Abstract
Recently, the absence of a core-fucose residue in the N-glycan has been implicated to be important for enhancing antibody-dependent cellular cytotoxicity (ADCC) activity of immunoglobulin G monoclonal antibodies (mAbs). Here, we first prepared anti-HER2 mAbs having two core-fucosylated N-glycan chains with the single G2F, G1aF, G1bF, or G0F structure, together with those having two N-glycan chains with a single non-core-fucosylated corresponding structure for comparison, and determined their biological activities. Dissociation constants of mAbs with core-fucosylated N-glycans bound to recombinant Fcγ-receptor type IIIa variant were 10 times higher than those with the non-core-fucosylated N-glycans, regardless of core glycan structures. mAbs with the core-fucosylated N-glycans had markedly reduced ADCC activities, while those with the non-core-fucosylated N-glycans had high activities. These results indicate that the presence of a core-fucose residue in the N-glycan suppresses the binding to the Fc-receptor and the induction of ADCC of anti-HER2 mAbs.
Immunoglobulin G (IgG) monoclonal antibodies (mAbs) targeting TNFα, CD20, HER2, and CCR4 are now widely used for therapeutic purposes.Citation1–5) In particular, adalimumab and infliximab, which are mAbs targeting TNFα, are prescribed for treatment of patients with rheumatoid arthritis, and rituximab, trastuzumab, and mogamulizumab, which are mAbs targeting CD20, HER2, and CCR4, respectively, are used for treatment of patients with cancers. IgG consists of two heavy chains and two light chains. It is functionally divided into the antigen-binding fragment (Fab), which recognizes a specific antigen, and fragment crystallizable (Fc)-region, which modulates immune cell activities such as complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC).Citation6,7) The Fc-region contains two N-glycans attached to each heavy chain.Citation8) These N-glycans affect not only the stability of the protein, but also the therapeutic efficacy of the interaction with Fc-receptors expressed on natural killer cells and macrophages.Citation9,10) Previous studies showed that the N-glycan attached to Asn297 of human serum IgG consists of at least 31 different forms,Citation11) and therapeutic mAbs generally contain N-glycans with a variety of forms dependent on the host cell types and their culture conditions. However, recent studies have shown that the therapeutic potential of mAbs such as rituximab and trastuzumab relies on certain forms of the N-glycans attached to the Fc-region.Citation12,13) Therefore, several groups, including ourselves, have developed methods to produce mAbs whose N-glycans contain the homogeneous structures. For instance, Wang and his group used a chemoenzymatic method for remodeling the N-glycans attached to rituximab produced in Chinese hamster ovary (CHO) cells.Citation14) We used endo-β-N-acetylglucosaminidase S (Endo S) to remove N-glycans from recombinant anti-HER2 mAb produced in silkworm cocoon and to transfer N-glycans with A2, G2, G0, and M3 structures (Supplement 1) to produce a mAb having two N-glycans with homogeneous structures. By using these mAbs, it was shown that their structures influence their binding to a variant Fcγ receptor type IIIa (FcγRIIIa-V158) and their ADCC activities.Citation15)
We determined the structures of N-glycans attached to anti-HER2 mAb (trastuzumab) produced in CHO cells by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis, and showed that the N-glycans are G0F (42.0% of the total N-glycans), G1F (34.1%), G2F (5.4%), G1 (4.7%), G0 (4.4%), GN1F (2.9%), G2 (2.1%), M5 (1.9%), G1GN1F (1.6%), and GN1 (0.9%) (Supplement 2), indicating that most of them are core-fucosylated N-glycans whose partial chemical structures are shown in Supplement 3. mAbs having N-glycans without a core-fucose residue have a higher ADCC activity than those having N-glycans with a core-fucose residue,Citation16) and the removal of a core-fucose residue enhances an ADCC activity by increasing binding to FcγRIIIa.Citation17,18) Therefore, if we can produce mAbs having N-glycans without a core-fucose residue, such mAbs should have the highest ADCC activity and could be useful for cancer treatment with high efficacy. In order to prove that mAbs having N-glycans with a core-fucose residue might show a low ADCC activity, we first established the method to prepare anti-HER2 mAbs having two N-glycans with homogeneous core-fucosylated bi-antennary complex-type structures using commercially available anti-HER2 mAbs produced in CHO cells and by endo-β-N-acetylglucosaminidase activity together with transglycosylation activity of Endo S. In the purification steps, Endo CC was included to remove the mAbs having non-core-fucosylated N-glycans. Finally, we determined binding constants to FcγRIIIa-V158 and ADCC activities of these anti-HER2 mAbs.
