Abstract
Superantigens (SAgs) are powerful T-cell stimulatory proteins. Because an atopic dermatitis (AD) model NC/Nga mice had two endogenous SAgs, namely minor lymphocyte-stimulating locus-1a (Mls-1a) and mouse mammary tumor virus (MMTV)(SHN), SAg-responsive T-cells bearing Vβ5.1, Vβ6, Vβ8.1, Vβ8.2, Vβ8.3, Vβ9, and Vβ11 should be endogenously deleted. Here, we discuss that the endogenous SAgs-expression may be involved in AD-sensitivity in NC/Nga mice.
Key words:
Superantigens (SAgs) bind to major histocompatibility complex (MHC) class II molecules and activate both CD4+ and CD8+ T-cells bearing variable regions of specific T-cell receptor β chains (TCR Vβ) domain.Citation1) Endogenous SAg of mouse mammary tumor virus (MMTV or Mtv) causes tolerance or the clonal deletion of T cells bearing a specific Vβ element, thus allowing proliferation of infected B cells and transmission of the virus to offspring.Citation1) Of the murine endogenous SAgs encoded by Mtv, the minor lymphocyte-stimulating locus-1a (Mls-1a), encoded by an open reading frame in the 3′ long terminal repeat of the Mtv-7 provirus, is expressed in AKR, CBA/J, C58, DBA/2, and NZB mice, where it induces tolerance or clonal deletion of T-cells bearing TCR Vβ6, Vβ8.1, and Vβ9.Citation1) TCR Vβ7 is also weakly stimulated by Mls-1a.Citation2) In contrast, human endogenous retroviruses type K (HERV-K), HIV, and human T cell leukemia virus (HTLV) are complex retroviruses with features similar to MMTV.Citation3) The HERV-K family also contains endogenous SAgs, suggesting their potential involvement in autoimmunity.Citation3,4) HERV-K family members (particularly HERV-K10 and HERV-K18), HTLV-1-related endogenous sequence (HRES)-1, endogenous retrovirus (ERV)-3, and HERV-E4-1 may also function in the pathogenesis of systemic lupus erythematosus.Citation5) Although HERV-K18 is recognized by human T-cells bearing TCR Vβ7 and Vβ13,Citation3,4) it is unknown whether other HERV are recognized by other T-cells bearing a specific Vβ element.
NC/Nga mice were established as an inbred strain by Dr K. Kondo in 1957 based on the Japanese fancy mice (Nishiki-Nezumi).Citation6) This strain has been reported to have the following biological characteristics: liver and kidney esterase isozyme patterns similar to those in DBA/2 mice and high susceptibility to X ray-irradiation and to anaphylactic shock from ovalbumin (OVA).Citation6) NC/Nga mice spontaneously develop atopic dermatitis (AD)-like disease under conventional conditions, but not under specific pathogen-free conditions.Citation7) Here, we show that the clonal deletion of T-cell repertoire with a specific TCR Vβ is due to the expression of two endogenous SAgs in NC/Nga mice, and discuss the role of endogenous SAgs in developing spontaneous AD-like disease in NC/Nga mice.
Animal; NC/Nga (H-2nc, previously unknown),Citation8) BALB/c (H-2d, Mls-1b), DBA/2 (H-2d, Mls-1a), and B10.BR (H-2k, Mls-1b) (male) (Japan SLC, Hamamatsu, Japan) were maintained in SPF conditions, and used at 8–10 weeks of age. All mice were maintained in our full-barrier animal facility under controlled temperature, humidity, and a 12-hour light/dark regimen. All experiments were approved by the Institutional Review Board (IRB) for Animal Studies of the Nippon Veterinary and Life-science University (NVLU), and were performed following the guidelines provided by the committee.
