Abstract
Maitake mushroom (Grifola frondosa [Dicks.] Gray) is generally cultured using the sawdust of broadleaf trees. The maitake strain Gf433 has high production efficiency, with high-quality of fruiting bodies even when 30% of the birch sawdust on the basal substrate is replaced with conifer sawdust. We performed metabolome analysis to investigate the effect of different cultivation components on the metabolism of Gf433 and Mori52 by performing CE–MS on their fruiting bodies in different cultivation conditions to quantify the levels of amino acids, organic acids, and phosphorylated organic acids. We found that amino acid and organic acid content in Gf433 were not affected by the kind of sawdust. However, Gf433 contained more organic acids and less amino acids than Mori52, and Gf433 also contained more chitin compared with Mori52. We believe that these differences in the metabolome contents of the two strains are related to the high production efficiency of Gf433.
The yield and the production efficiency of Maitake Gf433 (GL, GB) are higher than the existing strain Mori52 (MB). Gf433 contained more organic acids and less amino acids than MB.
Maitake (Grifola frondosa [Dicks.] Gray) is a popular mushroom in Asia with a superior flavor, taste, and texture. Moreover, maitake mushroom has long been considered to have positive physiological effects on the maintenance and improvement of health. Extensive research has demonstrated that maitake influences the progression of inflammatory bowel diseaseCitation1); modulationCitation2) and enhancementCitation3–5) of the immune system; anti-diabeticCitation6–8); cholesterol-loweringCitation9); antiatherogenicCitation10); and antihypertensiveCitation11) effects. Therefore, in light of its health-related benefits, maitake is now routinely consumed in Japan owing to the increase in health-conscious consumers.
Maitake is most efficiently cultivated using sawdust obtained from broadleaf trees such as beech (Fagus crenata Blume), oak (Quercus crispula Blume), and Betula spp. The sawdust from these trees has been routinely used for the preparation of basal substrate for maitake. Larch (Larix kaempferi [Lamb.] Carrière) is one of the most important conifers grown in Hokkaido, an island in northern Japan. Importantly, a stable supply of larch is available at all times. Moreover, the price of sawdust from larch is lower than that of sawdust from broadleaf trees. Therefore, the use of larch sawdust for the cultivation of maitake could lead to significant reduction in cost. However, larch contains phenol compounds such as taxifolin,Citation12,13) which inhibits the mycelial growth of the mushroom.Citation14)
Recently, Gf433 (variety denomination: Taisetsu hananomai No. 1) has been bred as a new strain of maitake, which shows high production efficiency with high-quality fruiting bodies even when 30% of the birch (Betula ermanii Cham.) sawdust in basal substrate was replaced by larch sawdust.Citation15) Because the growth of Gf433 is not affected by the presence of larch sawdust in the basal substrate, the metabolism of Gf433 is likely to be different from the existing varieties.
Metabolome analysis is a useful method for the estimation of levels of different metabolites,Citation16,17) for the seasonal evaluation of food,Citation18) and for metabolite comparison among different varietiesCitation19,20) in diverse environmental growth conditions.Citation21) Fly agaric (Amanita muscaria [L.] Hook.), a poisonous mushroom, has been studied to uncover the correlation between the chemical composition of its fruiting bodies and the topsoil contents using an 1H NMR-based metabolomics approach.Citation22) However, there has been little investigation on the metabolome analysis of chemical composition of fruiting bodies of edible mushrooms.
In the present study, we performed CE–MS to understand the differences in the metabolism and cultivation characteristics between Gf433 and a popular mushroom strain in Hokkaido, Mori52. The metabolome analysis compared the levels of the primary metabolites such as amino acids, organic acids, and phosphorylated organic acids (including mononucleotides involved in delicious taste) in the fruiting bodies of these two strains cultured on different sawdust containing media.
Materials and methods
Preparation of experimental samples
Gf433 strain of maitake used in this study was developed by the Forest Products Research Institute while the existing strain Mori52 was obtained from Mori & Co. Ltd., Japan. The basal substrate formulation for culturing these strains contained sawdust (27%), wheat bran (8%), and water (65%). The Gf433 strain was cultured using birch sawdust (GB) or a mixture of 70% birch and 30% larch sawdust (GL), while Mori52 strain was cultured using only birch sawdust (MB). The basal substrate was packed in a polypropylene bag and autoclaved at 121 °C for 30 min. After the blocks cooled down, they were inoculated with the spawn. The spawn-running process was carried out at 22 °C ± 1 °C and 70% ± 5% relative humidity for 52 days. These cultures were then transferred to a vegetative room and grown further at 18 °C ± 1 °C, 90% ± 5% relative humidity, and 350 lux on a 12 h light/dark schedule. The sawdust blocks were cultured in Forest Products Research Institute as described by Yoneyama et al.Citation15) The fruiting bodies were harvested when the tubes on the pilei matured and the resulting yield (fresh weight, FW) was measured. The pilei were immediately collected from the harvested fruiting bodies, frozen in liquid nitrogen, and stored at –80 °C. Harvesting period describes the period from the inoculation to the harvest. Production efficiency was calculated as the yield of fruiting bodies (in g FW/bag)/harvesting period (in days). The yield of each strain was statistically compared using Tukey’s test (p < 0.01).
