Abstract
Long non-coding RNA (lncRNA) plasmacytoma variant translocation 1(PVT1) was aberrantly expressed in various cancers and is associated with tumor prognosis. Here, we aim to investigate its function in prostate cancer. Small interfering RNA against PVT1 was transfected into prostate cancer cell lines and cell growth and apoptosis were analyzed. Our results showed that PVT1 was overexpressed in prostate cancer tissues and cells. Higher levels of PVT1 indicated poorer overall survival and disease-free survival. A significant association was found between PVT1 expression and tumor stage. Besides, PVT1 knockdown significantly inhibited prostate cancer growth in vivo and in vitro and promoted cell apoptosis. PVT1 knockdown also significantly upregulated the expression of cleaved caspase-3 and cleaved caspase-9, but downregulated the expression of c-Myc in prostate cancer cell lines. Our results suggest that PVT1 played an oncogenic role in prostate cancer and could be used as a potential biomarker for diagnosis of prostate cancer.
LncRNA PVT1 predicts prognosis and regulates tumor growth in prostate cancer.

Key words:
Prostate cancer is one of the most common cancers that develop in the male reproductive system. According to the cancer statistics in 2014,Citation1) prostate, lung and bronchus, and colorectal have become the three most commonly diagnosed types of cancer among men, accounting for about 50% of all newly diagnosed cancers. Among them, prostate cancer alone accounts for 27% of incident cases in men. It has become the top leading cause of cancer-related death in men with increasing morbidity in the developing world.Citation1–4) Although the therapeutic options, including radical prostatectomy or radiation, could successfully cure the majority of patients, disease recurrence (approximately 30–40%) is still the major factor impairing survival of the patients with advanced prostate cancer.Citation5–7) Therefore, it is urgently required to clarify the underlying mechanisms of prostate cancer development, and to identify a biomarker for the early detection of prostate cancer.
Long non-coding RNA (lncRNA) is an RNA molecule longer than 200 nucleotides, which does not have protein-coding capabilities.Citation8) Abnormal expression of lncRNAs is closely associated with various tumors and diseases.Citation9–12) LncRNAs have the potential to become molecular targets for tumor therapy. Evidence has accumulated that lncRNAs are often altered in human urologic cancers and are involved in tumor progression.Citation13) LncRNA plasmacytoma variant translocation 1(PVT1) was found to be overexpressed in gastric cancer, hepatocellular carcinoma, non-small cell lung cancer, ovarian, and breast cancer, and associated with the cell proliferation, apoptosis, lymph node invasion and metastasis, and tumor prognosis.Citation14–20) Recent studies found that PVT1 was upregulated in prostate cancer and identified PVT1 as an oncogene that increases the risk of prostate cancer.Citation21,22) However, the underlying mechanism of PVT1 in the development of prostate cancer was still unclear.
In this study, in order to explore the role of PVT1 in prostate cancer, we assessed its expression in prostate cancer tissues and cells, confirmed the correlation between PVT1 expression and the clinicopathological features. By small interfering RNA transfection, we found that PVT1 knockdown significantly inhibited prostate cancer growth in vivo and in vitro and promoted cell apoptosis. Our results suggest that PVT1 plays an oncogenic role in prostate cancer and could be used as a potential biomarker for the detection and treatment of prostate cancer.
Material and method
Patients
One hundred and fifty-two clinical samples of prostate cancer and thirty non-cancerous tissues were obtained from Subei People’s Hospital of Jiangsu province. The diagnosis of prostate cancer was performed according to World Health Organization criteria. Clinical characteristics of carcinoma samples (age, tumor stage, Gleason grade, nodal stage, serum PSA level, and resection margin) are recorded in Table . Informed consent for the collection of samples was obtained from all patients. Our study protocol was approved by the Medical Ethics Committee of JiangSu province (China).
Table 1. Clinical characteristics of the patients with prostate cancer.
Cell culture
The human prostate cancer cell lines BPH-1, PNT2, LNCaP, PC-3, and DU145 were purchased from the American Type Culture Collection (ATCC). Cultures were maintained at 37 °C in an atmosphere of 5% CO2.
