Abstract
In this study, the multi-walled carbon nanotubes (MWCNTs) were applied in lateral flow strips (LFS) for semi-quantitative and quantitative assays. Firstly, the solubility of MWCNTs was improved using various surfactants to enhance their biocompatibility for practical application. The dispersed MWCNTs were conjugated with the methamphetamine (MET) antibody in a non-covalent manner and then manufactured into the LFS for the quantitative detection of MET. The MWCNTs-based lateral flow assay (MWCNTs-LFA) exhibited an excellent linear relationship between the values of test line and MET when its concentration ranges from 62.5 to 1500 ng/mL. The sensitivity of the LFS was evaluated by conjugating MWCNTs with HCG antibody and the MWCNTs conjugated method is 10 times more sensitive than the one conjugated with classical colloidal gold nanoparticles. Taken together, our data demonstrate that MWCNTs-LFA is a more sensitive and reliable assay for semi-quantitative and quantitative detection which can be used in forensic analysis.
Graphical abstract
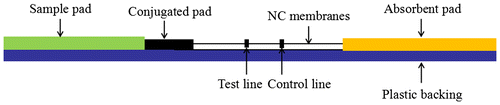
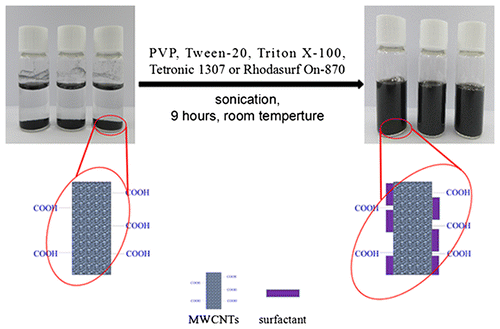
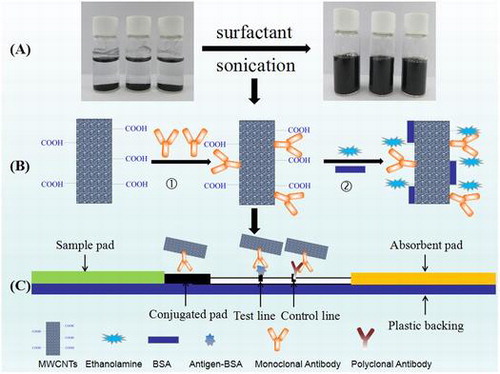
Schematic diagram: (A) Ultrasonic processing of MWCNTs in the media solutions. (B) MWCNTs conjugated with antibody in an non-covalent manner. (C) MWCNTs labeled lateral flow strips for detection.
Chromatographic lateral flow assays (LFA) is an analytical technique which effectively combines the excellent separation of the chromatography with the high sensitivity and specificity of conventional immunoassays. As a point-of-care diagnostic tool, LFA has been applied in detecting various agentsCitation1–3) with several advantages: simple, fast, low cost, versatile, and a prolonged shelf life.Citation4) Colloidal gold nanoparticles (AuNPs) are the most extensively used labels in the LFA,Citation5) which feature excellent biocompatibility, easy preparation, and easy combination with biomolecules.Citation6) However, the intensity of the red color of AuNPs is affected by the particle size of AuNPs.Citation7 AuNPs with small sizes, such as 30 nm, can reflect red light, giving them a red color. As the particle sizes increase, such as 80 nm, the gold nanoparticles absorb red light and reflect blue light, making the nanoparticles exhibit blue or purple. When the particle sizes are bigger than 100 nm, the particles become shiny and it is difficult to detect. Actually the size of the particle in the preparation is not uniform so that the color of different batches varies. The principle of quantitative LFA is that the red color of AuNPs is converted into gray scales by a digital camera and quantified by the image analysis software. The size variation of AuNPs which shows different gray pixels influences the accuracy of the quantitative detection. Hence, this size-related property of AuNPs limits their application in quantitative detection.
