Abstract
Egg productivity is declined by stress. It has been reported that some food supplements can improve the egg productivity due to a reduction of environmental stress. We evaluated the effect of fermented waste mushroom bed (FWMB) as a dietary additive on egg productivity. Hens were fed control food (control group, n = 100) or 3% FWMB-added food (FWMB group, n = 100) for 16 months. The number of eggs, soft-shelled eggs, and broken eggs were recorded for 15 months. We also evaluated stress-related markers (ovotransferrin, lipid peroxide, and the heterophil-to-lymphocyte ratio). The FWMB group had slightly increased egg production compared with control hens. The FWMB group produced significantly less broken and soft-shelled eggs than the control group. All stress-related markers were significantly lower in the FWMB group than in the control group. Gut flora was also affected by FWMB feeding. The increased egg production and decreased proportion of broken and soft-shelled eggs might be related to the prevention of stressful conditions by FWMB.
Hens were fed 3% FWMB added to the diet. Comparing the egg production between the control group and FWMB group in each month, blood stress markers were improved by FWMB feeding.
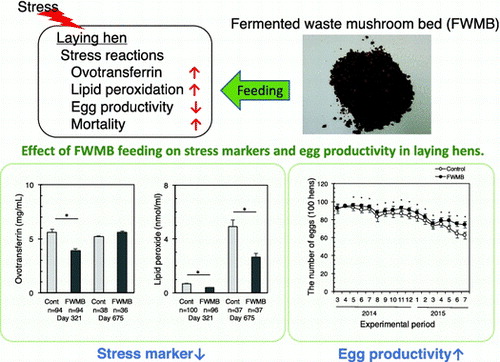
Hens on small-scale poultry farms may experience environmental stresses such as overcrowding, heat, and/or poor hygiene. Excessive stress causes several characteristic symptoms, including decreased egg production and increased mortality.Citation1) Moreover, these stresses should be removed to improve animal welfare. Although there is concern about excess stresses on hens in small-scale poultry farms, improvement of their environment is difficult due to cost. Development of interventions to decrease stress is important to not only increase productivity, but also to improve animal welfare. It has been suggested that ascorbic acid or green vegetable supplementation could be effective in mortality and declining egg production due to a reduction of environmental stress.Citation2,3) It means that poultry feed supplements could moderate hen’s stress.
In mushroom cultivation, mushroom beds are used as an artificial medium that contains nutritional sources such as corn cob, rice bran, or wheat bran. These mushroom beds then become industrial waste after the mushrooms are harvested. The amount of waste mushroom bed produced is about 300,000 tons per year in JapanCitation4); therefore, research has been conducted for the effective utilization of waste mushroom beds, such as a growing substrate for pot-cultured plants instead of peat moss,Citation5) floor litter for animal stalls, and reuse for mushroom beds. We developed a fermented waste mushroom bed (FWMB) to use as an additive feed for laying hens. Waste mushroom beds contain substances from mushrooms, and mushrooms produce several known bioactive compounds, including fiber, flavonoid, and vitamin D.Citation6–8) One type of fiber, β-glucan, affects the immune system,Citation6) flavonoids have an antioxidative effect,Citation9) and vitamin D promotes absorption of calciumCitation10); fibers also improve gut flora.Citation11) The purpose of the present study was to evaluate the effect of the FWMB on the egg productivity of hens.
We measured the plasma lipid peroxidation level, ovotransferrin concentration, and heterophil/lymphocyte ratio (H/L ratio) as general stress markers.Citation3) Lipid peroxide is the oxidized lipid-binding form of radical oxygen. Radical oxygen is produced by energy production in mitochondria, infection, and metabolism of arachidonic acid. Ovotransferrin is one of the acute inflammation proteinsCitation12); it increases due to infection, external injury, or stress. There are some reports that inflammation states are changed by breeding conditions.Citation13,14) The heterophil is the characteristic leucocyte of birds and rabbits (equivalent to the neutrophil in a large majority of mammals). Leucocyte production is controlled by the automatic nervous system. As the stress reaction activates the sympathetic system and inactivates the parasympathetic system, the heterophil concentration increases and the lymphocyte concentration decreases. Therefore, the H/L ratio is often used to evaluate stress in birds.Citation13) FWMB is a rich source of fiber which can influence gut flora. Recently, it is emphasized that gut flora is one of the key regulators of stress in human and rodents.Citation15) We also evaluated whether gut flora was affected by FWMB feeding.