Cell survival and apoptosis assay
Cell survival was measured using the MTT Cell Proliferation/Viability Assay kit (Sigma, Germany) according to the guidelines.
Apoptosis was evaluated using an Annexin-V-Fluos and Propidium Iodide (PI) Apoptosis Detection Kit (Sigma, Germany) and TUNEL Apoptosis Assay Kit (Roche, Switzerland) according to the manufacturer’s protocol.
Colony formation assay
Colony formation assay was based on our previous study.Citation23) Briefly, both uninfected and infected cells were cultured in 6-well plates (400/well). The culture medium was changed at regular intervals. The plates were incubated at 37 °C with 5% CO2 for 2 weeks. Cell colonies were stained with Giemsa solution and counted using a light microscope.
Tumor xenograft experiments
Equal numbers of prostate cancer cells expressing either PVT1 knockdown or control vectors were injected subcutaneously into the male BALB/c athymic nu/nu mice (Vital River). The tumors were harvested and weighed at the terminal time point of experiments.
Quantitative RT-PCR (qRT-PCR)
Total mRNA from prostate cancer tissues and cells was isolated using TRIzol Reagent (Takara, Japan) and reverse transcribed into cDNA using Prime Script RT reagent kit (Takara, Japan) according to the manufacturer’s protocol. Real-time PCR was performed for assessing the PVT1 mRNA level by the SYBR Premix Ex TaqTM II kit (Takara, Japan) on a Stratagene MX3005P system (Agilent, USA). β-actin served as an internal standard. The PCR primers used in this study were as previously described.Citation18)
Small interfering RNA transfection
Small interfering RNA (siRNA) were synthesized by Santa Cruz Biotechnology (Santa Cruz, CA) and transfected into three prostate cancer cell lines LNCaP, PC-3 and DU145 for suppressing the function of PVT1 using Lipofectamine 2000 (Invitrogen, USA). 48 h post-transfection, cells were harvested for qRT-PCR to determine the transfection efficiency.
Statistical analysis
Data were shown as mean ± SD. Comparisons of continuous data were analyzed using the independent t-test between the two groups, whereas categorical data were analyzed by the χ2 test. Predictors of differences in overall and disease-free survival were analyzed using Kaplan–Meier analyses. Statistical analysis was performed using SPSS software version 16.0. p < 0.05 was considered statistically significant.
Results
PVT1 is upregulated in prostate cancer tissues and cell lines
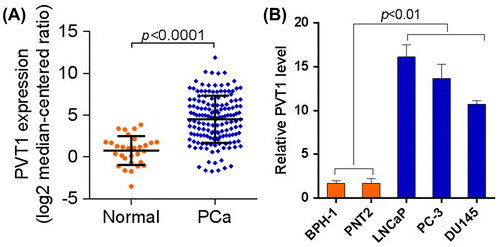
Previous studies have shown that lncRNA PVT1 is aberrantly expressed in various types of cancer, including lung, breast, gastric, and hepatocellular cancer. To explore the role of PVT1 in prostate cancer, we first determined the expression level of PVT1 in prostate cancer. As shown in Fig. (A), the mRNA level of PVT1 was significantly upregulated in prostate cancer tissues (p < 0.0001). In addition, it was found that PVT1 was significantly upregulated in prostate cancer cell lines, LNCaP, PC-3, and DU145 (Fig. (B), p < 0.01). Overall, PVT1 was upregulated in prostate cancer tissues and cell lines.