The carbon nanotubes (CNTs) have attracted a lot of public interest since the first report by Iijima et al.Citation8) in 1991. CNTs are seamless, hollow tubular body composed of graphene sheets. As a novel material, it has been shown outstanding electrical conductivity, mechanical strength, physical adsorption, and chemical inertness.Citation9) Over the last two decades, CNTs have a tremendous wide application in biomedicine,Citation10) environmental monitoringCitation11–12), and food safety.Citation13)
Compared with AuNPs, CNTs possess more binding sites owing to their high specific surface area, which are easy to conjugate with biomolecules, such as antibody to enhance detective sensitivity. Besides their lower cost, the single black color against the white background of CNTs makes them more suitable for quantification assays based on gray pixel processing.Citation14) However, the poor biocompatibility of CNTs limits their application in the LFA. Particularly, CNTs cannot be dissolved in aqueous solvents due to the hydrophobic property of CNTs and strong Van der waals attractions between the nanotubes. Surface functionalization has been used to enhance their dispersion in aqueous solution. Both covalent modification of the CNTs surface and adsorption of surfactants have been shown to improve their biocompatibilityCitation15–16) and solubility.Citation17) Importantly, by adding non-toxic surfactants not only increases CNTs solubility but also minimizes its surface structure defects. The hydrophobic part of the surfactant molecules adsorb onto the surface of the CNTs, while the hydrophilic parts interact with the aqueous medium to suppress aggregation into bundles and ropes.Citation18) Single-walled carbon nanotubes (SWCNTs) only contain a single graphite sheet, while multi-walled carbon nanotubes (MWCNTs) have several concentric graphene cylinders.Citation19) Although most of the previous work have been focused on SWCNTs due to their simple structureCitation20) and easily predicted physical property, MWCNTs also has been attracting more attention recently due to their stability for surface functionalization and better conductivity than SWCNTs.Citation21)
In this study, we explored the application of carboxylated MWCNTs as antibody labels in the LFA for rapidly and sensitively semi-quantitative and quantitative detection assays of MET. Four non-ionic surfactants (PVP, Rhodasurf On-870, Tween-20, and Triton X-100) and one zwitter-ionic surfactant (Tetronic 1307) were tested to suspend the MWCNTs. UV–vis spectroscopy was used to quantitatively characterize the dispersion process. The performance of adsorption and covalent conjugation were compared in the experiments. The quantitative accuracy and the sensitivity of MWCNTs were evaluated by the competitive assay and the sandwich assay, respectively. We report here, that comparing with AuNPs, MWCNTs showed robust improvement of their sensitivity and accuracy in semi-quantitative and quantitative LFA.
Materials and methods
Reagents
The monoclonal antibody against MET were obtained from Artron BioResearch Inc. (Canada). The antigens coupling with BSA and goat anti-mouse polyclonal antibody were from Arista Biologicals Inc. (Germany). Carboxylated MWCNTs (OD: 10–20 nm, Length: 10–30 μm, 2.00 wt% carboxyl group) were obtained from Beijing DK nano technology Co., Ltd. Bovine serum albumin (BSA), 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), and polyvinyl pyrrolidone (PVP) were purchased from Sigma (Barcelona, Spain). Tween-20, Triton X-100, Tetronic 1307, and Rhodasurf On-870 were purchased from BASF (Germany). The sonicator was manufactured by Ningbo Cientz Biotechnology Co., Ltd. of China. NC membrane (135 mm) and the glass fiber was purchased from Millipore. Inc.
The dispersion of MWCNTs in various surfactants
The dispersion of MWCNTs with various surfactants was prepared as follows. About 7.5 mg carboxylated MWCNTs were added into 20 mL deionized water. A series of concentrations of PVP, Tween-20, Triton X-100, Tetronic 1307, and Rhodasurf On-870 (all from 0.025 to 0.80 mg/mL) were added into the mixtures, respectively. Each mixture was sonicated at 190 W power level for an hour. The optical property of the solutions was analyzed by UV–Visible spectroscopy. The optimum proportion between MWCNTs and surfactants was obtained by selecting the maximum absorbance at 400 nm. After determining the optimal proportion, MWCNTs were dispersed with five surfactants, respectively and sonicated for 9 h. The solutions were stored at room temperature. The stability of the MWCNTs solutions was evaluated by both UV–Visible spectroscopy and transmission electron microscopy (TEM).
Production of MWCNTs-antibody conjugates
Abouts 1 mL of 0.75 mg/mL MWCNTs solution was centrifuged at 4000 rpm for 10 min to remove the precipitate. Then the solution was centrifuged at 15,000 rpm for another 20 min. The MWCNTs pellet was washed twice with DI water and finally, resuspended in 1 mL MES buffer with pH 6.0. 20 μg of antibodies against methamphetamine (competitive assay) or 100 μg of antibodies against HCG (sandwich assay) were added into the MWCNTs solution and incubated for 2 h at room temperature with shaking. The conjugates were blocked with 10 μL of 1 M ethanolamine for 30 min, followed with 100 μL of 100.0 g/L BSA for one more hour blocking. After washed twice with 1 mL of 0.1 M Borax containing 100.0 g/L BSA, the final conjugates were resuspended and stored at 4 °C for further use.