Materials and methods
Preparation of the FWMB
Fresh crushed waste mushroom (Flammulina velutipes) beds and dried okara (refuse of bean curd) were blended in a tank in a weight ratio of 9:1. Pediococcus pentosaceus Kirishima 1C and Lactobacillus fermentum Kirishima 1R were then added, and the mixture was incubated for 20 h at 35 °C. The FWMB was then dehydrated in a dryer (Model No. 30, Ochiai MFG, Shizuoka, Japan) and crushed by a crusher (Model A-2, Kokkosya, Aichi, Japan). The FWMB components are listed in Table . The FWMB also contained around one billion cfu/g of L. fermentum as dead bacteria. Vitamin D could not be detected in FWMB (data not shown).
Table 1. Constituents of FWMB.
Evaluation of egg productivity
The tested hens (Sonia sp.) were bred in a battery cage of an open-air poultry house. Hens were divided into two groups, with 100 in each group, and each group was housed in an area of 12 × 100 m. The control group was given general food (Powerful Layer-18), and the experimental group was given general food with 3% FWMB (w/w) added from the time that the hens were 133 days old (February 11 2014) to 668 days old (July 31 2015). Total egg production, the numbers of broken eggs and soft-shelled eggs, and the total egg weight per day were measured from 133 to 637 days old. The total number of broken eggs and soft-shelled eggs was defined as the number of abnormal eggs.
Measurement of stress markers
Blood was drawn from the wing vein when the hens were 321 days old (August 21 2014) and 637 days old (June 30 2015) and collected into Venoject vacuum tubes containing EDTA-Na (TERUMO, Tokyo, Japan). Some blood from each tube was cold-stored for blood smears, and the rest was centrifuged at 1000 × g for 5 min to prepare a plasma sample. The plasma was divided into several microtubes and stored at −80 °C. The H/L ratio was measured from the blood smear using the Diff–Quick method.Citation16) Heterophils and lymphocytes were counted with a microscope, and the H/L ratio was calculated. Ovotransferrin and lipid peroxide in plasma were measured using a commercial chicken ovotransferrin kit (Immunology Consultants Laboratory, Portland, OR) and TBARS kit (Cayman Chemical Company, Ann Arbor, Oxford Biomedical Research), respectively.
Detection of gut flora
The number of bacteria (Bifidobacterium spp., Lactobacillaceae spp., Bacteroides spp., Streptococcus spp., Staphylococcus spp., and Enterobacteriaceae spp.) in 2 g of feces that were excreted from 582 days old hens was examined. NBGT, TATAC, and PEES medium were prepared according to Mitsuoka’s method.Citation17) Culture media used for each bacteria type was as follows: BL blood agar media (Nissui Seiyaku, Tokyo, Japan) for Bifidobacterium spp., MRS agar media (KANTO CHEMICAL, Tokyo, Japan) for Lactobacillaceae spp., NBGT agar media (based on BS media, Nissui Seiyaku) for Bacteroides spp., TATAC agar media (based on BS media, Nissui Seiyaku) for Streptococcus spp., PEES agar media (based on Mannitol salt agar media, Nissui Seiyaku) for Staphylococcus spp., and DHL agar media (Nissui Seiyaku) for Enterobacteriaceae spp. Fresh feces were collected from six hens in each group. Two g of feces were dissolved in 18 mL of medium. This was then diluted to 10−3, 10−5, 10−7, and 10−9, and 30 μl of the diluted solutions and applied to each agar media. The agar media was dried and divided into four sections (one section for each dilution): Lactobacillaceae spp. were cultured in semi-sparking conditions for 4 days, Bifidobacterium spp. and Bacteroides spp. were cultured in anaerobic conditions for 4 days, Streptococcus spp. were cultured in aerobic conditions for 2 days, Staphylococcus spp. was cultured in aerobic conditions for 3 days, and Enterobacteriaceae spp. was cultured in aerobic conditions for 1 day. All bacteria were cultured at 37 °C.