Materials and methods
Chemicals
Hen egg yolk sialylglycopeptides and Endo CCCitation19) were obtained from Fushimi Pharmaceutical Co., Ltd. (Kagawa, Japan). Anti-HER2 mAb (trastuzumab; lot No. 13G020E) was obtained from Hoffmann-La Roche (Basel, Switzerland). Chitin resin, Remove-iT Endo D and β-galactosidase came from New England Biolabs (Ipswich, MA, USA).
Preparation of wild-type and mutant Endo
S. GST-tagged wild-type Endo S and its mutant enzyme (D233Q) were expressed in E. coli cells and purified as described.Citation14,20) GST-tagged wild-type Endo S was immobilized on NHS-activated SepharoseTM 4 Fast Flow beads (GE Healthcare Japan, Tokyo, Japan) according to the manufacturer’s instructions.
Preparation of oxazolinated glycans (G2-Oxa, G1a-Oxa, G1b-Oxa, and G0-Oxa)
G2-Oxa and G0-Oxa were prepared as described.Citation15) G1a-OH and G1b-OH were prepared from hen egg yolk sialylglycopeptides by treatments with mild acid, β-galactosidase, and Endo S. They were oxazolinated as described,Citation15,21) and their structures were determined by 1H-NMR analysis (data not shown).
Preparation of mAbs with core- and non-core-fucosylated N-glycans
Anti-HER2 mAb was digested with wild-type Endo S immobilized on the beads and with Remove-iT Endo D in 20 mM Tris-HCl buffer (pH 7.5) at 37 °C for 20 h. The Endo S and Endo D were removed from the reaction mixture by passage through a Micro Bio-Spin Empty Column (Bio-Rad, Hercules, CA, USA) and then purified by affinity chromatography using chitin resin. mAbs as glycan acceptors containing GlcNAcβ1→Asn, Fucα1→6GlcNAcβ1→Asn or both were isolated on Ab-Capcher ExTra beads (ProteNova, Kagawa, Japan), and incubated with oxazolinated glycans (G2-Oxa, G1a-Oxa, G1b-Oxa or G0-Oxa) as a glycan donor at a donor-to-acceptor ratio of 100:1 in the presence of GST-tagged Endo S (D233Q) as a transglycosidase in 50 mM Tris-HCl buffer (pH 7.0) at 37 °C for 1 h. The reaction mixture was passed through a column containing GST-Accept beads (Nacalai Tesque, Kyoto, Japan) to remove Endo S, and the mAbs were isolated on Ab-Capcher ExTra beads. To exclude the mAbs with non-core-fucosylated N-glycans, they were treated with Endo CC in 50 mM phosphate buffer (pH 6.0) at 37 °C for 24 h, and the mAbs were recovered on Ab-Capcher ExTra beads. Then the mAbs having two core-fucosylated N-glycan chains were separated from those having one N-glycan chain with the (±Fucα1→)GlcNAcβ1→Asn group and having two (±Fucα1→)GlcNAcβ1→Asn groups by high-performance liquid chromatography (HPLC) using a ProPacTM WCX-10 column (Thermo Fisher Scientific Inc., Waltham, MA, USA) and detected with Shimadzu HPLC system equipped with a UV detector (280 nm).Citation15,22) As standards, mAbs having two N-glycan chains, one N-glycan chain and one (±Fucα1→)GlcNAc group, and two (±Fucα1→)GlcNAc groups were prepared during the above process. The mAbs having non-core-fucosylated N-glycans with G2, G1a, G1b, and G0 structures were prepared as described.Citation15) Structures of N-glycans attached to the individual mAbs thus prepared were confirmed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using their glycopeptides as described.Citation15,23) A schematic illustration of preparation procedures as described above is shown in Supplement 4.