Preparation of AuNPs and AuNPs-antibody conjugates
The preparation of 20 nm AuNPs by a citrate reduction method was following a reported protocol.Citation2) Briefly, 2 mL of 1% HAuCl4 solution was added into 100 mL boiling water with vigorous stirring. About 2 mL of 1% sodium citrate solution was added immediately. The color of the solution changed from blue to red and the solution was kept boiling for another 15 min when it’s turning red allowing the solution to cool down at room temperature and it was stored at 4 °C. AuNPs-antibody conjugates were prepared as described by Masinde et al. with minor modification.Citation22) In brief, 8 μL of 0.1 M K2CO3 as a pH adjuster was firstly, added into 1.0 mL AuNPs solution, then 65 μg MET antibodies (competitive assay) or 230 μg HCG antibodies (sandwich assay) were added and incubated for 30 min. The conjugates were blocked with 20 μL of 100.0 g/L BSA for 15 min. The solution was centrifuged at 6000 rpm for 15 min and the supernatant was recentrifuged at 10,800 rpm for another 30 minutes. The supernatant was discarded and the precipitate was diluted with 200 μL diluent buffer (0.05 M Tris–HCl containing 10.0 g/L BSA, 0.4% Triton X-100, 5% trehalose, 10% sucrose, pH 8.2), and it was stored at 4 °C.
Preparation of the LFS
Lateral flow strips were prepared using nitrocellulose (NC) membranes which were fixed on a plastic backing. About 0.5 mg/mL MET-BSA conjugate was dispensed on the NC membrane as the test line (T line) for the competitive assay. For the sandwich assay, 1.0 mg/mL HCG monoclonal antibody was coated at T line. The control line (C line) for both assays was goat anti-mouse polyclonal antibodies. The coated NC membranes were dried at 37 °C overnight. About 50 μL MWCNT-Antibody Conjugates was diluted 10 times and sprayed on a 20 mm2 glass fiber and then dried at 37 °C overnight. The test strip was prepared as illustrated in Fig. .
Immunoassay
A series of standards were made by diluted MET or HCG controls in 0.1 M phosphate buffer (PBS). About 100 μL of standard was dipped into the sample hole. The lines appeared at T and C line positions. The pixel gray intensity of the test and control lines on the membrane of the test strip was measured by density reader (JSJ-803 Venture, Inc, Shanghai, China) after 30 min. The correlation between the target concentration and value of pixel gray intensity was analyzed using Origin (version 8.0) software.
Results and discussion
The dispersion of MWCNTs in various surfactants
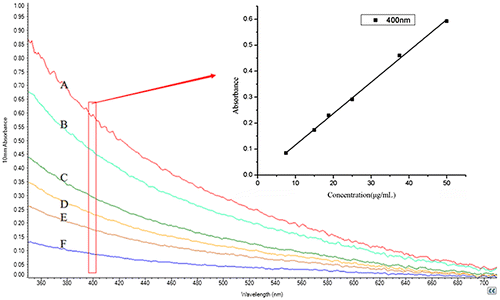
MWCNTs exhibit characteristic adsorption in UV–vis region, which is associated with the concentration of suspended MWCNTs in solution and the intensity of the corresponding absorption spectrum. In order to establish a benchmark of the relationship between absorbance and concentration, the MWCNTs solutions dispersed with Rhodasurf On-870 were diluted to 7.5, 15.0, 18.8, 25.0, 37.5, and 50.0 μg/mL, respectively and the absorbance was detected by UV–vis spectroscopy. We observed a linear relationship between the absorbance and concentrations of MWCNTs solutions (y = 0.0121x−0.0046, R2 = 0.998) at wavelength of 400 nm (Fig. ). The results were consistent with the previous report.Citation23) Hence, the absorbance of each solution at the wavelength of 400 nm was recorded to quantitatively characterize the dispersion process. The four non-ionic surfactants (Triton X-100, Tween-20, PVP, and Rhodasurf On-870) and zwitter-ionic surfactants (Tetronic 1307), we used in our study, were the most commonly used reagents in lateral flow strips due to their high hydrophilicity and safety for protein samples.
Fig. 2. The UV–vis absorbance spectra of the Rhodasurf On-870 dispersed MWCNT solutions with different concentrations: (A) 7.5 μg/mL, (B) 15.0 μg/mL, (C) 18.8 μg/mL, (D) 25.0 μg/mL, (E) 37.5 μg/mL, and (F) 50.0 μg/mL. The inset image: linear curve of concentration with absorbance at 400 nm.