The bacteria were distinguished by colony properties: Bifidobacterium spp. was white-brown and smooth, and grew in medium-big sized colonies of about 2 mm; Lactobacillaceae spp. was creamy white and smooth, and grew in medium colonies of about 1 mm; Bacteroides spp. was ash colored and smooth, and grew in small colonies of about 0.5 mm; Streptococcus spp. was red and smooth, and grew in medium colonies of about 1 mm; Staphylococcus spp. was white and smooth, and grew in medium colonies of about 1 mm; and Enterobacteriaceae spp. was red and smooth, and grew in medium-big sized colonies of about 2 mm.
Statistical analysis
We excluded the egg productivity data from February 2014, as this was when the hens began laying and so the number of eggs was much less than the other months. In statistical processing of all examinations, data that exceeded the 95% confidence limits was eliminated. Statistical testing was done via the unpaired Student’s t-test.
Results
Egg productivity
Sixteen hens in the control group and three hens in the FWMB group died during the study period; the reasons were not defined by veterinarians. Hence, the results were adjusted from raw numbers to the number per 100 hens. Fig. shows the monthly egg production per 100 hens. The number of eggs in the FWMB group was significantly large in all months except March and April 2014. The average number of eggs produced from one hen in her life (668 days) was 430 in the control group and 450 eggs in the FWMB group. Table lists the egg productivity. The FWMB group had significantly fewer soft-shelled eggs and broken eggs compared with the control group. There was no difference in egg weight between the control group and the FWMB group.
Fig. 1. Effect of FWMB feeding on egg production.
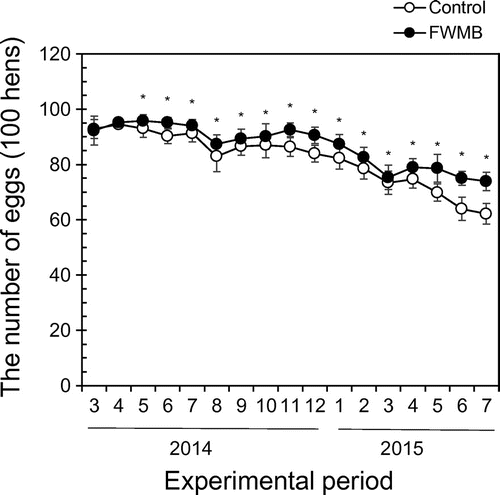
Table 2. Egg production of hens.
Stress markers
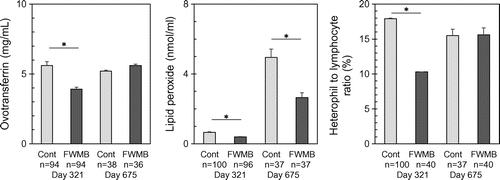
Fig. shows the plasma ovotransferrin concentration, lipid peroxide level, and H/L ratio in hens. All three markers were significantly lower in the FWMB group than those in the control group at 321 days old hens. The lipid peroxide level, but not the ovotransferrin and H/L ratio, was also significantly lower in the FWMB group than the control group. The plasma concentrations of vitamin E and A at 637 days old hens were not significantly different in the control and FWMB groups (data not shown).
Fig. 2. Effect of FWMB feeding on stress-related biomarkers in blood.