Flow cytometry; Peripheral blood mononucleated cells (PBMCs) were isolated from mouse blood using Lymphoprep (ProGen, Heidelberg, Germany). For FACS analysis, PBMCs suspended in PBS were stained with a mixture of FITC or PE-conjugated CD4 (clone; GK1.5) or CD8 (clone; 53-6.7) and FITC-conjugated Vβ8.1 8.2 (clone; KJ16-133.18), PE-conjugated Vβ5.1 5.2 (clone; MR9-4), PE-conjugated Vβ6 (clone; RR4-7)-specific mAbs, PE-conjugated Vβ9 (clone; MR10-2)-specific mAbs, or PE-conjugated Vβ11 (clone; KT11)-specific mAbs (BioLegend, St. Louis, MO). Two-color analysis was conducted by FACS (FACSCalibur, Nippon Becton Dickinson, Japan).
RT-PCR; CD4+ T-cells, CD8+ T-cells, B-cells (3 × 105/well), or myeloid cells were enriched from LNs using anti-mouse CD4, anti-mouse CD8, anti-mouse B220, or anti-mouse CD11b Magnetic Particles-DM (BD Biosciences). Antigen-presenting cells (APCs) were used as maturated myeloid cells cultured with treatment of 20 ng/ml recombinant (r)GM-CSF (PeproTech, Rocky Hill, NJ) and 20 ng/ml rIL-4 (PeproTech) for 72 h at 37°C in 5% CO2. These RNA were extracted using ISOGEN (Nippon Gene, Tokyo, Japan). First strand cDNA was synthesized using 1 µg of isolated RNA template, M-MLV reverse transcriptase, Oligo (dT)15 primer, RNase inhibitor and dNTP according to the manufacturer’s protocol (Nippon Gene). The primers (20 nM) for PCR were sense (5′-GTCAAAGAACAGGTGCAAGGAC-3′) and antisense (5′-AAGGGATCGAAGCCAACGCG-3′) for Mls-1a (443 bp), sense (5′-TCTGCGCACAAACGGATGAG-3′) and antisense (5′-AAGGGGGCATCTGTTGGTCT-3′) for MMTV(SHN) (572 bp) (GenBank: X78590.1), sense (5′-ATGGCTTCTGTGGCTACAGACC-3′) for Vβ2, sense (5′-AAGGACAAAAAGCAAAGATGAGG-3′) for Vβ3, sense (5′-AACACTGCCTTCCCTGACCC-3′) for Vβ5.1, sense (5′-CAAAAACTGACCTTGAAATGTCAA-3′) for Vβ6, sense (5′-AGAATGTTTTGCTGGAATGTGGA-3′) for Vβ7, sense (5′-GAAAGGTGACATTGAGCTGTCAC-3′) for Vβ8.1, sense (5′-GGAAAGGTGACATTGAGCTGTAAT-3′) for Vβ8.2, sense (5′-GAAAGGTGACATTGAGCTGTCAC-3′) for Vβ8.3, sense (5′-CTTCTGTCTTCTTGCAGCCACTT-3′) for Vβ9, sense (5′-TGCCTCTTGGGAATAGGCC-3′) for Vβ10, sense (5′-TGCTTCTTGAGAGCAGAACCAA-3′) for Vβ11, antisense (5′-GCAATCTCTGCTTTTGATGGCT-3′) for Cβ,Citation9) and sense (5′-ACCACAGTCCATGCCATCAC-3′), and antisense (5′-TCCACCACCCTGTGCTGTA-3′) for GAPDH (981 bp). The PCR conditions of Mls-1a, MMTV(SHN), Vβ5.1, and Vβ6 using Taq DNA polymerase (BioAcademia, Osaka, Japan) were as follows: 36 cycles at 98 °C for 10 s, 56.5 °C for 30 s, and 72 °C for 70 s, then the annealing temperature for Vβ2, Vβ3, Vβ7, and Vβ8.1 are 61 °C, and the annealing temperature for Vβ8.2, Vβ8.3, Vβ9, Vβ10, and Vβ11 are 58.5 °C. The products were diluted five-fold with loading buffer consisting of xylene cyanol and bromophenol blue dyes and were electrophoresed on 2% agarose gel.
Statistical analysis; Statistical analysis was performed by ANOVA using Excel (Microsoft) and StatPlus (AnalystSoft, Alexandria, VA). A p-value < 0.05 was considered significant.