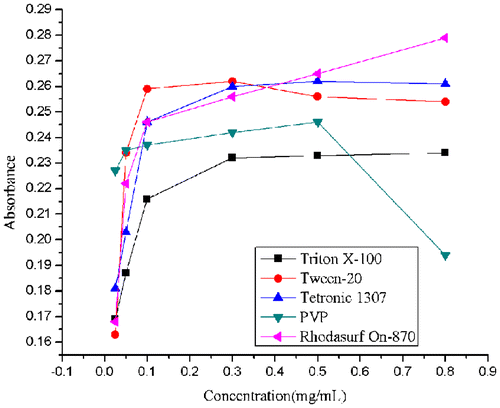
The amount of surfactants is a key factor directly affecting the solubility of MWCNTs.Citation24) To optimize the condition for the maximum solubility, we used different amount of surfactants and monitored the solubility of the MWCNTs. Fig. showed that the UV–vis absorbance of the different MWCNTs suspensions at 400 nm. The absorbance of MWCNTs in all five suspensions continuously increased as more surfactants were added. As seen in Fig. , the absorbance values of Tetronic 1307, Triton X-100, and Tween-20 tend to be stable when the concentration of the surfactant reached to 0.3 mg/mL. For PVP, the absorbance value peaks at 0.5 mg/mL and decreases at 0.8 mg/mL. For Ohodasurf On-870, the absorbance values were still increasing until 0.8 mg/mL, but started to decrease at 1.0 mg/mL (not shown in the figure, the absorbance value was 0.233 at 1.0 mg/mL). The data showed that the optimal concentration is 0.3 mg/mL for Tetronic 1307, Tween-20, and Triton X-100, 0.5 mg/mL for PVP, as well as, 0.8 mg/mL for Rhodasurf On-870. The best proportion of surfactants to MWCNTs was used for the following research. Critical micelle concentration (CMC) is an important physicochemical parameter of surfactants.Citation25) When the concentration of surfactants becomes larger than CMC, the addition of surfactants has no more obvious positive or even a negative effect on the solubility of MWCNTs. This is mainly because that excess surfactants may break the equilibrium that established between the MWCNTs and the surfactants during ultrasonic processing. Therefore, the optimal concentration of the surfactants should be lower than CMC.Citation20)
Fig. 3. Absorbance of MWCNTs solutions at 400 nm after ultrasonic treatment with surfactants of a series of concentrations (all from 0.025 to 0.80 mg/mL).
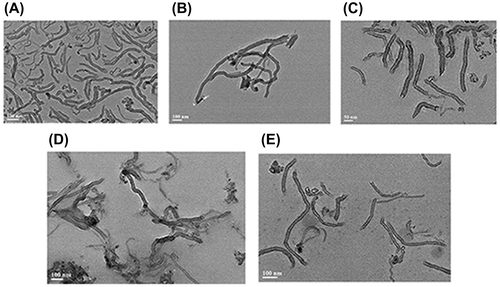
To determine the influence of ultrasonication time to the dispersion of MWCNTs, the absorbance was measured each hour during the sonication. It was shown that the absorbance of five solutions increased as the sonication continued (Fig. ). According to Strano,Citation25) ultrasonic treatment is such a process in which ultrasonic waves provide high shear force at the bundle ends of CNTs. The spaces between the bundles and the individual CNTs could form sites for surfactant adsorption. When the individual CNT move through Brownian motion, the surfactants continue permeating along the CNTs until separation (Fig. ). In the absence of ultrasonic waves and surfactants, the individual CNTs are reattached by van der Waals force to form an unstable solution. In our study, there was a positive correlation between the absorbance and the ultrasonic duration in the different suspensions. The results suggested the ultrasonic treating time is a significant influence factor for the dispersion of MWCNTs. The solutions was treated for 9 h and were monitored by TEM. TEM image (Fig. ) indicated that the average length of MWCNTs was 100–200 nm, the shorter length gives larger specific surface area, providing more binding sites for interaction with biomolecules thus, enhancing the sensitivity of the assays. After ultrasonic treatment for 9 h, all surfactants we used resulted in better dispersion for nanotubes. Hence, we selected 9 h as the processing time.
Fig. 4. Absorbance of MWCNTs solutions at 400 nm after ultrasonic treatment at different time point after preparation.
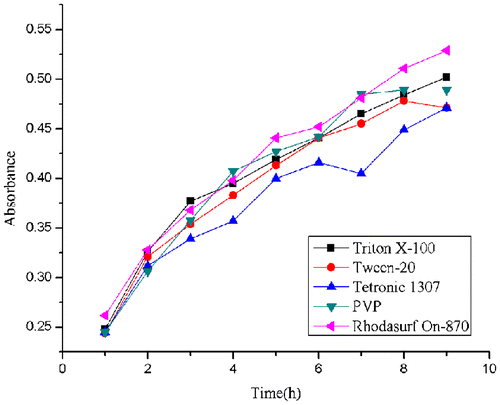
Fig. 6. TEM image of MWCNTs dispersed with (A) Triton X-100, (B) Tween-20, (C) Tetronic 1307, (D) PVP, and (E) Rhodasurf On-870.