Gut flora
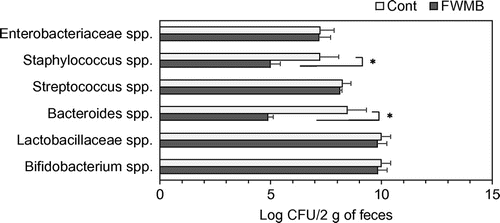
Fig. shows the number of each bacteria species in 2 g feces. The FWMB group had significantly lower numbers of Bacteroides spp. and Staphylococcus spp. than the control group. The control group had 3700 times more Bacteroides spp. and 170 times more Staphylococcus spp. compared to the FWMB group. There were no significant differences between the control and FWMB groups in the numbers of Bifidobacterium spp., Lactobacillaceae spp., Streptococcus spp. and Enterobacteriaceae spp.
Fig. 3. Effect of FWMB feeding on gut microbiota.
Discussion
Adding FWMB to the feed improved egg production and decreased egg loss due to broken or soft shells by one-sixth. The FWMB-fed hens each produced an average of 20 extra eggs in their lifetime compared with the control food-fed hens. Moreover, hens fed FWMB prevented the waste of 18 eggs per 100 hens each month. Although the differences in the present study were slight between the control and FWMB groups, these will make big differences on large-scale poultry farms.
Factors affecting egg production or condition include nutrients, stresses, aging, and diseases. In the present study, the nutritional content of the FWMB might have had a positive effect on the hens, as the two groups of hens were of the same species and age and were kept in the same conditions. We compared the stress conditions between the two groups and found that the FWMB group had a significantly lower H/L ratio, ovotransferrin concentration, and lipid peroxidation level compared to the control group at 321 days old hens. When chickens experience stresses or infection, they produce radical oxygen to maintain homeostasis. However, excessive stresses and continuous infection cause the production of excess radical oxygen in the chickens.Citation1) In the present study, the increase of lipid peroxide level may be influenced by excessive stresses. At 675 days, only lipid peroxide level was significantly lower in FWMB group compared to control group. If the lipid peroxide level was dependent on only stress, the lipid peroxide level should be almost the same level. We suggest that lipid peroxide level is increased by not only stress but also other factors. Further experiments are needed to elucidate the reason why FWMB decreased lipid peroxide level. Moreover, FWMB improved the gut flora. The amount of Bacteroides spp. and Staphylococcus spp. were drastically decreased by FWMB feeding. Staphylococcus spp. causes diarrhea,Citation18) while Bacteroides spp. and Streptococcus spp. seem to be related to a weakened immune system.Citation19) The FWMB may improve the stresses and gut flora conditions, and these better conditions in the FWMB-fed hens might be related to egg productivity and condition.
One of the beneficial factors of FWMB was thought to be due to the fiber content. Fiber has a prebiotic effect and a defecation promoting effect, and so it improves gut flora.Citation11) The FWMB was a fermented product produced by L. fermentum. Therefore, the FWMB contained a number of L. fermentum. Wang et al.Citation20) reviewed the beneficial effect of antioxidants in probiotic bacteria and found that oxidative stress can decrease immune function and worsen the gut flora. Further analyses are needed to determine whether fiber and/or L. fermentum can decrease lipid peroxide levels.
FWMB slightly increased egg production and significantly decreased the number of broken and soft-shelled eggs. FWMB also decreased the stress markers and improved the gut flora; these changes might be related to egg productivity and condition.