Food composition and chitin content analysis
The harvested fruit-body was freeze-dried, powdered, and used for the analysis. Food composition (protein, lipid, ash, minerals, vitamins, and dietary fiber) analysis was conducted by Japan Food Research Laboratories (Tokyo, Japan) according to the Japanese Food Labeling Standards (Cabinet Office Ordinance No. 10 of 2015, http://www.caa.go.jp/foods/pdf/150320_kijyun.pdf).
The chitin in the fruiting bodies was fractionated, hydrolyzed, and measured as N-acetylglucosamine. The chitin content was calculated by multiplying 0.92 by N-acetylglucosamine content. Chitin was fractionated using the method by Yanase.Citation23) Chitin fraction thus obtained, was hydrolyzed using 6 N HCl autoclaved at 114 °C for 6 h. N-acetylglucosamine levels were determined according to the method of Boas.Citation24) All analyses were performed in triplicate.
CE–MS analysis
Metabolites (amino acids, organic acids, and phosphorylated organic acids) were quantified using CE–MS as described by Miyagi et al.Citation16) (with minor modifications). The pilei from five sawdust blocks were collected as samples, and five such samples were prepared from MB, GB, and GL, respectively. Each sample was crushed in a homogenizer AM-1 (Nihonseiki Kaisha, Ltd., Tokyo, Japan) with liquid nitrogen. 50 mg of frozen sample was added to 0.2 mL of methanol for inactivation of enzymes. Then 0.2 mL ultrapure water containing 100 μM 1, 4-piperazine diethane sulfonic acid (PIPES) and 100 μM methionine sulfone (internal standard) was added. After centrifugation at 4 °C, 15,000 rpm for 5 min, the supernatant was transferred to a 3 kDa cut-off filter (Merck Millipore, Billerica, MA, USA). After another round of centrifugation of the filtrate at 4 °C, 12,000 rpm for 30 min, the filtrate was used for CE–MS analysis.
CE–MS analysis was performed by Agilent CE–MS system (CE; G1600AX Capillary Electrophoresis, MS; G1956B LC/MS, Agilent Technologies, Waldbronn, Germany). For the quantification of levels of anionic compounds, polyethylene glycol-coated DB-WAX capillaries (100 cm × 50 μm i.d., Agilent Technologies) were used with 20 mM ammonium acetate (pH 8.5) as the running buffer and applied voltage of 25 kV. However, for cationic compound estimation, uncoated fused silica capillaries (100 cm × 50 μm i.d., GL Sciences, Japan) were used with 1 M formic acid (pH 1.9) as the running buffer and applied voltage of 25 kV. For the stabilization of MS analysis, the sheath solution containing 5 mM ammonium acetate in 50% (v/v) methanol for anionic compounds; and 0.1% formic acid in 50% (v/v) methanol for cationic compounds, was applied to the capillary at 8 μL/min using an isocratic pump (1100 series system, Agilent Technologies) equipped with a 1:100 splitter. During each electrophoresis round the glass capillary voltage was ± 3500 V and the drying nitrogen gas was adjusted at 320 °C. The flow was maintained at 8 L/min for 20–30 min. The quantitative accuracy of compounds investigated was determined by measuring known concentrations of 59 targeted compounds (organic acids, amino acids and phosphorylated organic acids) using the Agilent ChemStation Software (Rev. A. 10.01).
Data analysis
Statistical analyses of metabolite contents were performed using the Tukey–Kramer HSD test (p < 0.05) (Tukey 1953, Kramer 1956) using JMP 11.2.1 software (SAS Institute, Cary, NC, USA). Multivariate analyses were carried out using the Statistical Package for the Social Sciences (SPSS v22.0, IBM, NY, USA). Principal component analysis was based on the correlation matrix (case = each strain; variables = metabolites). Hierarchical clustering analysis was performed with squared Euclidean distance and average linkage method between groups. The metabolite contents were normalized by the Z-score obtained from these analyses. The heatmap was generated using Microsoft Excel 2010.