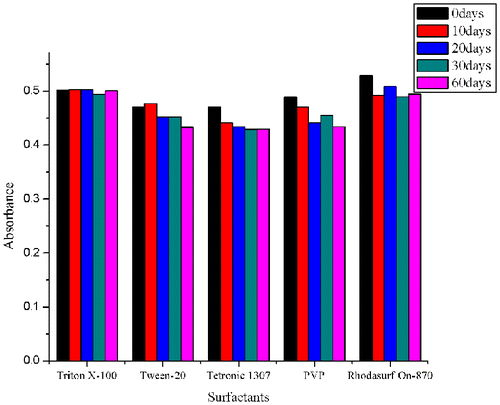
The stability of the dispersion stored at room temperature was also investigated by measuring the absorbance on the 10th, 20th, 30th, and 60th day after initial dispersion. The data shows there was no obvious decline of the absorbance values in five solutions after two months (Fig. ). The coefficient of variation (CV) of Triton X-100, Tween-20, Tetronic 1307, Rhodasurf On-870, and PVP are 2.45, 3.45, 3.96, 2.21, and 3.45%, respectively, which all fits the criteria for making lateral flow test. Therefore, we demonstrated that all five surfactants can significantly improve the dispersion of the MWCNTs as well as maintaining the stability of suspensions. Among the five surfactants, Rhodasurf On-870 not only showed the highest solubility of MWCNTs when dispersing MWCNTs, but also had the smallest CV value in the long-term stability of solutions, and was chosen for the subsequent conjugation study.
The production of MWCNT-antibody conjugates
The effective binding of MWCNTs and antibody is important for LFA. The antibody can be immobilized on the surface of the CNT by covalent conjugate and passively absorption.Citation26) Covalent immobilization of antibody with EDC/NHS chemistry has been widely used because of its stable and durable attachment. Covalent method is based on the covalent reaction between the amino groups of the antibody and the carboxyl groups on the MWCNTs, in which the coupling agents EDC and NHS were used. The absorption method is a direct reaction between MWCNTs with antibody due to its natural affinity for proteins, including hydrophobic and electrostatic interactions. In order to optimize the antibody conjugation, these two different conjugate methods, covalent conjugate and passively absorption were compared in two experiments. First, different concentrations of coupling agents (0.4 M EDC and 0.1 M NHS added into 1 mL of pH 6.0 MES buffer, then diluted to 50, 10, 5, 1, and 0.5% from the original amount) were added to MWCNTs solutions to activate the carboxyl group of MWCNTs, then the mixtures were incubated with 20 μg MET antibodies. In another experiment, the same incubation procedure was carried out with the MWCNTs in MES buffer without EDC/NHS. All seven conjugates were used in the follow-up lateral flow assay under the same condition. The strips were tested with 100 ng/mL MET, the signal intensity of T line to characterize the binding amount of antibody to the MWCNTs. The stronger the T line signals, we consider the more the amount of antibody binding to the MWCNTs. The values of T line have shown in Fig. , in which the strongest test signal values were obtained in the absence of EDC/NHS among all seven strips. The increase in the amount of EDC/NHS results in a gradual decrease of the test signal value. The Coomassie Plus (Bradford) Assay Kit was also used to detect the concentration of antibodies in the MWCNTs supernatant after incubation with 40 μg MET antibodies. We measured three supernatants which were incubated with 1 and 5% EDC/NHS or without EDC/NHS. The results showed that the lowest concentration was obtained in the supernatants without EDC/NHS, followed by high concentration in the supernatants with 1 and 5% EDC/NHS. The results demonstrated that antibody can be effectively absorbed onto the surface of MWCNTs, the amount of antibody binding to MWCNTs are more in the absence of EDC/NHS than with it. The use of EDC/NHS did not improve the binding of antibody to MWCNTs and may lead to some loss of protein activity.Citation27) The possible formation of cross-linked protein aggregates impeded the conjugating of antibody to MWCNTs, which lead to the decrease of test line values (T-value: the digital signal was converted by signal intensity of the test line) in the lateral flow assay. These results were supported by the previous report.Citation26) Therefore, the attachment between the antibody and MWCNTs depended on its strong adsorption but not covalent conjugation in our study.