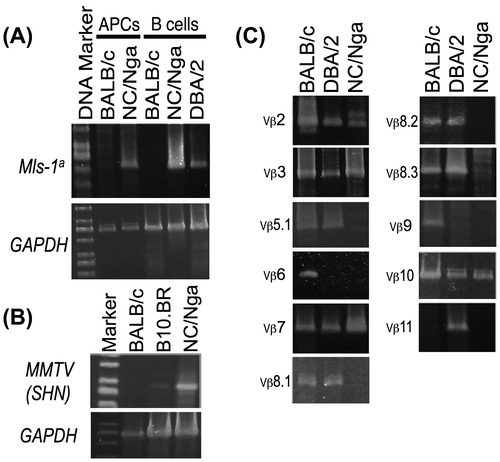
The Mls-1a and MMTV(SHN) genes were detected in mRNA of NC/Nga mice B-cells or antigen-presenting cells (APCs) using RT-PCR (Fig. (A) and (B)). Although the clonal deletion of TCR Vβ6 and Vβ9 by Mls-1a expression in NC/Nga mice was assumed to be the same as in control DBA/2 mice (Mtv-1, Mtv-6, Mtv-7, and Mtv-13), other T-cells bearing TCR Vβ5.1, Vβ8.1, Vβ8.2, Vβ8.3, and Vβ11 should be also deleted in NC/Nga mice CD4+ T-cells (Fig. (c)). Then, when the expression of Vβ chains on also thymocytes and LN cells in NC/Nga strain was compared, the results indicated before and after clonal deletion of periferal T cells in NC/Nga mice. (Supplemental Fig. S1). Because MMTV(SHN) mRNA was expressed in B10.BR or NC/Nga mice but not BALB/c mice (Fig. (B)), the clonal deletion of a specific TCR Vβ by MMTV(SHN) should also be induced. Besides, the PCR products of Mls-1a and MMTV(SHN) were confirmed by the sequence analysis (Supplemental Fig. S2). Furthermore, TCR Vβ11, but not Vβ5.1, was deleted in BALB/c mice (Mtv-6, Mtv-8, and Mtv-9), although it is reported that the expression of Mlsf in BALB/c mice induced the deletion or tolerance of T-cells bearing TCR Vβ5.1, Vβ11, and Vβ12.Citation10)
Fig. 1. The expression of TCR Vβ repertoires and endogenous superantigens in NC/Nga mice. (A) Endogenous superantigen Mls-1a-specific expression was measured by RT-PCR using APCs purified from BALB/c and NC/Nga mice and B-cells purified from BALB/c (Mls-1b), NC/Nga (unknown), and DBA/2 (Mls-1a) mice, (B) The mRNA expression of MMTV(SHN) in B-cells purified from BALB/c, B10.BR, and NC/Nga mice were measured using RT-PCR, and (c) The mRNA expression of TCR Vβ repertoires (Vβ2, Vβ3, Vβ5.1, Vβ6, Vβ7, Vβ8.1, Vβ8.2, Vβ8.3, Vβ9, Vβ10, and Vβ11) in CD4+ T-cells purified from BALB/c, DBA/2, and NC/Nga mice was measured using RT-PCR. Data are representative of two independent experiments.
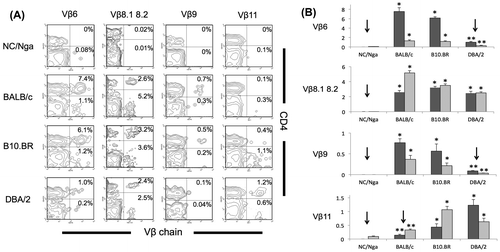
RT-PCR results were then confirmed using FACS analysis. T-cell repertoires of TCR Vβ6, Vβ8.1, Vβ8.2, Vβ9, and Vβ11 were clearly deleted in NC/Nga mice. However the deletion of Vβ5.1 in NC/Nga mice was not confirmed by FACS analysis because the population of CD4+ T cells was detected by mAb specific to Vβ5.1 5.2 (data not shown). Vβ11 was deleted in also BALB/c mice (p < 0.05, ANOVA). Vβ6 and Vβ9 were deleted in DBA/2 mice having Mls-1a (p < 0.05, ANOVA; Fig. (A) and (B)).