Results
Yield of fruiting bodies and the production efficiency
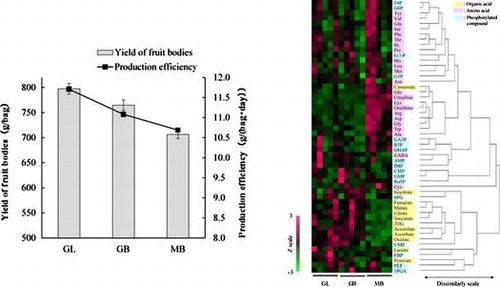
Harvesting period was calculated from the time of inoculation of strains to the time of tube maturity in the pilei. The harvesting periods for Gf433 strain cultured in birch sawdust (GB), Gf433 strain cultured in a mixture of 70% birch and 30% larch sawdust (GL), and Mori52 strain cultured in birch sawdust (MB) were 69.0 ± 1.1, 68.1 ± 1.5, and 66.1 ± 1.2, respectively. Regardless of the kind of sawdust used, Gf433 needed 2–3 days more for attaining maturity than MB strains. The yield of fruiting bodies for GB and GL were 8.2 and 12.9% higher than that of MB, respectively (Fig. ). Altogether, we observe that regardless of the kind of sawdust used in substrate, the yield of Gf433 was significantly higher than that of MB, and that the production efficiency of GL was the highest of all.
Fig. 1. Yield of fruiting bodies and production efficiency for Gf433 strains GL and GB and Mori52 (MB).
Comparison of metabolite levels among different strains and diverse basal substrates
Upon comparison of food composition between the different basal substrates for GL, GB, and MB, we observed that the levels of some minerals were altered between the different substrates. For instance, phosphorus content of GB and MB was higher than that of GL, while the calcium content of GL was higher than those of GB and MB. Moreover, water-insoluble dietary fiber contents of GL and GB were higher than that of MB, thereby suggesting that the high dietary fiber content is a characteristic of the Gf433 strain. Food composition of all the three kinds of fruiting bodies are shown in Table . The content of chitin, which is the major nitrogen compound in mushrooms, in GL, GB, and MB were 3.9 ± 0.1, 3.7 ± 0.2, and 3.1 ± 0.1 g/100 g dry weight, uncovering the difference in chitin levels between the different strains.
Table 1. Food composition of maitake (dry weight basis).
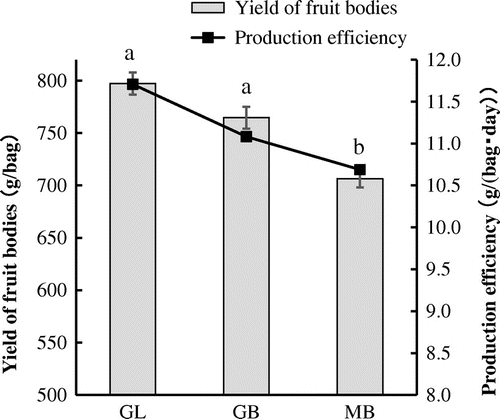
To investigate the influence of the strain identity and the basal substrate composition on the levels of amino acids, organic acids, and phosphorylated organic acids, the fruiting bodies were subjected to CE–MS-based metabolomics. The results showed that 57 metabolites (except for glycolate and glyoxylate) were detected. We found that malate was the predominant organic acid found in all the three kinds of fruiting bodies, followed by fumarate and citrate (Fig. (A)). The contents of each of the organic acid were different in different fruiting body classes. In GL and GB, the contents of aconitate, ascorbate, citrate, oxalate, and succinate were significantly higher than those in MB. In addition, the contents of 2-oxoglutarate (2OG) and fumarate in GB were significantly higher than those in MB. Only the cinnamate content in MB was the highest among all classes. Importantly, the total organic acid contents were 17.7 μmol/g in GL, 18.0 μmol/g in GB, and 15.3 μmol/g in MB, suggesting that the total organic acid content in Gf433 is higher than that in MB.
Fig. 2. Quantitative comparison of the content of organic acids (A), amino acids (B), and nucleic acids (C) in the fruiting bodies of maitake.
Amino acids contents in the fruiting bodies were also different among the different strains (Fig. (B)). In GL and GB, Glu was the predominant amino acid, followed by Orn, Gln, and Asp. In MB, the non-proteinogenic amino acid, Orn was the predominant, followed by Glu and Asp. The contents of Ala, Arg, Asp, Glu, Lys, Orn, Gly, Cit, and Trp were significantly lower in GB and GL than in MB. Total amino acid content in GL, GB, and MB were 7.7 ± 0.1 μmol/g, 7.3 ± 0.7 μmol/g, and 11.0 ± 1.6 μmol/g, respectively, showing that GL and GB have significantly lower total amino acid content compared to MB (Tukey–Kramer HSD test [p < 0.05]).