MWCNTs-based lateral flow immunoassay
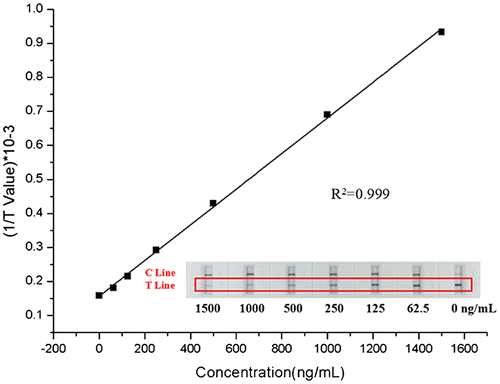
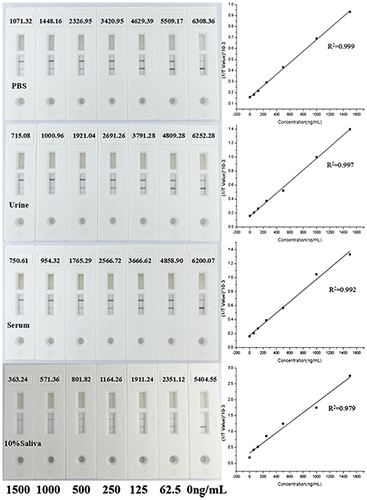
For the quantitative detection for MET, we developed a competitive lateral flow test assay, in which MET monoclonal antibody was conjugated to MWCNTs and MET-BSA conjugate was coated on the NC membrane as the T line. The data from the reader showed a linear relationship between the concentrations of MET and the signal intensity of test line (y = 5.237E−4x + 0.157, R2 = 0.999). And the linear range was between 62.5 and 1500 ng/mL (Fig. ). To evaluate the performance of MWCNT-LFS in complex sample matrix, we added a series of MET (62.5, 125, 250, 500, 1000, and 1500 ng/mL) into human serum, urine, and saliva, respectively. The test line values from different sample matrixes were compared with the signals in 0.01 M PBS (Fig. ). They all exhibited an excellent linear relationship between the test line signal and MET concentrations. Compared with in 0.01 M PBS, the test line values declined in all complex samples matrix. The worst performance was obtained in saliva samples diluted to 10% with 0.01 M PBS, in which the ratio of two signals at 1000 ng/mL was 39.5%. In contrast, the ratio for serum sample is 65.9% and for urine is 69.1%. This could be explained by the matrix-induced response existed in these matrix. There might be two main reasons for the decrease of the intensity of the test line. One reason is the pH difference of different samples, the pH of urine ranges between 4.4 and 8.0, the blood varies between 7.35 and 7.45, and the saliva is between 5.3 and 7.8.Citation28) The variance of pH could affect the stability of the MWCNTs-antibody conjugates and their release on the binding pad, resulting in a reduction in signal intensity. Another reason could be the complex components from the real sample would adsorbed onto the MWCNTs, especially in saliva which including the large viscosity of enzymes and proteins, which preventing the MWCNTs from binding to the target substance. In addition, the adsorption of components would make MWCNTs particles become larger and deposited in the NC bottom, hindering its flow in the NC membranes. Although the signal intensity were low in real sample matrixes, they could be improved by increasing the buffer capacity of the sample pad and purification of the samples to get rid of the complex components.
The sensitivity of the MWCNTs lateral flow strips
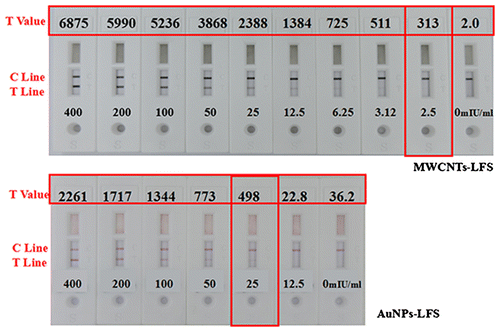
Although AuNPs are the most common labels used for LFS, the low sensitivity limits its wide applications. To compare the sensitivity of MWCNTs with traditional colloidal gold, the MWCNTs or AuNPs were conjugated with HCG antibody as previous described. The sensitivity of this method was evaluated in a sandwich assay by conjugating MWCNTs with HCG antibody due to its easy-to-do analysis than competitive assay. In the lateral flow assay, the minimal detecting concentration of HCG by MWCNTs-based test is 2.5 mIU/mL, while AuNPs-based is 25 mIU/mL (Fig. ). It means that the MWCNTs-based label is 10 times more sensitive than the AuNPs which is consistent with other research.Citation14,29) Owing to their high specific surface area and high contrast of “black on white” test results, MWCNTs would be used as a more sensitive label for LES-based quantitative detection of lower concentrations analytes.
The accuracy of the MWCNTs lateral flow strips
The accuracy is very important for all immunoassays, especially for the quantitative assays. There are many factors affecting the accuracy of the lateral flow strips, such as raw materials, labeling methods, manufacturing processing and environment that causing variations between or within batches of the assay. CV is the value for evaluating the accuracy of the assay. After comparing five different batches (six parallel for each batch) of MWCNTs-based with the AuNPs-based lateral flow test strips, we found that the CV of MWCNTs-based test is 2.9%, while for AuNPs is 11.3% (Table ). Usually we made the gold colloid particles by the method using citrate acid in which the particle size is controlled by the amount of citrate used. The big CV of AuNPs-based test was introduced due to the size variation of the gold colloid particles which showing different colors when captured by the camera. And there is no such problem in MWCNTs-based test which makes it more suitable for quantitative assay.
