Abstract
Microwave-assisted extraction was employed for the isolation of polysaccharides from Posidonia oceanica (PPO). The extracting parameters were optimized adopting response surface methodology. The highest polysaccharide yield (2.55 ± 0.09%), which is in concordance with the predicted value (2.76%), was obtained under the following conditions: extraction time 60 s, liquid–solid ratio of 50:1 (mL/g) and power of 800 W. This polysaccharide, with molecular weight of 524 KDa, characterized by gas chromatography–mass spectrometry showed that PPO was mainly composed of galactose, glucose, and arabinose with molar percentages 25.38, 24.37, and 21.64%, respectively. The pharmacological evaluation of PPO using animal models at the dose of 100 mg/kg indicated a significant anti-inflammatory activity with a percentage of inhibition of edema of 54.65% and a significant antinociceptive activity with 78.91% inhibition of writhing for peripheral analgesic activity and an increase in the hot plate reaction time for central analgesic activity.
Microwave-assisted extraction and pharmacological activities of polysaccharides from Posidonia oceanica
The ocean, which covers about 71% of our planet’s surface, support many different sort of plant lifeCitation1). Various carbohydrates from marine species have numerous beneficial biological activitiesCitation2). Posidonia oceanica is a marine plant endemic to the Mediterranean basin that grows along the coast and extend from surface to 30–40 m of depth, according to the transparency of waterCitation3). It is appeared as beams from 4 to 8 broad sheets from approximately 1 cm (1 ± 0.2 cm) and long from 20 to 80 cm, on average, but being able to reach more than one meter. P. oceanica had a foremost role in marine ecosystem dynamics such as providing food and shelter source for several species, while stabilizing the sea floor. Previous studies on the composition of different kinds of seaweeds have determined that seagrasses have the third highest energy content and organic matter of all marine plants studiedCitation4,5).
In general, hot-water method has been used for classical extraction of carbohydrates, and has been widely investigatedCitation6). Nonetheless, it should be noted that hot water extraction of carbohydrates shows disadvantages including lower extraction efficiency, longer extraction time, and higher temperature. It is essential to find a favorable technique for extracting polysaccharides economically that gives high yield and strong activity. Nowadays, alternative extraction techniques such as microwave-assisted extraction (MAE), ultrasonic and enzyme-assisted extraction with enhanced yields or shorter time has been reportedCitation7,8). In this regard, MAE has received a great attention because it has many benefits with less solvent, shorter time, higher extraction rate, and superior products qualityCitation9). Many researches have been mentioned on the isolation of carbohydrates from natural sources by MAECitation10,11). Response surface methodology (RSM) is the most used statistical technique to assess the relationship between experimental and observed resultsCitation12). To the best of our cognition, there were no studies available in the literature concerning the optimization of MAE of carbohydrates from P. oceanica by RSM.
The objective of this paper was to study the effects of extraction time, microwave output power and liquid–solid ratio on total polysaccharide yield. Then, the extract has been analyzed using FT-IR analysis, size exclusion chromatography (SEC), and gas chromatography coupled to mass spectrometer (GC–MS) analysis. Finally, anti-inflammatory and antinociceptive activities (acetic acid induced and hot plate) of this isolated polysaccharide were evaluated in vivo.
Materials and methods
Materials and chemicals
P. oceanica leaves were collected on Monastir (Marina), Tunisia, in August 2014. Before use, they were washed three times with water to remove surface sand and dirt. Then, they were dried and ground in a laboratory dry blender to make fine powder. Finally, dried powder was extracted 3 times with 80% of ethanol at 75 °C until boiling to defat and remove some soluble and colored materials. All chemicals and solvents used in this research were purchased from Sigma-Aldrich and Chennai.
Extraction of polysaccharides
Extraction experiments of crude polysaccharide from P. oceanica were carried out in a StartSynth multimode microwave instrument producing controlled irradiation at 2.45 GHz (Milestone S.r.l., Sorisole, Italy). Each sample of P. oceanica was extracted with an aqueous solution of chloridric acid (10−2 M) in different parts of liquid–solid ratio (mL/g), extraction time (s), and power (W). After microwave heating, the mixture was centrifuged at 3500 rpm for 15 min and the supernatant was precipitated with 3 times volume of 95% (v/v) ethanol, stirred vigorously and left 10 hours at 4 °C. The precipitate was dissolved in distilled water and then lyophilized. The extraction yield of crude polysaccharide was calculated as follows:(1)
where m0 (g) is the weight of dried polysaccharide and m (g) is the weight of dried P. oceanica powder.