Phosphorylated organic acid analysis showed that AMP was the predominant entity in maitake (Fig. (C)), while the other mononucleotides as well as the total mononucleotide content were not significantly different between MB, GL, and GB.
Finally, the analysis of free sugar contents using HPLC indicated that trehalose, glycerol, and glucose were the major components in the strains. Moreover, the levels of these sugars were not significantly different among the three samples tested (data not shown).
Principal component analysis using metabolite datasets
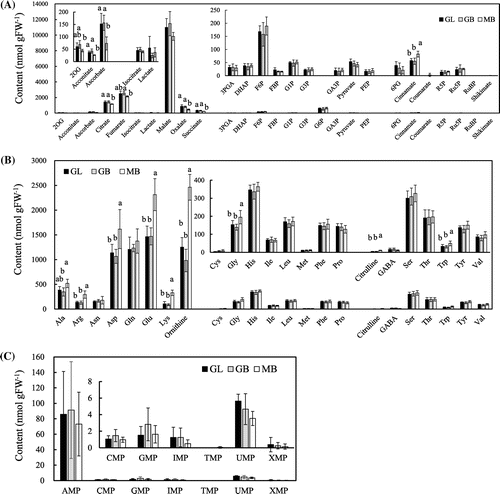
For precise assessment of the differences among the three samples, the levels of amino acids, organic acids, and phosphorylated organic acids were subjected to principal component analysis (Fig. ). The first and second components exhibited 38.8 and 19.4% of the total variation, respectively, thereby representing 58.2% of the total variance (Fig. (A)).
Fig. 3. Principal component analysis of metabolite contents in fruiting bodies of Gf433 and Mori52.
When these principal component scores were plotted, the plots of GL, GB, and MB segregated into two clusters according to the strains: the clusters for GL and GB, and the clusters for MB, indicating that these clusters were not divided according to the kind of sawdust in the basal substrate These two clusters were mainly divided by the first principal component, amino acids (Fig. (B) and (C)) Overall, these results show that the metabolite contents were different between the two strains, and that the metabolites in Gf433 were less affected by the kind of sawdust compared to other strains.
Hierarchical clustering analysis
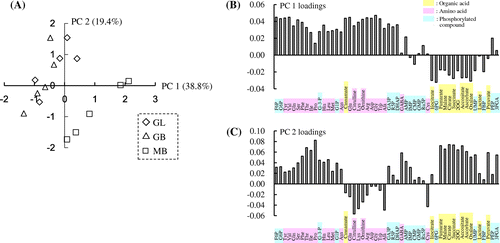
To visualize the alteration in the levels of each of the metabolites, hierarchical clustering analysis was performed in the basis of the amino acid, organic acid, and phosphorylated organic acid levels. The result showed that there were mainly two large clusters (Fig. (B)). One cluster contained amino acids and the other cluster contained organic acids. The first cluster was further divided into two sub-clusters comprising amino acids and phosphorylated compounds.
Fig. 4. Metabolite profiles in the fruiting bodies of Gf433 and Mori52.
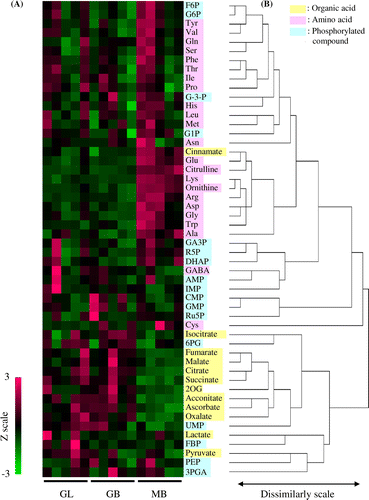
The Z-scores of the overall amino acids were especially high in two samples of MB. In these two samples, the Z-scores of Tyr, Val, Gln, Ser, Phe, Thr, Ile, Leu, Met, and Asn, which belong to the same cluster, were high. In addition, the Z-scores of the cluster composed of Glu, Cit, Lys, Orn, Arg, Asp, Gly, Trp, and Ala were high in more than three samples of MB (Fig. (A)). In GL and GB, the Z-scores of the former cluster were slightly high in some samples, but those in the latter cluster were low in each of the samples. On the other hand, the Z-scores of organic acids were low in MB, but high in GL and GB. The heatmap for all metabolites corresponding to each sample is presented in Fig. (A).
Discussion
For the cultivation of maitake a broadleaf tree is generally used for preparing the basal substrate in the culture medium. However, when maitake is cultured in substrate containing conifer sawdust, the pilei become small and a decrease in the yield is observed.Citation15) We could show that, if a part of the birch sawdust of the basal substrate is replaced by sawdust from a conifer such as larch, Gf433 did not show a decrease in yield and production efficiency (Fig. ). Thus, Gf433 has different characteristics from the existing strain Mori52.Citation15) These results suggest that the difference in growth between Mori52 and Gf433 could be the result of the difference in their metabolism.