Fig. 2. FACS analysis of the expression of TCR Vβ6, Vβ8.1–8.2, Vβ9, and Vβ11. (A) Samples were stained using a mixture of mAbs specific to CD4 and TCR Vβ6, Vβ8.1–8.2, Vβ9, or Vβ11 in PBMCs of NC/Nga, BALB/c, B10.BR, and DBA/2 mice and analyzed using FACS. An example of a two-color panel is shown, (B) The percentages of TCR Vβ6, Vβ8.1–8.2, Vβ9, or Vβ11-positive populations in CD4+ cells (dark gray) or CD8+ cells (light gray) of NC/Nga, BALB/c, B10.BR, and DBA/2 mice (n = 4) are shown. Significant downregulation (down arrow) by clonal deletion is indicated.
Because physiological barrier dysfunctions of the skin, high numbers of Staphylococcus aureus among the skin surface bacterial flora, and an increased hypersensitivity to itch were reported in AD patients,Citation11) the effects of applying exogenous SAg Staphylococcal enterotoxin B (SEB) to the skin of AD model NC/Nga mice have been investigated.Citation12) However, the effect of SEB on NC/Nga mice may be reviewed because it was identified that endogenous SAgs Mls-1a and MMTV(SHN) were expressed in these mice. Although it was reported that TCR Vβ8+ T-cells, but not Vβ2.1+and Vβ7.1+ T-cells, were absent in NC/Nga mice,Citation12) it is considered to be caused by the endogenous clonal deletion by Mls-1a. The expression of the Mtv-7 provirus product, Mls-1a, induces the deletion or tolerance of TCR Vβ6, Vβ8.1, and Vβ9.Citation1) Our results indicated NC/Nga mice lack T-cells bearing TCR Vβ5.1, Vβ6, Vβ8.1, Vβ8.2, Vβ8.3, Vβ9, and Vβ11 and contain MMTV(SHN) in addition to Mls-1a. Because the expression of Mtv-8, Mtv-9, and Mtv-11 proviruses product, Mlsf, induces the deletion or tolerance of TCR Vβ5.1, Vβ11, and Vβ12,Citation10) and the expression of Mtv-4 provirus product, MMTV(SHN), induces the deletion or tolerance of TCR Vβ7, Vβ8.1, Vβ8.2, and Vβ8.3,Citation13) we suggest that NC/Nga mice also contain Mlsf in addition to Mls-1a and MMTV(SHN). In contrast, SEB stimulates Vβ3+, Vβ7+, Vβ8.1+, Vβ8.2+, Vβ8.3+, or Vβ17+ T-cells in mice.Citation12) Interestingly, SEB-induced autoreactive T-cell responses were blocked by IL-10 production from tolerized Vβ8+ T-cells in CBA/J mice (Mls-1a).Citation14) A similar phenomenon may also occur in NC/Nga mice. SEB-induced Th1 dominant state mediated by IL-12 or IL-18 was inhibited by the absence of Vβ8+ T-cells in NC/Nga mice, resulting in a Th2 dominant state.Citation12) Recent studies conducted on the pathogenesis, biomarkers, and itch in AD have reported the potential role of Staphylococcus aureus, IL-18, nerve growth factor (NGF), and semaphorin 3A in the pathogenesis and treatment of AD.Citation15) To investigate this, NC/Nga mice have been used as an AD model. Therefore, the correct characterization of NC/Nga mice is important when interpreting the in vivo responses.
Author contribution
K. O-T. designed the experiments, and performed the experiments. The manuscript was written by K. O-T. All authors participated in the discussion of the data and in the production of the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
The supplemental material for this paper is available at https://doi.org/10.1080/09168451.2017.1374829.
Supplemental_Figure.pdf
Download PDF (1.3 MB)Acknowledgment
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors would like to thank Enago for the English language review.