Moreover, the extract obtained under the optimum conditions was purified before the physico-chemical characterization and the evaluation of activities. In order to eliminate salts and compounds of low molecular weight (oligosaccharides, mineral and salts), the crude polysaccharide was extensively dialyzed using dialysis tubing with molecular weight cut off 14 kDa against distilled water for four days at 4 °C until conductivity equaled that of the distilled water. Finally, the dialysate was freeze dried.
Experimental design
In this research, three factors-three level Box-Behnken design (BBD) was utilized using MAE to study the individual and interactive effects of variables to extract the crude polysaccharide from P. oceanica. Process variables and their ranges are given in Table . Extraction time (X1), liquid–solid ratio (X2), and microwave power (X3) are selected as independent variables, whereas extraction yield of crude polysaccharide (Y) is selected as response.
Table 1. Process variables and their ranges.
Table shows the whole designed experiment is formed of 17 trial points in arbitrary order. Regression analysis was carried out and fitted into the empirical quadratic second-order polynomial model:(2)
Table 2. Box Behnken Design (BBD) matrix of the three variables in coded units and response values for the extraction rate of the polysaccharides.
where Y is the response, β0 is the model intercept coefficient, βi, βii, and βij are interaction coefficient of linear, quadratic, and the second-order terms, respectively, Xi and Xj are variables (i and j range from 1 to 3).
All the statistical analyses were realized using the software STATISTICA (version 7.0) for the experimental design and regression analysis of experimental data.
Chemical analysis
Protein content was determined according to AOAC method 981.10Citation13). Total sugar was estimated by Dubois method, using glucose as the standardCitation14). The uronic acid content was quantified by the carbazole methodCitation15).
ATR-FTIR analysis
In order to study the structural characteristics of the isolated polysaccharide, FT-IR spectra were recorded with PerkinElmer Spectrum Two ATR-FTIR. It was obtained between the frequency range of 4000–500 cm−1 and it was subsequently analyzed to determine possible bond types and functional groups.
Nuclear magnetic resonance
The isolated polysaccharide was dissolved in deuterium oxide D2O (99.96%) before NMR analysis. 1H NMR spectra were recorded using an AVANCE-300 NMR spectrometer (Brucker Inc., Rheinstetten, Germany) at 40 °C. The tetramethylsilane (TMS) was employed as an internal standard.
Analysis of monosaccharides composition by GC–MS
Neutral sugars composition of PPO was determined according to the procedure of Mkadmini et al.Citation16). After lyophilization, 0.45 mg of PPO was dissolved in 2 M trifluoroacetic acid (1 mL) and hydrolyzed at 70 °C for 2 h in a sealed tube in nitrogen atmosphere. Then, 100 μL of D-myo-inositol at a concentration of 1800 μg/mL in deionized water was added to the hydrolysate. The released monosaccharides were transformed to their trimethylsilyl derivatives by adding 100 μL of dry pyridine and 100 μL of N, O-bis (trimethylsilyl)-trifluoroacetamide to the dried sample. The residue was dissolved in 100 μL of dichloromethane and the solution stored at 4 °C before GC–MS analysis (Agilent Technologies, 5975C inert MSD with its Triple-Axis Detector, Germany). The detector condition was 70 eV. The temperatures used were 150 °C for the MS Quad, 230 °C for the MS Source, and 250 °C for the transfer line.
Size exclusion chromatography
SEC was used to determine the weight-average molecular weight (Mw), the dispersity (Ð = Mw/Mn) and the z-average gyration radius (Rg) of the extracted polysaccharide. A mobile phase (0.1 M aqueous LiNO3 solution) is pumped at 0.5 mL/min (LC10 Ai Shimadzu, Japan) through two OHPAK SB 804 and 806 HQ columns (Shodex) in seriesCitation17). The polymer solution (PPO) at 0.5 g/L injected through a 100 μL loop was eluted, separated, and detected using three detectors in series: a multi-angle light scattering (Down HELEOS II, Wyatt Technology), viscometer detector (VD) (Viscoster II, Wyatt Technology, CA, USA) and a differential refractive index (DRI) (RID 10 A).
