JW, Son JY, Oh SM, et al. An herbal formula, CGX, exerts hepatotherapeutic effects on dimethylnitrosamine-induced chronic liver injury model in rats. World J Gastroenterol. 2006;12:6142–6148.10.3748/wjg.v12.i38.6142
- Inami M, Fukushima A, Ueno T, et al. Reduction of dimethylnitrosamine-induced liver fibrosis by the novel gene regulator PI polyamide targeting transforming growth factor β1 gene. Biol Pharm Bull. 2015;38:1836–1842.10.1248/bpb.b15-00328
- Nagase S, Isobe H, Ayukawa K, et al. Inhibition of nitric oxide production increases dimethylnitrosamine-induced liver injury in rats. J Hepatol. 1995;23:601–604.10.1016/0168-8278(95)80068-9
- Lukivskaya O, Lis R, Zwierz K, et al. Effect of the nitric oxide donor and the nitric oxide synthase inhibitor on the liver of rats with chronic hepatitis induced by dimethylnitrosamine. Pol J Pharmacol. 2004;56:599–604.
- Amirtharaj GJ, Thangaraj KR., Kini A, et al. Acute liver injury induced by low dose dimethylnitrosamine alters mediators of hepatic vascular flow. Toxicol Rep. 2014;1:707–717.10.1016/j.toxrep.2014.09.001
- McKim SE, Gabele E, Isayama F, et al. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology. 2003;125:1834–1844.10.1053/j.gastro.2003.08.030
- Matthews JR, Botting CH, Panico M, et al. Inhibition of NF-κB DNA binding by nitric oxide. Nucleic Acids Res. 1996;24:2236–2242.10.1093/nar/24.12.2236
- Peng HB, Libby P, Liao JK. Induction and stabilization of IκBα by nitric oxide mediates inhibition of NF-κB. J Biol Chem. 1995;270:14214–14219.10.1074/jbc.270.23.14214
- Wills PJ, Suresh V, Arun M, et al. Antiangiogenic effect of lygodium flexuosum against N-nitrosodiethylamine-induced hepatotoxicity in rats. Chem Biol Interact. 2006;164:25–38.10.1016/j.cbi.2006.08.021
- Hong SW, Jung KH, Lee HS, et al. Suppression by fucoidan of liver fibrogenesis via the TGF-β/Smad pathway in protecting against oxidative stress. Biosci Biotechnol Biochem. 2011;75:833–840.10.1271/bbb.100599
- Hernandez-Ledesma B, Davalos A, Bartolome B, et al. Preparation of antioxidant enzymatic hydrolysates from α-lactalbumin and β-lactoglobulin. Identification of active peptides by HPLC-MS/MS. J Agric Food Chem. 2005;53:588–593.10.1021/jf048626 m