Author contribution
M.K., H.M., T.O., and H.K conceived the project. M.K. and H.M. designed the feeding program and measured egg production. S.Y., T.O., and H.K. collected blood. S.Y., T.O., A.O., K.O., and H.K. performed biochemical analyses. S.Y. and T.O. performed microbial measurements. All authors analyzed the data and discussed the result. S.Y. and H.K. performed statistical analyses and wrote the manuscript. All authors read and approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
We thank Kelly Zammit, BVSc, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
References
- Star L, Kemp B, Van den Anker I, et al. Effect of single or combined climatic and hygienic stress in four layer lines: 1. Performance. Poult Sci. 2008;87:1022–1030.10.3382/ps.2007-00142
- Cheng TK, Coon CN, Hamre ML. Effect of environmental stress on the ascorbic acid requirement of laying hens. Poult Sci. 1990;69:774–780.10.3382/ps.0690774
- Kullu SS, Das A, Bajpai SK, et al. Egg production performance, egg yolk antioxidant profile and excreta concentration of corticosterone in golden pheasants (Chrysolophus pictus) fed diets containing different levels of green vegetables. J Anim Physiol Anim Nutr (Berl). 2016;101:e31–e42.
- Yoneyama S. Q&A. Rinsanshi Dayori. 2007;9:11.
- Sendi H, Mohamed MT, Anwar MP, et al. Spent mushroom waste as a media replacement for peat moss in Kai-Lan (Brassica oleracea var. Alboglabra) production. Sci World J. 2013;2013:258562.
- Schwartz B, Hadar Y. Possible mechanisms of action of mushroom-derived glucans on inflammatory bowel disease and associated cancer. Ann Transl Med. 2014;2:19.
- Robaszkiewicz A, Bartosz G, Lawrynowicz M, et al. The role of polyphenols, β-carotene, and lycopene in the antioxidative action of the extracts of dried, edible mushrooms. J Nutr Metab. 2010;2010:173274.
- Valverde ME, Hernández-Pérez T, Paredes-López O. Edible mushrooms: improving human health and promoting quality life. Int J Microbiol. 2015;2015:376387.
- Manach C, Scalbert A, Morand C, et al. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747.
- Tang BM, Eslick GD, Nowson C, et al. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370:657–666.10.1016/S0140-6736(07)61342-7
- Simpson HL, Campbell BJ. Review article: dietary fibre-microbiota interactions. Aliment Pharmacol Ther. 2015;42:158–179.10.1111/apt.13248
- Rath NC, Anthony NB, Kannan L, et al. Serum ovotransferrin as a biomarker of inflammatory diseases in chickens. Poult Sci. 2009;88:2069–2074.10.3382/ps.2009-00076
- Ebrahimzadeh SK, Farhoomand P, Noori K. Immune response of broiler chicken fed diets supplemented with different level of choromium methionine under heat stress condition. Asian-Australas J Anim Sci. 2012;25:256–260.
- Skinner JG. International standardization of acute phase proteins. Vet Clin Pathol. 2001;30:2–7.10.1111/vcp.2001.30.issue-1
- Rea K, Dinan TG, Cryan JF. The microbiome: a key regulator of stress and neuroinflammation. Neurobiol Stress. 2016;4:23–33.10.1016/j.ynstr.2016.03.001
- Melillo G, Balzano G, Stefanelli F, et al. Ultrasonic nebulization of hypertonic solution: a new method for obtaining specimens from nasal mucosa for morphologic and biochemical analysis in allergic rhinitis. Allergy. 1998;53:794–797.
- Mitsuoka T, Ohno K, Benno Y, et al. The fecal flora of man. IV. Communication: comparison of the newly developed method with the old conventional method for the analysis of intestinal flora (author’s transl). Zentralbl Bakteriol Orig A. 1976;234:219–233.
- Suzuki Y, Kobayashi M, Matsushita S, et al. Detection of the staphylococcal enterotoxin D-like gene from staphylococcal food poisoning isolates over the last two decades in Tokyo. J Vet Med Sci. 2015;77:905–911.10.1292/jvms.15-0028
- Cao Y, Rocha ER, Smith CJ. Efficient utilization of complex N-linked glycans is a selective advantage for Bacteroides fragilis in extraintestinal infections. Proc Nat Acad Sci USA. 2014;111:12901–12906.10.1073/pnas.1407344111
- Wang Y, Wu Y, Wang Y, et al. Antioxidant properties of probiotic bacteria. Nutrients. 2017;9:pii. E521.