Pharmacological evaluation
Male and female Swiss albino mice (20–25 g) and Wistar rats (150–200 g) purchased from Pasteur Institute (Tunis, Tunisia) were used. They were housed in groups in plastic cages at 20–25 °C and keeped on a standard pellet diet with free access to water.
Anti-inflammatory activity
The anti-inflammatory activity of the extracted polysaccharide from P. oceanica (PPO) was evaluated using carrageenan induced rat paw edema test. Wistar rats were splitted into groups of six animals each. The edema was induced by injecting 0.05 mL of 1% carrageenan into the subplantar region of the left hind pawCitation18).
PPO (50 or 100 mg/kg) and reference drug (Diclofenac, 25 mg/kg) were administered intraperitoneally (i.p.) 30 min before the injection of carrageenan. The control group received saline water (2.5 mL/kg, i.p.). Before carrageenan injection, measurement of paw size was done using Plethysmometer (Ugo Basile No. 7140) and then 1, 2, 3, 4, and 5 h later after carrageenan injection. Percentages of inhibition of edema were obtained for each lot using the following ratio:(3)
where Vt is the average volume for each group and V0 is the average volume obtained for each group before any treatment.
Acetic acid induced writhing in mice
The acetic-acid writhing test was used to evaluate peripheral analgesic activity according to the method of Koster et al.Citation19). Mice were selected one day before each test and were distributed into groups (n = 6 per group). One group served as control was pretreated subcutaneous (s.c.) with 0.9% saline (10 mL/kg); the second group was pretreated with the reference drug, acetylsalicylate of lysine (ASL) at the dose of 200 mg/kg and the lasting groups were pretreated with PPO at the doses of 50 and 100 mg/kg by the same route. Thirty min after administration of test compounds, all groups received 1% of acetic acid (10 mL/kg) by intraperitoneal administration. The intensity of nociception was measured by counting the total number of writhes that take place between 0 and 30 min after the injection. The percentages of inhibition were calculated according to the following formula:(4)
Hot-plate method
Hot-plate method was used to evaluate the central analgesic activity of PPO against thermal noxious stimuli in mice. The manipulation was carried out according to Lajili et al.Citation20). Each mouse was placed on a hot-plate analgesiameter (Ugo Basile, Cold-hot plate, No. 35100, Varese, Italy), which was maintained at 55 ± 0.5 °C. The time of latency (s) was recorded between placement of animal inside the cylinder up to the jumping of the paws or licking. Animals showing a reaction time upper than 10 s on hot-plate pretesting were excluded from the test analysis. Mice were divided into groups of six. The control group received 0.9% of saline solution (10 mL/kg), standards groups received reference drugs, Tramadol at the dose of 25 and 50 mg/kg and Paracetamol at the dose of 100 and 200 mg/kg. The other groups were injected with 10 mL/kg of PPO at the doses of 50 and 100 mg/kg. All test compounds were administrated subcutaneous. The latency time was measured at 30, 60, 90, and 120 min after the administration of compounds.
Statistical analysis
Results were analyzed using One Way ANOVA (Fisher LSD post hoc test) and expressed as mean ± s.e.m, using SPSS Statistics Software (SPSS for Windows software release18.0). Difference between means of control and treated groups were considered significant at p < 0.05.
Results and discussion
Model building and statistical analysis
In order to maximize the combined effects of independent variables extraction time, liquid–solid ratio and microwave power on the isolated polysaccharide from P. oceanica by RSM, a BBD of 17 runs was carried out arbitrarily (Table ). As shown in Table ,it was concluded that only the significant factors including linear-term coefficients (X1, X2, X3) and interaction-term coefficients (X1X2, X1X3) with a correlation coefficient (R2 = 0.8935) are given by the second-order polynomial equation between predicted response (the yield of polysaccharide) and the extraction variables.(5)
Table 3. Regression coefficients and analysis of variance (ANOVA) of the predicted second order polynomial model for the extraction yield of polysaccharide from P. oceanica.
where Y was the polysaccharide yield (%), and X1, X2, and X3 were the coded parameters for extraction time, liquid–-solid ratio and microwave power, respectively. A summary of the analysis of variance for the selected quadratic regression model was shown in Table . The smaller the p-value, the more significant the corresponding coefficients is. The obtained results showed that the F-value was 18.464, and p-value was less than 0.0001, which implied that the response surface quadratic model was significant. Additionally, the value of the determination coefficient R2 was 0.8935 implied that the sample variation of 89.35% for the extraction yield of PPO was attributable to the independent variables. From these results, we can conclude that the developed mathematical models have the ability to interpret the present extraction process very robustly.