PVT1 expression predicts prognosis and is associated with tumor stage of prostate cancer patients
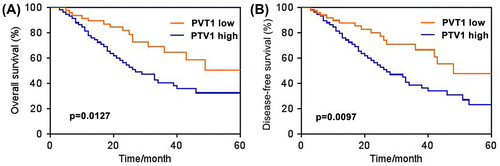
Next, we investigated whether PVT1 expression was correlated with the prognosis of prostate cancer patients. Kaplan–Meier survival analysis showed that PVT1 expression was associated with the survival of prostate cancer patients (Fig. (A) and (B)). High PVT1 level group (>25th percentile) indicated poorer overall survival and disease-free survival than the low-level group (<25th percentile). In addition, the association of PVT1 expression with the clinicopathological features of patients with prostate cancer is summarized in Table . There was no correlation between PVT1 expression and patient age, Gleason grade, nodal stage, serum PSA level, and resection margin (p > 0.05). But a significant association was found between PVT1 expression and tumor stage (p < 0.01). In summary, these results implied that the expression level of PVT1 may be associated with the disease progression of prostate cancer.
Fig. 2. High level of PVT1 expression indicated poor prognosis. Kaplan–Meier curve comparing time to survival between prostate cancer cells with high (>25th percentile) and low (<25th percentile) PVT1 expression. (A) Overall survival. (B) Disease-free survival. n = 52 in PVT1 low group, n = 59 in PVT1 high group.
PVT1 knockdown inhibits prostate cancer growth in vivo and in vitro
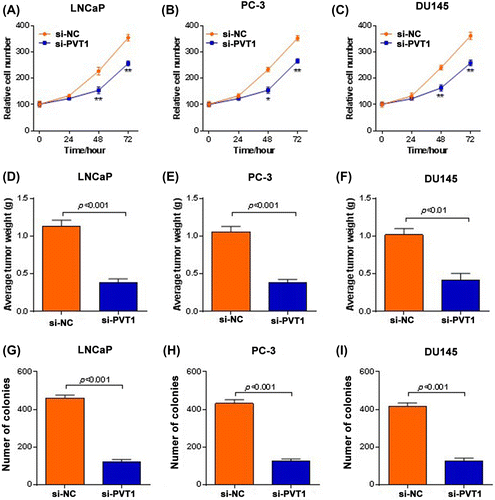
To further investigate the effect of PVT1 on the tumor progression in prostate cancer, we knocked down PVT1 in three prostate cancer cell lines (LNCaP, PC-3 and DU145). We found that PVT1 knockdown in prostate cancer significantly inhibited the cancer growth and transformation in vitro and in vivo. As shown in Fig. (A)–(C), cell proliferation was significantly inhibited in three prostate cancer cell lines when PVT1 was knocked-down in vitro (p < 0.01 or p < 0.001). PVT1 knockdown also significantly suppressed the tumor growth of prostate cancer in vivo (Fig. (D)–(F); p < 0.01 in LNCaP and PC-3 cells and p < 0.001 in DU145 cell). Besides, colony formation was significantly inhibited in three prostate cancer cell lines when PVT1 was knocked-down in vitro (Fig. (G)–(I); p < 0.001).
Fig. 3. PVT1 knockdown inhibited prostate cancer growth in vivo and in vitro. LNCap, PC-3 and DU145 cells were infected with control siRNA (si-NC) or siRNA targeting PVT1 (si-PVT1). (A-C) PVT1 knockdown inhibited prostate cancer cell proliferation. Relative cell numbers were evaluated using MTT analysis at the indicated time points. *p < 0.05, **p < 0.01 vs. si-NC. (D–F) PVT1 knockdown suppresses tumor growth in vivo. LNCap, PC-3 and DU145 cells infected with si-NC or si-PVT1 were applied to xenograft mice experiment. The tumors were harvested and weighed at the end of the experiment. (G-I) PVT1 knockdown decreased colony formation. LNCap, PC-3 and DU145 cells infected with si-NC or si-PVT1 were subjected to soft agar colony formation assay and colony numbers were evaluated 2 weeks later. *p < 0.05 vs. si-NC and **p < 0.01 vs. si-NC.