Table 1. Comparison of the precision of the MWCNTs-based lateral flow test with AuNPs-based lateral flow test for the negative samples.
The stability of the MWCNTs-based lateral flow assay
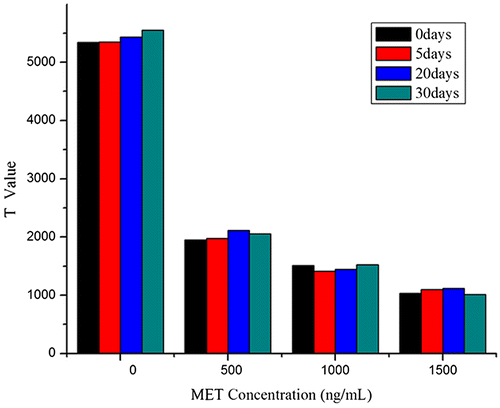
The stability of LFA is another important issue which could be affected by the activity of antibody conjugation, the NC membrane and the storage conditions. Among all these factors, the antibody activity is the most pivotal one to the assay performance. The influence of temperature on the antibody stability is usually the first consideration when we evaluate the stability of the lateral flow strips. To evaluate the stability of the lateral flow strips, we stored them at 37 °C and measured test line signals for detecting MET (0, 500, 1000, and 1500 ng/mL) at different time points. Result reveals that the CV of strips are 3.46%. As shown in Fig. , there is no significant change for the intensity of the test line after MWCNTs-labeled LFS was stored at 37 °C for 30 days indicating its high stability.
Conclusions
In this study, we established a novel LFA based on the new material MWCNTs for the semi-quantitative and quantitative assays. Using five different surfactants, we improved the dispersion of MWCNTs and achieved a satisfactory bio-compatibility. Rhodasurf On-870 showed the best performance among the five surfactants and was chosen for the subsequent conjugation study. In addition, we established that the conjugation of antibody to MWCNTs can be realized by a simple absorption procedure, which not only save the cost of the work, but also result in improvements in performance of LFA. Compared with AuNPs-based assays, the MWCNT-based assay has improved sensitivity and accuracy. For the accuracy evaluation, our data indicated that the monochromatic carbon nanotube is more reliable and suitable than the red gold nanoparticles quantitative assays using the “gray pixel” process. Unlike the traditional labeling reagent, such as gold colloid, MWCNTs are chemically more stable and easier to handle. Therefore, MWCNTs-LFA could be a novel and sensitive assays which can be used in forensic analysis or other biological analysis.
Author contributions
Conceived and designed the experiments: SWj, HXl, ZLb. Performed the experiments: SWj, HXl, LJ. Contributed to data collection: SWj, HXl. Wrote the manuscript: SWj, HXl. Reviewed the manuscript: HXl, ZYr, LJz, ZLb.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
We thank Shanghai Venture Biotechnology, Co; Ltd. for helping with lateral flow strips and providing MET-BSA conjugate.
References
- Zhou W, Kong W, Dou X, et al. An aptamer based lateral flow strip for on-site rapid detection of ochratoxin A in Astragalus membranaceus. J Chromatogr B. 1022;2016:102–108.
- Niu K, Zheng X, Huang C, et al. A colloidal gold nanoparticle-based immunochromatographic test strip for rapid and convenient detection of Staphylococcus Aureus. J Nanosci Nanotechnol. 2014;14:5151–5156.10.1166/jnn.2014.8703
- Anfossi L, Giovannoli C, Giraudi G, et al. A lateral flow immunoassay for the rapid detection of ochratoxin A in wine and grape must. J Agric Food Chem. 2012;60:11491–11497.10.1021/jf3031666
- Noguera P, Posthuma-Trumpie GA, van Tuil M, et al. Carbon nanoparticles in lateral flow methods to detect genes encoding virulence factors of Shiga toxin-producing Escherichia coli. Anal Bioanal Chem. 2011;399:831–838.10.1007/s00216-010-4334-z
- Mak WC, Beni V, Turner APF. Lateral-flow technology: from visual to instrumental. Trac Trends Anal Chem. 2015;79:297–305.
- Tang Y, Xu X, Liu X, et al. Development of a lateral flow immunoassay (LFA) strip for the rapid detection of 1-aminohydantoin in meat samples. J Food Sci. 2011;76:T138–T143.10.1111/jfds.2011.76.issue-6
- Alqadi K, Saadah B. Gold nanoparticles: size vs. color. 2015. Geo.sc.chula.ac.th.
- Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58.10.1038/354056a0
- Ajayan PM. Nanotubes from carbon. Chem Rev. 1999;99:1787–1800.10.1021/cr970102 g
- Wang Z, Wang Y, Xu H, et al. Carbon nanotube-filled nanofibrous membranes electrospun from poly(acrylonitrile-co-acrylic acid) for glucose biosensor. J Phys Chem C. 2009;113:2955–2960.10.1021/jp807047s
- Zhang S, Shao T, Bekaroglu SSK, et al. Adsorption of synthetic organic chemicals by carbon nanotubes: effects of background solution chemistry. Water Res. 2010;44:2067–2074.10.1016/j.watres.2009.12.017
- Yu X, Luo T, Zhang Y, et al. Adsorption of lead(II) on O2-plasma-oxidized multiwalled carbon nanotubes: thermodynamics, kinetics, and desorption. ACS Appl Mater Interfaces. 2011;3:2585–2593.10.1021/am2004202
- Liu G, Chen H, Peng H, et al. A carbon nanotube-based high-sensitivity electrochemical immunosensor for rapid and portable detection of clenbuterol. Biosens Bioelectron. 2011;28:308–313.10.1016/j.bios.2011.07.037
- Posthuma-Trumpie GA, Wichers JH, Koets M, et al. Amorphous carbon nanoparticles: a versatile label for rapid diagnostic (immuno)assays. Anal Bioanal Chem. 2012;402:593–600.10.1007/s00216-011-5340-5
- Ramanathan T, Fisher FT, Ruoff RS, et al. Amino-functionalized carbon nanotubes for binding to polymers and biological systems. Chem Mater. 2005;17:1290–1295.10.1021/cm048357f
- Dyke CA, Tour JM. Overcoming the insolubility of carbon nanotubes through high degrees of sidewall functionalization. Chem A Eur J. 2004;10:812–817.10.1002/(ISSN)1521-3765
- Lin Y, Taylor S, Li H, et al. Advances toward bioapplications of carbon nanotubes. J Mater Chem. 2004;14:527.10.1039/b314481j
- Britz DA, Khlobystov AN. Noncovalent interactions of molecules with single walled carbon nanotubes. Chem Soc Rev. 2006;35:637.10.1039/b507451 g
- Tasis D, Tagmatarchis N, Bianco A, et al. Chemistry of carbon nanotubes. Chem Rev. 2006;106:1105–1136.10.1021/cr050569o
- Abera A, Choi J. Point-of-care immunoassay system using carbon nanotube labels. Doct Diss. 2010:9–10.
- Liu C, Choi J. Improved dispersion of carbon nanotubes in polymers at high concentrations. Nanomaterials. 2012;2:329–347.10.3390/nano2040329
- Masinde LA, Sheng W, Xu X, et al. Colloidal gold based immunochromatographic strip for the simple and sensitive determination of aflatoxin B1 and B2 in corn and rice. Microchim Acta. 2013;180:921–928.10.1007/s00604-013-1008-5
- Lee JU, Huh J, Kim KH, et al. Aqueous suspension of carbon nanotubes via non-covalent functionalization with oligothiophene-terminated poly(ethylene glycol). Carbon. 2007;45:1051–1057.10.1016/j.carbon.2006.12.017
- Yu J, Grossiord N, Koning CE, et al. Controlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solution. Carbon. 2007;45:618–623.10.1016/j.carbon.2006.10.010
- Strano MS, Moore VC, Miller MK, et al. The role of surfactant adsorption during ultrasonication in the dispersion of single-walled carbon nanotubes. J Nanosci Nanotechnol. 2003;3:81–86.10.1166/jnn.2003.194
- Gao Y, Kyratzis I. Covalent immobilization of proteins on carbon nanotubes using the cross-linker 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide—a critical assessment. Bioconjugate Chem. 2008;19:1945–1950.10.1021/bc800051c
- Scida K, Stege PW, Haby G, et al. Recent applications of carbon-based nanomaterials in analytical chemistry: critical review. Anal Chim Acta. 2011;691:6–17.10.1016/j.aca.2011.02.025
- Baldrich E, Muñoz FX. Carbon nanotube wiring: a tool for straightforward electrochemical biosensing at magnetic particles. Anal Chem. 2011;83:9244–9250.10.1021/ac201137q
- Yao L, Teng J, Zhu M, et al. MWCNTs based high sensitive lateral flow strip biosensor for rapid determination of aqueous mercury ions. Biosens Bioelectron. 2016;85:331–336.10.1016/j.bios.2016.05.031