Table 4. Analysis of variance (ANOVA) of the second order polynomial model for the extraction yield of polysaccharide.
Response surface plot and contour plot analysis
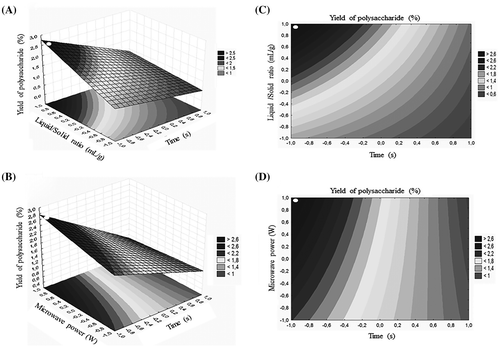
In this present research, as shown in Fig. , the 3D response surface plot and contour plot explain the extraction yield of carbohydrates from P. oceanica as function of any two independent variables while the third variable was fixed at 0 level. Fig. (A) and (C) graphed the effect of extraction time (X1) and ratio of solution to solid (X2) and their extractions on polysaccharide yield (%), when microwave power (X3) was fixed at 800 W. The result revealed that the polysaccharide yield augmented to the maximum value with increased ratio of solution to raw material but with a further increase in extraction time, extraction yield decreases slightly. As shown in Fig. (B) and (D) the polysaccharide yield increased evidently as microwave power increased but shorter or longer times led to decrease polysaccharide yield.
Multi-response optimization and verification of predictive model
According to BBD results, the optimum values of the tested independent variables of polysaccharide from P. oceanica were determined as follows: extracting time of 60 s, liquid–solid ratio of 50 mL/g and power of 800 W. Under these optimal conditions, the theoretical polysaccharide yield reached to the maximum as 2.76%. To validate the adequacy of the model equation for predicting the polysaccharide yield value, experimental rechecking was carried out using the above-mentioned conditions. A mean value of carbohydrate yield of 2.55% (n = 5) was obtained from real experiments, slightly less than the predicted value (2.76%) demonstrated the validation of the optimized conditions.
Physico-chemical characterization
The water soluble crude polysaccharide (PPO) was extracted from P. oceanica under optimum conditions with a yield of 2.55% using microwave-assisted extraction. The physicochemical properties and chemical compositions of the isolated polysaccharide at optimal conditions were summarized in Table . The total sugar, uronic acid, and protein content were 53.40, 22.97, and 1.19%, respectively. Based on the analysis of monosaccharide using GC–MS, PPO was a heteropolysaccharide and composed of galactose, glucose, arabinose, rhamnose, xylose, and fucose with the molar ratio of 25.38, 24.37, 21.64, 11.83, 10.88, and 5.90%, respectively. The results also indicate that galactose is the major monosaccharide component as detailed in Table . In comparison with other studiesCitation21), it was noted that some monosaccharides are commonly found in P. oceanica including glucose, galactose, xylose and the difference in the composition may be due to the extraction, the purification and the separation conditions or to environmental factorsCitation22).
Table 5. Physico-chemical characteristics and monosaccharide composition of P. oceanica.
SEC/MALS/DRI was used to determine the distribution and range of molecular weight of PPO. As shown in Table , the low values of the z-average gyration radius (Rg = 24 nm) and intrinsic viscosity ([η] = 77.2 mL g−1) relative to the high value of the weight-average molecular weight (Mw = 524 KDa) show that PPO possesses the conformation of a random coil.