PVT1 knockdown promoted prostate cancer cell apoptosis in vitro
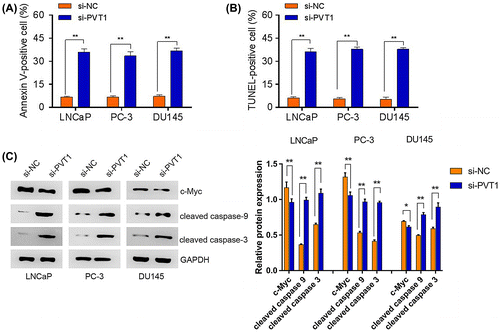
To determine whether PVT1 knockdown inhibited prostate cancer growth through an apoptotic pathway, we detected the apoptosis condition in prostate cancer cell lines by fluorescence-activated cell sorting (FACS) analysis when PVT1 was knocked down. It was found that PVT1 knockdown significantly accelerated prostate cancer cells apoptosis (Fig. (A)–(B), p < 0.01). Activation of caspases is an essential prerequisite for induction of apoptosis.Citation24) Thus, the expression levels of cell apoptosis-related proteins were also detected, we found that PVT1 knockdown significantly upregulated the expression of cleaved caspase-3 and cleaved caspase-9 as compared to the control in prostate cancer cell lines (Fig. (C), p < 0.01). Besides, it is well known that PVT1 is a large non-coding locus adjacent to c-Myc, and is recognized as a cancerous gene co-amplified with c-Myc in various cancers.Citation25) Here, we found that PVT1 knockdown significantly downregulated the expression of c-Myc as compared to the control (Fig. (C), p < 0.01 in LNCaP and PC-3 cells; p < 0.05 in DU145 cell).
Fig. 4. PVT1 knockdown promoted prostate cancer cell apoptosis. LNCaP, PC-3 and DU145 cells were infected with si-NC or si-PVT1 (A) PVT1 knockdown induced prostate cell apoptosis by Annexin-V-FLUOS staining. (B) PVT1 knockdown induced prostate cell apoptosis by TUNEL staining. (C) PVT1 knockdown significantly upregulated the expression of cleaved caspase-3 and cleaved caspase-9, but downregulated the expression of c-Myc as compared to the control in prostate cancer cell lines. *p < 0.05 vs. si-NC and **p < 0.01 vs. si-NC.
Discussion
PVT1, which is located at 8q24.21, was found to be upregulated in a diverse range of cancer types.Citation14–20) Ectopic expression of PVT1 has been identified as a powerful predictor of tumor progression and patient survival in lung cancer, hepatocellular carcinoma, and colorectal cancer.Citation16,17,19) Additionally, it exerted regulatory functions in various biological processes, such as proliferation, apoptosis, mobility, and invasion.Citation15,16,18–20) Research has reported that PVT1 was overexpressed in prostate cancer and promoted the risk of prostate cancer and the underlying mechanism was also explored. For example, PVT1 was previously identified as an oncogene that increases the risk of prostate cancer.Citation21,22) Liu et al. indicated that lncRNA PVT1 regulates prostate cancer cell growth by inducing the methylation of miR-146a.Citation26)
In this study, the significant upregulation of PVT1 was found in prostate cancer tissues and cell lines compared to the normal tissues and cells. High expression of PVT1 was found to be associated with poorer prognosis and advanced tumor stage, suggesting that PVT1 expression could be identified as an independent prognostic factor in prostate cancer. These findings are consistent with the results previously seen in lung cancer and hepatocellular carcinoma (HCC).Citation16,17) In HCC, overexpression of PVT1 was associated with a poor recurrence-free survival and a higher recurrence rate.Citation17) Increased PVT1 expression was significantly correlated with histological grade and overall survival in non-small cell lung cancer.Citation16) These results indicate that PVT1 plays an important role in the development of human cancers.