Surface plasmon resonance analysis
The interaction between the mAbs having N-glycans with defined homogeneous structures and recombinant human FcγRIIIa-V158 (Novoprotein, Summit, NJ, USA) was determined in 10 mM HEPES buffer (pH 7.4) containing 0.15 M NaCl, 3 mM EDTA, and 0.05% surfactant P 20 (HBS-EP buffer) by a single-cycle kinetic method in a Biacore X100 surface plasmon resonance (SPR) analyzer (GE Healthcare). Protein A was immobilized on a CM5 sensor chip according to the manufacturer’s instructions, and then mAb having N-glycans with each defined structure was immobilized on the chip by protein A. Recombinant human FcγRIIIa-V158 at 0.0016, 0.008, 0.04, 0.2, or 1.0 μM dissolved in HBS-EP buffer was loaded at a flow rate of 30 μl/min to allow interaction with the mAb for 120 s, and then replaced with HBS-EP buffer to dissociate the FcγRIIIa that interacted with the chip for 600 s. The dissociation constant (KD) of each mAb in the steady state was determined in Biacore X100 software.
Determination of ADCC activity of the mAbs
To examine the ADCC activity of the mAbs having N-glycans with each defined structure, we used an ADCC Reporter Bioassay Core Kit (Promega, Fitchburg, WI, USA) according to manufacturer’s instructions and the method published previously.Citation24) HER2-positive SK-BR-3 cells (5,000 cells) as a target and Jurkat/FcγRIIIa/NFAT-Luc cells as an effector mixed at the ratio of 1:15 were placed in a 96-well plate and incubated with different concentrations of the mAbs. After incubation at 37 °C for 20 h, Bio-Glo™ Luciferase Assay Reagent (Promega) was added and the mixture was incubated at room temperature for 5 min. The luminescence units were determined with a TriStar2 multidetection microplate reader (Berthold Technologies, Wildbach, Germany). In this system, ADCC activity was evaluated by determining elevation levels of relative luminescence derived from the NFAT-Luc reporter gene-inserted effector cells upon activation with mAbs added.
Results
Preparation of anti-HER2 mAbs having two N-glycan chains with a single core-fucosylated structure
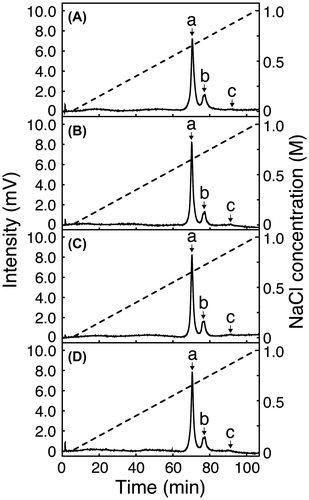
To examine the function of the core-fucose residue attached to N-glycans of mAbs, we prepared four types of mAbs having two N-glycan chains with G2F, G1aF, G1bF, and G0F structures, respectively (Fig. ). Since the mAbs produced in CHO cells contain N-glycans with a mixture of structures in terms of core-fucosylation, β4-galactosylation, β-N-acetylglucosaminylation, and α-mannosylation,Citation25) we first treated them with GST-tagged Endo S immobilized on beads and then with Endo D, which, unlike Endo S, hydrolyzes oligo-mannose-type glycans present in a small quantity.Citation15) The mAbs thus obtained contained two (±Fucα1→6)GlcNAcβ1→Asn groups. They were then incubated with an oxazolinated glycan-donor (G2-Oxa, G1a-Oxa, G1b-Oxa or G0-Oxa whose details are shown inCitation15)) in the presence of GST-tagged Endo S variant (D233Q), which can transfer effectively the glycan moiety to the GlcNAc residue of (±Fucα1→6)GlcNAc groups of the mAbs by transglycosylation.Citation14,15) Since transglycosylated mAbs contained core-fucosylated and non-core-fucosylated N-glycans together with untransglycosylated acceptors, their structures