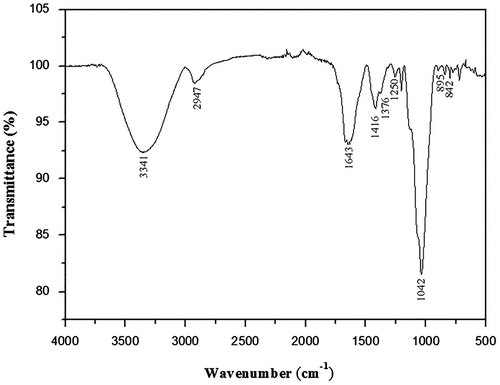
ATR-FTIR spectroscopy is a helpful technique that provides information of characteristic organic groups in polysaccharides. As shown in Fig. , a large absorption band at around 3341 cm−1was associated to the stretching vibration of O–H which is characteristic of glycosidic structures of sugar residues. A weak absorption band at 2947 cm−1 was referred to stretching vibration of C–H in the sugar ring. Also, the band at 1042 cm −1 was assigned to C–OC of glycosidic structureCitation23). Uronic acids were indicated by the strong absorption band at 1643 cm−1 (C=O) and two medium size bands at 1376 cm−1 and 1416 cm−1 (O–C=O)Citation24). In addition, the presence of an absorption band at 1250 cm−1 can be attributed to the stretching vibration of non-symmetrical C–O–C. Finally, the two characteristic absorption bands at about 842 and 895 cm−1 are arised from α-anomer and β-anomer form of the pyranoid ring, respectivelyCitation25). These results indicated that PPO possessed typical absorption peak of carbohydrates.
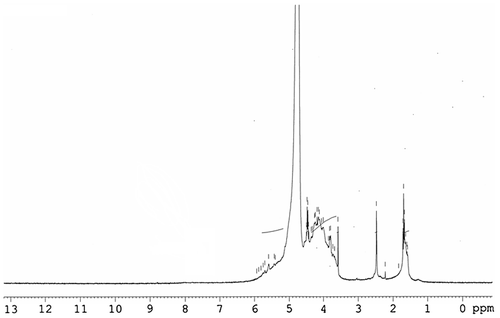
In order to better understand and confirm the sugars units of the extracted polysaccharide, we proceed by NMR 1H spectroscopy. Based on the literature, 1H NMR spectrum showed typical characteristic signals of polysaccharides (Fig. ), which are crowded in a tight region between 3.5 and 5.7 ppm, indicating the presence of sugar residuesCitation26). Signals between δ 3.6 and 4.1 ppm may be attributed to characteristic resonances of ring protons (H2–H5). Signals at δ 4.0–4.7 ppm are characteristic of β-anomeric protonsCitation27).Whereas a signal at δ 5.24 ppm, which was overlapping with the signal of the solvent, belonged to the α-anomeric protonsCitation28).
Anti-inflammatory activity of PPO
The anti-inflammatory potential of PPO was assessed at a concentration of 50 and 100 mg/kg, using carrageenan-induced paw edema. Edema was reduced by PPO in a dose–dependent manner (Table ). In the group treated with 100 mg/kg, PPO showed a significant activity with a reduction in paw edema of 54.65% comparable to that of diclofenac (57.08%). Its known that carrageenan-induced edema implied different chemical mediators including serotonin, histamine, leukotrienes, and prostaglandinsCitation29). The anti-inflammatory activity exhibited by PPO suggest that this substance could modulate the effects of these mediators by antagonizing their action or inhibiting their productions. Previous research has shown that the red seaweed Hypnea musciformis synthesizes polysaccharide fraction that reduced the inflammatory response by modulating neutrophil migration, which is dependent on the NO pathwayCitation30). Rodrigues et al.Citation31), investigating a sulfated polysaccharide from green seaweed C. cupressoides, demonstrated that its anti-inflammatory activity involves neutrophil migration, which suggests the pertinent act for this polysaccharide in interfering in the acute inflammation response. These reports demonstrate that each polymer could exhibit diverse biological action in vivo.
Table 6. Anti-inflammatory effect of the intraperitoneal administration of polysaccharides extracted from P. oceanica in carrageenan-induced rat paw edema test in comparison to control group (0.9% saline) and reference drug (Diclofenac).