Additionally, the results showed that PVT1 knockdown significantly inhibited cell growth and colony formation in prostate cancer cell lines in vitro. In vivo, remarkable suppression of tumor growth was also observed after PVT1 was knocked down. Moreover, PVT1 knockdown significantly accelerated prostate cancer cells apoptosis. Our findings suggest that PVT1 plays an oncogenic role in the progression of prostate cancer by prompting cell growth and inhibiting cell apoptosis, which is consistent with previous research in other cancers.Citation15,16,18–20)
We also found that PVT1 knockdown significantly upregulated the expression of cleaved caspase-3 and cleaved caspase-9, but downregulated the expression of c-Myc as compared to the control. This suggested that PVT1 could inhibit prostate cancer cell apoptosis by upregulating cleaved caspase-3 and cleaved caspase-9. Besides, the underlying mechanism was partially associated with the expression of c-Myc. It is accepted that PVT1 is a cancerous gene co-amplified with c-Myc in various cancers.Citation25) Consistently, Peng et al.Citation27) indicated that the effect of lncRNA PVT1 on proliferation and apoptosis of human pancreatic cancer cell was partially through regulating c-Myc expression. Besides, Riquelme et al.Citation28 suggested that c-Myc and PVT1 CNG promote a malignant phenotype of malignant pleural mesothelioma, with c-Myc copy number gain stimulating cell proliferation, and PVT1 both stimulating proliferation and inhibiting apoptosis.
In addition to PVT1, other lncRNAs also have a similar regulatory function in prostate cancer.Citation13) PCGEM1 is a highly prostate-specific lncRNA that is overexpressed in prostate cancer.Citation29) Ectopic high expression of PCGEM1 was found to promote prostate cancer cell proliferation, inhibit cell apoptosis, and is associated with high risk of prostate cancer.Citation30) These results provide evidence that lncRNA is closely associated with the progression of various kinds of human cancers and diseases. The potential molecular mechanism that lncRNA PVT1 enhances carcinogenesis in prostate cancer is a prime candidate for further investigation.
In summary, our results indicated that PVT1 expression was associated with prognosis and tumor stage, and concluded that PVT1 plays an oncogenic role in prostate cancer. It may be identified as a new biomarker and a possible target for prostate cancer diagnosis and therapy.
Author contributions
Jin Yang and Cuirong Li designed the study, performed the experiments; Ashley Mudd analyzed the data; Xiao Gu drafted the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics. CA: Cancer J Clin. 2014;64:9–29.
- Bilusic M, Heery C, Madan RA. Immunotherapy in prostate cancer: emerging strategies against a formidable foe. Vaccine. 2011;29:6485–6497.10.1016/j.vaccine.2011.06.088
- Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2012;62:220–241.10.3322/caac.v62:4
- Xue G, Ren Z, Chen Y, et al. A feedback regulation between miR-145 and DNA methyltransferase 3b in prostate cancer cell and their responses to irradiation. Cancer Lett. 2015;361:121–127.10.1016/j.canlet.2015.02.046
- Roehl KA, Han M, Ramos CG, et al. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–914.10.1097/01.ju.0000134888.22332.bb
- Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–2131.10.1016/j.juro.2007.03.003
- Boorjian SA, Eastham JA, Graefen M, et al. A critical analysis of the long-term impact of radical prostatectomy on cancer control and function outcomes. Euro Urol. 2012;61:664–675.10.1016/j.eururo.2011.11.053
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159.10.1038/nrg2521
- Harries L. Long non-coding RNAs and human disease. Biochem Soc Trans. 2012;40:902–906.10.1042/BST20120020
- Spizzo R, Almeida MI, Colombatti A, et al. Long non-coding RNAs and cancer: a new frontier of translational research?. Oncogene. 2012;31:4577–4587.10.1038/onc.2011.621
- Wang P, Ren Z, Sun P. Overexpression of the long non-coding RNA MEG3 impairs in vitro glioma cell proliferation. J Cell Biochem. 2012;113:1868–1874.10.1002/jcb.v113.6
- Ellis BC, Molloy PL, Graham LD. CRNDE: a long non-coding RNA involved in cancer, neurobiology, and development. Front Genet. 2012;3:270. PMCID: PMC3509318. doi:10.3389/fgene.2012.00270.
- Martens-Uzunova ES, Böttcher R, Croce CM, et al. Long noncoding RNA in prostate, bladder, and kidney cancer. Euro Urol. 2014;65:1140–1151.10.1016/j.eururo.2013.12.003
- X-y Fang, H-f Pan, R-x Leng, et al. Long noncoding RNAs: novel insights into gastric cancer. Cancer Lett. 2015;356:357–366.