Notes
Abbreviations: Sags, superantigens; TCR Vβ, variable regions of T cell receptor β chains; APCs, antigen-presenting cells; MHC, major histocompatibility complex; AD, atopic dermatitis; Mls, minor lymphocyte-stimulating locus; MMTV, mouse mammary tumor virus; HERV, human endogenous retroviruses; mAbs, monoclonal antibodies; SEB, Staphylococcal enterotoxin B.
References
- Acha-Orbea H, Held W, Waanders GA, et al. Exogenous and endogenous mouse mammary tumor virus superantigens. Immunol Rev. 1993;131:5–25.10.1111/imr.1993.131.issue-1
- Waanders GA, McDonald HR. Hierarchy of responsiveness in vivo and in vitro among T cells expressing distinct Mls-1a-reactive V beta domains. Eur J Immunol. 1992;22:291–293.10.1002/(ISSN)1521-4141
- Conrad B, Weissmahr RN, Böni J, et al. A human endogenous retroviral superantigen as candidate autoimmune gene in type I diabetes. Cell. 1997;90:303–313.10.1016/S0092-8674(00)80338-4
- Sutkowski N, Conrad B, Thorley-Lawson DA, et al. Epstein-barr virus transactivates the human endogenous retrovirus HERV-K18 that encodes a superantigen. Immunity. 2001;15:579–589.10.1016/S1074-7613(01)00210-2
- Nelson P, Rylance P, Roden D, et al. Viruses as potential pathogenic agents in systemic lupus erythematosus. Lupus. 2014;23:596–605.10.1177/0961203314531637
- Kondo K, Nagami T, Tadokoro S. Comparative cellular and species radiosensitivity. In: Bond PV, Sugawara T, editors. Tokyo: Igakushoin; 1969. p. 20.
- Matsuda H, Watanabe N, Geba GP, et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol. 1997;9:461–466.10.1093/intimm/9.3.461
- Ohkusu-Tsukada K, Yamashita T, Tsukada T, et al. Low expression of a Ddm7/Ldm7-hybrid mutant (D/Ldm7) in the novel haplotype H-2nc identified in atopic dermatitis model NC/Nga mice. Gene Immun. Forthcoming.
- Wettstein P, Strausbauch M, Therneau T, et al. The application of real-time PCR to the analysis of T cell repertoires. Nucleic Acids Res. 2008;36:e140.
- Foo-Phillips M, Kozak CA, Principato MA, et al. Characterization of the Mlsf system. II. Identification of mouse mammary tumor virus proviruses involved in the clonal deletion of self-Mlsf-reactive T cells. J Immunol. 1992;149:3440–3447.
- Stalder JF, Fleury M, Sourisse M, et al. Local steroid therapy and bacterial skin flora in atopic dermatitis. Br J Dermatol. 1994;131:536–540.
- Habu Y, Seki S, Takayama E, et al. The mechanism of a defective IFN-gamma response to bacterial toxins in an atopic dermatitis model, NC/Nga mice, and the therapeutic effect of IFN-gamma, IL-12, or IL-18 on dermatitis. J Immunol. 2001;166:5439–5447.10.4049/jimmunol.166.9.5439
- Luther S, Shakhov AN, Xenarios I, et al. New infectious mammary tumor virus superantigen with V beta-specificity identical to staphylococcal enterotoxin B (SEB). Eur J Immunol. 1994;24:1757–1764.10.1002/(ISSN)1521-4141
- Ito K, Takaishi H, Jin Y, et al. Staphylococcal enterotoxin B stimulates expansion of autoreactive T cells that induce apoptosis in intestinal epithelial cells: regulation of autoreactive responses by IL-10. J Immunol. 2000;164:2994–3001.10.4049/jimmunol.164.6.2994
- Ikezawa Z, Komori J, Ikezawa Y, et al. A role of Staphyococcus aureus, interleukin-18, nerve growth factor and semaphorin 3A, an axon guidance molecule, in pathogenesis and treatment of atopic dermatitis. Allergy Asthma Immunol Res. 2010;2:235–246.10.4168/aair.2010.2.4.235