Acetic acid writhing test
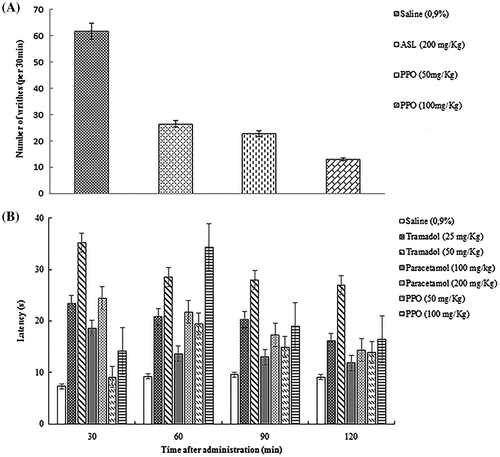
The acetic acid induced writhing reaction has widely been used as a screening tool for the evaluation of peripheral analgesia activity because of its sensitivity and simplicityCitation32). Dose-dependent and significant decreases in the number of abdominal writhing were observed after the administration of PPO. As shown in Fig. (A), at doses of 50 and 100 mg/kg, PPO reduced significantly the number of writhing by 63.25 and 78.91%, respectively. ASL, acting as a reference peripherally acting drug, at dose of 200 mg/kg reduced the number of writhing by 57.02%. Collier et al.Citation33) have demonstrated that acetic acid induced indirectly the release of endogenous mediators sensitive to non-steroidal anti-inflammatory agents (NSAIDs) and opioids. Suseem et al.Citation34) reported also that the significant decrease in acetic acid-induced writhes may be through central mechanisms concerning receptor systems or peripherally mediated via inhibition of synthesis and/or release of endogenous pro-inflammatory substances such as leukotrienes, prostaglandins, bradykinin, substance P in the peritoneal fluid. In this work, animals that were pretreated with PPO amended the analgesic response induced by acetic acid in a dose-dependent manner, suggesting that the analgesic action of PPO could be by inhibiting the release of mediators in response to acetic acid.
Hot plate test
For a possible specific central action of polysaccharides, in which the opioid agents exert their antinociceptive effects through spinal receptors and supra spinalCitation35), PPO was evaluated at 50 and 100 mg/kg during the 120 min of observation (Fig. (B)). PPO produced a maximum significant increase in the reaction latencies of animals against thermal stimuli at 60 min with a latency time of 19.37 s and 34.30 s, respectively. In comparison, Paracetamol (200 mg/kg) and Tramadol (50 mg/kg) were used as reference drugs and the maximum of anti-nociceptive activity was observed at 30 min with a latency time of 24.45 s and 35.16 s, respectively. Our results showed that PPO exhibited an important anti-nociceptive activity associated with supraspinal mechanisms, which might be related to its ability of scavenge oxygen free radicals (OFR) like and OH since the accumulation of oxygen free radicals (OFR) will increase the amount of Ca2+ in cells which is known to play a key role during the course of inducing the painCitation36). Otherwise, the test is a specific central antinociceptive activity in which opioid agents exert their antinociceptive actions via the central nervousCitation37), Rodrigues et al.Citation31) reported that polysaccharide isolated from green seaweed C. cupressoides was able to considerably delay the response to thermal stimuli.
This finding confirmed the result of the writhing test and shows that the anti-nociceptive activity of the compounds seems to be related to the involvement of the peripheral mechanisms as well as the central ones.
Conclusion
In the present research, extraction of polysaccharides from P. oceanica was investigated under different extraction condition such as power, extracting time, and liquid–solid ratio. Maximum extraction yield of carbohydrates (2.55%) from P. oceanica is found to be as follows: extraction time of 60 s, liquid–solid ratio of 50:1 (mL/g) and power of 800 W. GC–MS indicate the existence of different monosaccharides and FTIR confirms the presence of different functional groups in extracted carbohydrates. MAE is found to be a suitable technique to extract the carbohydrates from P. oceanica with anti-inflammatory and antinociceptive potential. Further studies are under investigation to elucidate the molecular mechanism of pharmacological effects of PPO.
Author contributions
Yosra Ben Salem contributes to the extraction of polysaccharides from P. oceanica and to the chemical characterization. Amal Abdelhamid carried out pharmacological activities. Khaoula Mkadmini Hammi participated in the optimization of the extraction conditions and the validation of the model using RSM. Didier Le Cerf made contribution to the discussion of SEC/MALS/VD/DRI results. Hatem Majdoub and Abderrahman Bouraoui were the supervisors and designed the study. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgment
The authors are grateful to Dr. Christophe Rihouey for technical assistance (SEC/MALS/VD/DRI analysis).