In the present study, we performed a systematic metabolome analysis using CE–MS and compared the profiles of organic acid, amino acid, and phosphorylated organic acid contents between Gf433 and Mori52 strains to uncover the strain-dependence and the influence of the kinds of sawdust on the metabolic characteristics of these strains. The principal component analysis and heatmap-based analysis revealed differences in metabolic profiles of Mori52 and Gf433 strains; however, no significant differences were observed between Gf433 cultured on GL and GB (Figs. (A), (A)). Therefore, the overall metabolite profile is strongly affected by the strain, while in Gf433, the metabolome seems to be minimally influenced by the type of sawdust used in the substrate.
Free amino acids in the fruiting bodies of the rotting fungi are derived from the biosynthesis using nitrogen sources, such as protein and amino acid nitrogen in the culture medium. Kitamoto et al.Citation25) and Terashita et al.Citation26) reported the changes in the distribution of nitrogen compounds in Polyporus arcularius during the fruiting body development. In the early growth stage of P. arcularius, most of the nitrogen incorporated from the culture medium served as mycelial proteins, and the rest was accumulated in the vegetative mycelium as nucleic acids, free amino acids, or chitin. When the fruiting body formation started, the accumulated mycelial proteins were hydrolyzed by proteases in the mycelium. The free amino acids, thus, formed upon hydrolysis were the main nitrogen source, while the small amount of free amino acids was incorporated from the culture medium. These amino acids should be translocated into the fruiting body for their utilization for biosynthesis of nitrogen compounds useful in growth and spore formation. When the fruiting body matured, the amino acid translocation may have stopped and a large content of amino acids accumulated in the vegetative mycelium.
The amount of proteinogenic amino acids in Mori52 was higher than that in Gf433. In addition, Mori52 characteristically contained a large amount of non-proteinogenic amino acids such as Orn. Mori52 also accumulated more Glu and Orn than Gf433 (Figs. (B), (A)). Glu and Gln are used as a nitrogen source for the biosynthesis of many components, such as purines and pyrimidines. However, the levels of phosphorylated organic acid including purines and pyrimidines were not significantly different between the different strains (Fig. (C)).
Chitin content of Gf433 was higher than that of Mori52, which might influence the texture of the pilei. Chitin is a major cell wall component of maitake together with β-glucan. Chitin is synthesized using Gln as a nitrogen source. First, F6P is synthesized from G6P by glucose-6-P isomerase. F6P is then converted into glucosamine-6-P (the precursor of chitin) by transferring the ammonia from Gln. Gln is converted to Glu in this reaction (Fig. (A)).Citation27,28) Furthermore, in the case of mushrooms, chitin synthesized in the vegetative mycelium is hydrolyzed by chitinase at the start of fruiting body formation, and the chitin hydrolysates are quickly served to the fruiting body for the chitin biosynthesis.Citation25,29) In P. arcularius, the chitin hydrolysates provided about 70% of the substrates for chitin biosynthesis, and most of the residual substrates were served from the vegetative mycelium as amino sugars.Citation25) In Lentinula edodes, chitin is hydrolyzed to β-N-acetylglucosamine in the vegetative mycelium at the fruiting body formation stage, and used as a substrate for chitin synthesis and as an energy source.Citation29)
Fig. 5. Chitin synthesis pathway [Citation27] (A) and the metabolic map of organic acids and amino acids (B).
![Fig. 5. Chitin synthesis pathway [Citation27] (A) and the metabolic map of organic acids and amino acids (B).](/cms/asset/dc2a961a-4266-4348-8f19-f98278074876/tbbb_a_1387049_f0005_b.gif)
We found that the nitrogen contents analyzed by the Kjeldahl method were similar between Gf433 and Mori52. However, Gf433 contained low levels of amino acids, but it contained higher levels of chitin than in Mori52. Consequently, we suggest that Gf433 might consume more amino acids for chitin or nitrogen compounds synthesis in vegetative mycelium than Mori52. Therefore, in the fruiting body of Gf433, the amino acids translocated from vegetative mycelium was less, while the chitin accumulated in the cell wall was more, when compared with Mori52. In order to elucidate the mechanism of translocation of nitrogen compounds on the each growth stage of G. frondosa, further examination on the time course of nitrogen compounds in cultured vegetative mycelium and fruiting body is needed. Chitin is also a food component with hypocholesterolemic properties.Citation30,31) In addition, it has been reported that the oral administration of chitin nanofibers activated the functions of gut microorganisms and increased the plasma level of ATP.Citation32) These functionalities are thus expected to be higher in Gf433, which contains more chitin.