- Zhang Z, Zhu Z, Zhang B, et al. Frequent mutation of rs13281615 and its association with PVT1 expression and cell proliferation in breast cancer. J Genet Genom. 2014;41:187–195.10.1016/j.jgg.2014.03.006
- Yang Y-R, Zang S-Z, Zhong C-L, et al. Increased expression of the lncRNA PVT1 promotes tumorigenesis in non-small cell lung cancer. Int J Clin Exp Pathol. 2014;7:6929–6935.
- Ding C, Yang Z, Lv Z, et al. Long non-coding RNA PVT1 is associated with tumor progression and predicts recurrence in hepatocellular carcinoma patients. Oncol Lett. 2015;9:955–963.
- Ding J, Li D, Gong M, et al. Expression and clinical significance of the long non-coding RNA PVT1 in human gastric cancer. OncoTargets Ther. 2014;7:1625–1630.10.2147/OTT
- Takahashi Y, Sawada G, Kurashige J, et al. Amplification of PVT-1 is involved in poor prognosis via apoptosis inhibition in colorectal cancers. Br J Cancer. 2014;110:164–171.10.1038/bjc.2013.698
- Guan Y, Kuo W-L, Stilwell JL, et al. Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Clin Cancer Res. 2007;13:5745–5755.10.1158/1078-0432.CCR-06-2882
- Soubra A, Konety B, Bagchi A. MP61-06 increased PVT1 expression correlates with advanced stage and hormone resistance of prostate cancer. J Urol. 2015;193:e748–e749.10.1016/j.juro.2015.02.2187
- Meyer KB, Maia A-T, O’Reilly M, et al. A functional variant at a prostate cancer predisposition locus at 8q24 is associated with PVT1 expression. PLoS Genet. 2011;7:e1002165.10.1371/journal.pgen.1002165
- Wang Y, Wu X, Ou L, et al. PLCε knockdown inhibits prostate cancer cell proliferation via suppression of Notch signalling and nuclear translocation of the androgen receptor. Cancer Lett. 2015;362:61–69.
- Ummanni R, Lehnigk U, Zimmermann U, et al. Immunohistochemical expression of caspase-1 and -9, uncleaved caspase-3 and -6, cleaved caspase-3 and -6 as well as Bcl-2 in benign epithelium and cancer of the prostate. Exp Ther Med. 2010;1:47–52.10.3892/etm_00000008
- Guo K, Yao J, Yu Q, et al. The expression pattern of long non-coding RNA PVT1 in tumor tissues and in extracellular vesicles of colorectal cancer correlates with cancer progression. Tumour Biol J Int Soc Oncodev Biol Med. 2017;39:1010428317699122.
- Liu HT, Fang L, Cheng YX, et al. LncRNA PVT1 regulates prostate cancer cell growth by inducing the methylation of miR-146a. Cancer Med. 2016;5:3512–3519.10.1002/cam4.900
- Peng J, Huang F, Zhuang Y, et al. Effect of LncRNA-PVT1 on proliferation and apoptosis of human pancreatic cancer cell line HPAF-Ⅱ. Chin J Gastroenterol. 2016;21:138–143.
- Riquelme E, Suraokar MB, Rodriguez J, et al. Frequent coamplification and cooperation between C-MYC and PVT1 oncogenes promote malignant pleural mesothelioma. J Thorac Oncol. 2014;9:998–1007.10.1097/JTO.0000000000000202
- Srikantan V, Zou Z, Petrovics G, et al. PCGEM1, a prostate-specific gene, is overexpressed in prostate cancer. Proc Nat Acad Sci. 2000;97:12216–12221.10.1073/pnas.97.22.12216
- Petrovics G, Zhang W, Makarem M, et al. Elevated expression of PCGEM1, a prostate-specific gene with cell growth-promoting function, is associated with high-risk prostate cancer patients. Oncogene. 2004;23:605–611.10.1038/sj.onc.1207069