References
- Torbatinejad NM, Annison G, Rutherfurd-Markwick K, et al. Structural constituents of the seagrass Posidonia australis. J Agric Food Chem. 2007;55(10):4021–4026.10.1021/jf063061a
- Imjongjairak S, Ratanakhanokchai K, Laohakunjit N, et al. Biochemical characteristics and antioxidant activity of crude and purified sulfated polysaccharides from Gracilaria fisheri. Biosci Biotechnol Biochem. 2016;80(3):524–532.10.1080/09168451.2015.1101334
- Plis A, Lasek J, Skawińska A, et al. Thermo-chemical properties of biomass from Posidonia Oceanica. Chem. Pap. 2014;68(7):879–889.
- Nicotri M. Factors involved in herbivore food preference. J Exp Mar Biol Eco. 1980;42(1):13–26.10.1016/0022-0981(80)90163-X
- Thimdee W, Deein G, Sangrungruang C, et al. Sources and fate of organic matter in Khung Krabaen Bay (Thailand) as traced by δ 13 C and C/N atomic ratiosand C/N atomic ratios. Wetlands. 2003;23(4):729–738.10.1672/0277-5212(2003)023[0729:SAFOOM]2.0.CO;2
- Wang Z, Luo D, Ena C. Optimization of polysaccharides extraction from Gynostemma pentaphyllum Makino using uniform design. Carbohydr Polym. 2007;69(2):311–317.10.1016/j.carbpol.2006.10.013
- Zhao L, Wang K, Wang D, et al. Response surface methodology (RSM) for optimization of the microwave-assisted extraction of polysaccharides from Catathelasma ventricosum. Agric J. 2012;7(4):255–259.
- Zhang R, Su D, Hou F, et al. Optimized ultra-high-pressure-assisted extraction of procyanidins from lychee pericarp improves the antioxidant activity of extracts. Biosci Biotechnol Biochem. 2017;81(8):1–10.
- Haiyan W, Huanyun L, Shuai H. Microwave-assisted extraction and purification research of tea polyphenols. Chin Agric Sci Bull. 2010;19:017.
- Zheng X, Fangping Y, Chenghai L, et al. Effect of process parameters of microwave assisted extraction (MAE) on polysaccharides yield from pumpkin. J North Agric Univ. 2011;18(2):79–86.
- Zeng WC, Zhang Z, Gao H, et al. Characterization of antioxidant polysaccharides from Auricularia auricular using microwave-assisted extraction. Carbohydr Polym. 2012;89(2):694–700.10.1016/j.carbpol.2012.03.078
- Chen J, Sun C, Han L, et al. Extraction of crude polysaccharides from Duchesnea indica (Andrews) Focke: optimization by response surface methodology. Biosci Biotechnol Biochem. 2015;79(8):1246–1256.10.1080/09168451.2015.1025689
- AOAC. Official methods of analysis. 15th ed. Arlington (VA): Association of Official Analytical Chemists; 1990. p. 2.
- DuBois M, Gilles KA, Hamilton JK, et al. Colorimetric method for determination of sugars and related substances. Anal Chemistry. 1956;28(3):350–356.10.1021/ac60111a017
- Bitter T, Muir HM. A modified uronic acid carbazole reaction. Anal Biochem. 1962;4(4):330–334.10.1016/0003-2697(62)90095-7
- Hammi KM, Hammami M, Rihouey C, et al. Optimization extraction of polysaccharide from Tunisian Zizyphus lotus fruit by response surface methodology: composition and antioxidant activity. Food Chem. 2016;212:476–484.