The contents of some organic acids, such as oxalate, succinate, citrate, fumarate, 2OG, aconitate, and ascorbate were significantly higher in Gf433 strain than in Mori52. These organic acids are synthesized via glycolysis and the TCA cycle (Fig. (B)). Because organic acid content was lower and amino acid level was higher in Mori52, the organic acids are possibly being used for amino acids synthesis rather than energy production. On the other hand, organic acid levels in Gf433 were higher even in the substrate comprising sawdust from larch. Therefore, we propose that the differences in levels of organic acids and amino acids between these strains may be involved in the higher production efficiency of Gf433 compared to Mori52 (Fig. ). These effects could also be indirect as energy production is essential for the growth of maitake and decreased energy production may result in the low yield of Mori52. In Arabidopsis thaliana, the growth was suppressed by stress (low pH) on rhizosphere, and the decrease in organic acids content and the accumulation of amino acids were induced.Citation33) Cordyceps pruinosa mycelia cultivated under various media and light conditions showed differences in growth and metabolic profiles of amino acids, organic acids, and sugar etc.Citation34) The low production efficiency might induce the amino acid accumulation in Mori 52. There has been a little evidence on the relation with growth and the flow of metabolites in mushrooms. Therefore, the time course analysis of growth, primary metabolite, and secondary metabolites, such as chitin and the correlation analysis on these compounds in vegetative mycelium and fruiting body would reveal the relation among metabolites, growth, and energy production.
Mononucleotides and amino acids are the taste determinants in mushroom. The equivalent umami concentration (EUC) is the concentration of monosodium glutamate (mg MSG/100 g) equivalent to the umami intensity of that given by the mixture of MSG and 5′-nucleotide.Citation35–37) The EUC of maitake was calculated based on the amounts of umami components in mushrooms: Asp, Glu, and 5′-nucleotides. We found that the EUC of GL, GB, and MB were 41.4 ± 12.3, 47.6 ± 20.2, and 63.4 ± 20.0 g MSG/100 g wet weight, respectively, and that no significant difference was recognized among them (Tukey–Kramer HSD test [p < 0.05]). These results indicated that the EUC was not different among the different strains tested even when the sawdust composition of the substrate was changed.
1H NMR-based metabolomics of fly agaric (Amanita muscaria) fruiting bodies revealed that the presence of organic matter in the topsoil was affected by the metabolome of the fruiting bodies.Citation22) In the present study, maitake were cultured on basal substrate which contained sufficient organic matter; therefore, the amino acid and organic acid contents in the fruiting bodies would be minimally affected by the kind of sawdust used. In addition, since the product efficiency of Gf433 was not affected by the larch sawdust, the sensitivity of Gf433 to phenolic growth inhibitors (such as taxifolin present in larch) is possibly very low.
Altogether, CE–MS-based metabolomics of the fruiting bodies revealed the characteristic differences between maitake strains. It is expected that the metabolomics of not only fruiting bodies but also vegetative mycelia will reveal the influence of the dynamics of nitrogen compounds and mononucleotides on the growth and maturity process of fruiting bodies.
Conclusions
In this study, the metabolome analysis revealed the differences between maitake strains, which have different cultivation characteristics. The levels of each metabolite were different among the strains, but they were not affected by the kinds of sawdust used in the substrate of Gf433. Compared with Mori52, Gf433 showed higher production efficiency and contained more organic acids and less amino acids. These differences in the metabolite contents could affect the production efficiency, especially of nitrogen compounds and energy production.
Author contributions
MS, AM, MK-Y, and YT designed experiments, MS, AM, SY, and SG performed experiments, MS, AM, MK-Y, and YT analyzed data, MS, AM, and YT wrote the paper.
Funding
This work was supported by the Science and technology research promotion program for agriculture, forestry, fisheries and food industry.
Disclosure statement
The authors of this manuscript have no competing interests. They do not have any other interests that influence the results and discussion of this paper.
Notes
Abbreviations: CE–MS, capillary electrophoresis-mass spectrometry, AMP, adenosine monophosphate; CMP, cytidine monophosphate; DHAP, dihydroxyacetone phosphate; FBP, fructose-1,6-bisphosphate; F6P, fructose-6-phosphate; GMP, guanosine monophosphate; G1P, glucose-1-phosphate; G3P, glycerol-3-phosphate; G6P, glucose-6-phosphate; GABA, γ-amino butyric acid; GA3P, glycelaldehyde-3-phosphate; IMP, inocine monophosphate; 3PGA, 3-phosphoglycerate; 6PG, 6-phosphogluconate; PEP, phosphoenolpyruvate; RuBP, ribulose-1,5-bisphosphate; Ru5P, ribulose-5-phosphate; R5P, ribose-5-phosphate; 2OG, 2-oxoglutarate; TMP, thymidine monophosphate; UMP, uridine monophosphate; XMP, xanthosine monophosphate.