- Chaouch MA, Hafsa J, Rihouey C, et al. Depolymerization of polysaccharides from Opuntia ficus indica: Antioxidant and antiglycated activities. Inter J Biol Macromol. 2015;79:779–786.10.1016/j.ijbiomac.2015.06.003
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Exp Biol Medi. 1962;111(3):544–547.10.3181/00379727-111-27849
- Amir M, Shikha K. Synthesis and anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation activities of some new 2-[(2, 6-dichloroanilino) phenyl] acetic acid derivatives. Eur J Med Chem. 2004;39(6):535–545.10.1016/j.ejmech.2004.02.008
- Lajili S, Deghrigue M, Bel Haj Amor H, et al. In vitro immunomodulatory activity and in vivo anti-inflammatory and analgesic potential with gastroprotective effect of the Mediterranean red alga Laurencia obtusa. Pharm Biol. 2016;54(11):2486–2495.10.3109/13880209.2016.1160937
- Silva J, Dantas-Santos N, Gomes DL, et al. Biological activities of the sulfated polysaccharide from the vascular plant Halodule wrightii. Rev Bras Pharmacol. 2012;22(1):94–101.10.1590/S0102-695X2011005000199
- Chang S, Hsu B, Chen B. Structural characterization of polysaccharides from Zizyphus jujuba and evaluation of antioxidant activity. Inter J Biol Macromol. 2010;47(4):445–453.10.1016/j.ijbiomac.2010.06.010
- Xu Y, Cai F, Yu Z, et al. Optimisation of pressurised water extraction of polysaccharides from blackcurrant and its antioxidant activity. Food Chem. 2016;194:650–658.10.1016/j.foodchem.2015.08.061
- Sayari N, Balti R, Mansour MB, et al. Anticoagulant properties and cytotoxic effect against HCT116 human colon cell line of sulfated glycosaminoglycans isolated from the Norway lobster (Nephrops norvegicus) shell. Biomed Pharmacother. 2016;80:322–330.10.1016/j.biopha.2016.03.027
- Kacuráková M, Capek P, Sasinkova V, et al. FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohydr Polym. 2000;43(2):195–203.10.1016/S0144-8617(00)00151-X
- Cui SW. Food carbohydrates: chemistry, physical properties, and applications. Taylor and Francis, Boca Raton. 2005;1:105–157.
- Sellimi S, Kadri N, Barragan-Montero V, et al. Fucans from a Tunisian brown seaweed Cystoseira barbata: structural characteristics and antioxidant activity. Int J Biol Macromol. 2014;66:281–288.10.1016/j.ijbiomac.2014.02.041
- Kolsi R, Fakhfakh J, Krichen F, et al. Structural characterization and functional properties of antihypertensive Cymodocea nodosa sulfated polysaccharide. Carbohyd Polym. 2016;151:511–522.10.1016/j.carbpol.2016.05.098
- Weiner R, Thakur M. Imaging infection/inflammations: pathophysiologic basis and radiopharmaceuticals. Q J Nucl Med Mol Imaging. 1999;43(1):2.
- de Brito TV, Prudêncio RdS, Sales AB. Anti-inflammatory effect of a sulphated polysaccharide fraction extracted from the red algae Hypnea musciformis via the suppression of neutrophil migration by the nitric oxide signalling pathway. J Pharm Pharmacol. 2013;65(5):724–733.10.1111/jphp.12024
- Rodrigues JA, Vanderlei E, Silva LM, et al. Antinociceptive and anti-inflammatory activities of a sulfated polysaccharide isolated from the green seaweed Caulerpa cupressoides. Pharmacol Rep. 2012;64(2):282–292.10.1016/S1734-1140(12)70766-1
- Smiderle F, Olsen L, Carbonero E, et al. A 3-O-methylated mannogalactan from Pleurotus pulmonarius: Structure and antinociceptive effect. Phytochem. 2008;69(15):2731–2736.10.1016/j.phytochem.2008.08.006
- Collier H, Dinneen L, Johnson CA, et al. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol. 1968;32(2):295–310.
- Suseem S, Saral M, Gregory M. Evaluation of the analgesic activity of ethyl acetate, methanol and aqueous extracts of Pleurotuseous mushroom. Asian Pacific J Tropic Med. 2011;4(2):117–120.
- Rinaldi S, Silva DO, Bello F, et al. Characterization of the antinociceptive and anti-inflammatory activities from Cocos nucifera L. (Palmae). J Ethnopharmacol. 2009;122(3):541–546.10.1016/j.jep.2009.01.024
- Ye C, Han N, Teng F, et al. Extraction optimization of polysaccharides of Schisandrae Fructus and evaluation of their analgesic activity. Inter J Biol Macromol. 2013;57:291–296.10.1016/j.ijbiomac.2013.03.025
- Nemirovsky A, Chen L, Zelman V, et al. The antinociceptive effect of the combination of spinal morphine with systemic morphine or buprenorphine. Anesth Analg. 2001;93(1):197–203.10.1097/00000539-200107000-00039