References
- Lee JS, Park SY, Thapa D, et al. Grifola frondosa water extract alleviates intestinal inflammation by suppressing TNF-α production and its signaling. Exp Mol Med. 2010;42:143–154.10.3858/emm.2010.42.2.016
- Inoue A, Kodama N, Nanba H. Effect of maitake (Grifola frondosa) D-fraction on the control of the T lymph node Th-1/Th-2 proportion. Biol Pharm Bull. 2002;25:536–540.10.1248/bpb.25.536
- Kodama N, Komuta K, Sakai N, et al. Effects of D-fraction, a polysaccharide from Grifola frondosa on tumor growth involve activation of NK cells. Biol Pharm Bull. 2002;25:1647–1650.10.1248/bpb.25.1647
- Kodama N, Murata Y, Nanba H. Administration of a polysaccharide from Grifola frondosa stimulates immune function of normal mice. J Med Food. 2004;7:141–145.10.1089/1096620041224012
- Harada N, Kodama N, Nanba H. Relationship between dendritic cells and the D-fraction-induced Th-1 dominant response in BALB/c tumor-bearing mice. Cancer Lett. 2003;192:181–187.10.1016/S0304-3835(02)00716-4
- Preuss HG, Echard B, Bagchi D, et al. Enhanced insulin-hypoglycemic activity in rats consuming a specific glycoprotein extracted from maitake mushroom. Mol Cell Biochem. 2007;306:105–113.10.1007/s11010-007-9559-6
- Hong L, Xun M, Wutong W. Anti-diabetic effect of an α-glucan from fruit body of maitake (Grifola frondosa) on KK-Ay mice. J Pharm Pharmacol. 2007;59:575–582.10.1211/jpp.59.4.0013
- Horio H, Ohtsuru M. Maitake (Grifola frondosa) improve glucose tolerance of experimental diabetic rats. J Nutr Sci Vitaminol (Tokyo). 2001;47:57–63.10.3177/jnsv.47.57
- Fukushima M, Ohashi T, Fujiwara Y, et al. Cholesterol-lowering effects of maitake (Grifola frondosa) fiber, shiitake (Lentinus edodes) fiber, and enokitake (Flammulina velutipes) fiber in rats. Exp Biol Med. 2001;226:758–765.10.1177/153537020222600808
- Mori K, Kobayashi C, Tomita T, et al. Antiatherosclerotic effect of the edible mushrooms Pleurotus eryngii (Eringi), Grifola frondosa (Maitake), and Hypsizygus marmoreus (Bunashimeji) in apolipoprotein E-deficient mice. Nutr Res. 2008;28:335–342.10.1016/j.nutres.2008.03.010
- Kabir Y, Yamaguchi M, Kimura S. Effect of shiitake (Lentinus edodes) and maitake (Grifola frondosa) mushrooms on blood pressure and plasma lipids of spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo). 1987;33:341–346.10.3177/jnsv.33.341
- Takehara T, Sasaya T. Studies on the extractives of larch: Phenolic constituents from sapwood of Larix leptolepis GORD. Res Bull College Exp For Hokkaido Univ. 1979;36:681–693.
- Sasaya T, Takehara T, Miki K, et al. Phenolic constituents of Larix leptolepis Gord. Res Bull College Exp For Hokkaido Univ. 1980;37:837–860.
- Rudman P. The causes of natural durability in timber – part XI. Some tests on the fungi toxicity of wood extractives and related compounds. Horzforschung. 1963;17:54–57.10.1515/hfsg.1963.17.2.54
- Yoneyama S, Gisusi S, Harada A, et al. Screening of new maitake (Grifola frondosa) variety for using cultivation on larch (Larix leptolepis) wood as the substrate. J For Prod Res Inst. 2006;20:21–26. Japanese.
- Miyagi A, Takahashi H, Takahara K, et al. Principal component and hierarchical clustering analysis of metabolites in destructive weeds; polygonaceous plants. Metabolomics. 2010;6:146–155.10.1007/s11306-009-0186-y
- Miyagi A, Uchimiya M, Kawai-Yamada M, et al. An antagonist treatment in combination with tracer experiments revealed isocitrate pathway dominant to oxalate biosynthesis in Rumex obtusifolius L. Metabolomics. 2013;9:590–598.10.1007/s11306-012-0486-5
- Sugimoto M, Koseki T, Hirayama A, et al. Correlation between sensory evaluation scores of Japanese sake and metabolome profiles. J Agric Food Chem. 2010;58:374–383.10.1021/jf903680d
- Kårlund A, Hanhineva K, Lehtonen M, et al. Nontargeted metabolite profiles and sensory properties of strawberry cultivars grown both organically and conventionally. J Agric Food Chem. 2015;63:1010–1019.10.1021/jf505183j
- Röhlig RM, Eder J, Engel K-H. Metabolite profiling of maize grain: differentiation due to genetics and environment. Metabolomics. 2009;5:459–477.10.1007/s11306-009-0171-5
- Miyagi A, Uchimiya H, Kawai-Yamada M. Synergistic effects of light quality, carbon dioxide and nutrients on metabolite compositions of head lettuce under artificial growth conditions mimicking a plant factory. Food Chem. 2017;218:561–568.10.1016/j.foodchem.2016.09.102
- Deja S, Wieczorek PP, Halama M, et al. Do differences in chemical composition of stem and cap of Amanita muscaria fruiting bodies correlate with topsoil type? PLoS ONE. 2014;9:e104084.10.1371/journal.pone.0104084
- Yanase M. 4. Carbohydrate [I]. In: Japan Society for Food Science and Technology, editor. Food analysis method. Tokyo: Korin; 1982. p. 205–207. Japanese.
- Boas NF. Method for the determination of hexosamines in tissues. J Biol Chem. 1953;204:553–563.
- Kitamoto Y, Matsumoto T, Hosoi N, et al. Nitrogen metabolism in Favolus arcularius: changes in cellular nitrogen compounds during development of the mycelium and fruit-bodies. Trans Mycol Soc Jpn. 1980;21:237–244.
- Terashita T, Kitamoto Y, Matsumoto T, et al. Nitrogen metabolism in Favolus arcularius: changes in composition of free- and protein-amino acids during development of the mycelium and fruiting bodies. Trans Mycol Soc Jpn. 1984;25:187–198.
- Merzendorfer H. The cellular basis of chitin synthesis in fungi and insects: common principles and differences. Eur J Cell Biol. 2011;90:759–769.10.1016/j.ejcb.2011.04.014
- Jennings DH. Nitrogen, phosphorus and sulfur utilization by fungi. In: Boddy L, Marchant R, Read DJ, editors. Nitrogen. Cambridge: Cambridge University Press; 1988. p. 1–31.
- Iwamoto T, Nakai M, Sasaki T, et al. β-N-acetylglucosaminidase production by a strain of basidiomycetes and its physiological functions. Hakkokogaku Kaishi. 1990;68:107–115.
- Azuma K, Nagae T, Nagai T, et al. Effects of surface-deacetylated chitin nanofibers in an experimental model of hypercholesterolemia. Int J Mol Sci. 2015;16:17445–17455.10.3390/ijms160817445
- van der Gronde T, Hartog A, van Hees C, et al. Systematic review of the mechanisms and evidence behind the hypocholesterolaemic effects of HPMC, pectin and chitosan in animal trials. Food Chem. 2016;199:746–759.10.1016/j.foodchem.2015.12.050
- Azuma K, Izumi R, Kawata M, et al. Effects of oral administration of chitin nanofiber on plasma metabolites and gut microorganisms. Int J Mol Sci. 2015b;16:21931–21949.10.3390/ijms160921931
- Hachiya T, Sugiura D, Kojima M, et al. High CO2 triggers preferential root growth of Arabidopsis thaliana via two distinct systems under low pH and low N stresses. Plant Cell Physiol. 2014;55:269–280.10.1093/pcp/pcu001
- Oh TJ, Hyun SH, Lee SG, et al. NMR and GC-MS based metabolic profiling and free-radical scavenging activities of Cordyceps pruinosa mycelia cultivated under different media and light conditions. PLoS ONE. 2014;9:e90823.10.1371/journal.pone.0090823
- Yamaguchi S, Yoshikawa T, Ikeda S, et al. The synergistic taste effect of monosodium glutamate and disodium 5′-guanylate. Nippon Nōgeikagaku Kaishi. 1968;42:378–381. Japanese.10.1271/nogeikagaku1924.42.6_378
- Yamaguchi S, Yoshikawa T, Ikeda S, et al. Measurement of the relative taste intensity of some L-α-amino acids and 5’-nucleotides. J Food Sci. 1971;36:846–849.10.1111/jfds.1971.36.issue-6
- Lee Y-L, Jian S-Y, Mau J-L. Composition and nonvolatile taste components of Hypsizigus marmoreus. LWT Food Sci Technol. 2009;42:2594–2598.