and amounts were determined by LC-MS/MS analysis. The results showed that these samples contain mAbs having core-fucosylated N-glycans with respective structures together with non-core-fucosylated N-glycans and (±Fucα1→6)GlcNAc group(s) by 8–12% (Fig. ). In order to eliminate those with non-core-fucosylated N-glycans and (±Fucα1→6)GlcNAc group(s), transglycosylated mAbs were treated with Endo CC that hydrolyzes non-core-fucosylated N-glycans,Citation19) and then subjected to chromatography using a ProPacTM WCX-10 column which can separate mAbs having two full N-glycan chains (peak a), one N-glycan chain and one (±Fucα1→6)GlcNAc group (peak b), and two (±Fucα1→6)GlcNAc groups (peak c) (Fig. ). Glycopeptides were obtained from mAbs included in peak a of each sample by trypsin digestion, and their N-glycan structures were confirmed by LC-MS/MS.Citation23) The results showed that peaks a in panels (A), (B), (C), and (D) of Fig. contain only N-glycans with G2F, G1aF, G1bF, and G0F structures, respectively. Using similar methods as above, mAbs having N-glycans with a G2, G1a, G1b, or G0 structure were prepared as controls described in detail.Citation15)
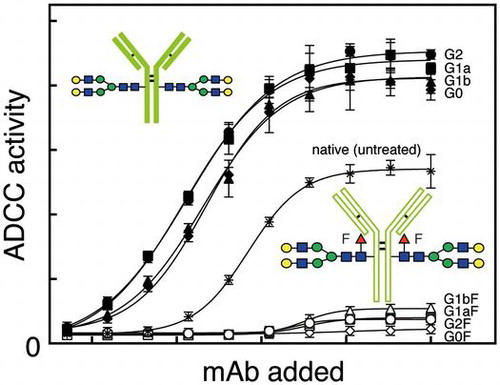
Fig. 1. Structures of bi-antennary complex-type glycans defined in the present study. Anti-HER2 mAbs contain core-fucosylated N-glycans with two galactose residues (G2F), one galactose residue on the Manα1→6Man arm (G1aF), one galactose residue on the Manα1→3Man arm (G1bF) or no galactose residue (G0F); and non-core-fucosylated N-glycans with two galactose residues (G2), one galactose residue on the Manα1→6Man arm (G1a), one galactose residue on the Manα1→3Man arm (G1b) or no galactose residue (G0). +Fuc, core-fucosylated and –Fuc, non-core-fucosylated.
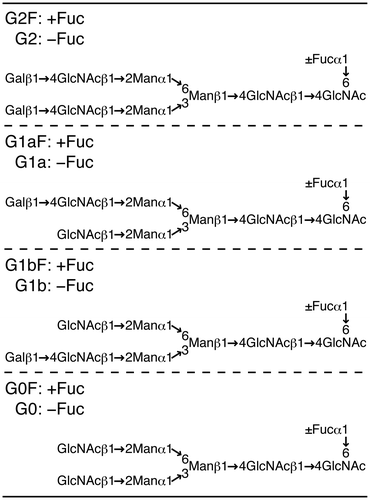
Fig. 2. Relative amounts of transglycosylated products and untransglycosylated acceptors in each mAb sample. Glycopeptides were obtained from mAbs transglycosylated by Endo S variant and their N-glycan structures were confirmed by LC-MS/MS.Citation23) Individual N-glycans are indicated with initials used in Fig. and (±Fucα1→)GlcNAc group(s) with asterisk.
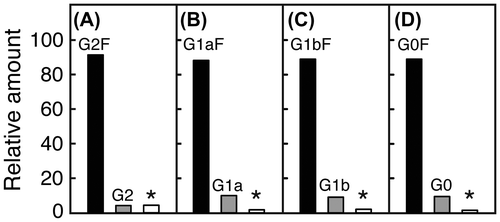
Fig. 3. Isolation of mAbs having two N-glycan chains by cation-exchange column chromatography. mAbs incubated with Endo S variant in the presence of G2-Oxa (A), G1a-Oxa (B), G1b-Oxa (C), or G0-Oxa (D) were digested with Endo CC followed by chromatography using a ProPacTM WCX-10 column to separate mAbs having two N-glycan chains (peak a), one N-glycan chain and one (±Fucα1→)GlcNAc group (peak b), and two (±Fucα1→)GlcNAc groups (peak c), which were prepared as standards. Dotted line indicates concentration of NaCl.
Binding of the mAbs to FcγRIIIa
FcγR interacts with the Fc-region of IgG and induces a variety of effects on target cells.Citation26) Among FcγRs, FcγRIIIa is expressed on the surface of natural killer cells and macrophages, and its interaction with the antibodies induces ADCC of the target cells.Citation27) The N-glycan attached to Asn297 in the IgG1-Fc-region affects the binding affinity of the Fc-region to FcγRIIIa;Citation26) in particular, the removal of a core-fucose residue from the N-glycans dramatically enhances the binding activity.Citation26,27)
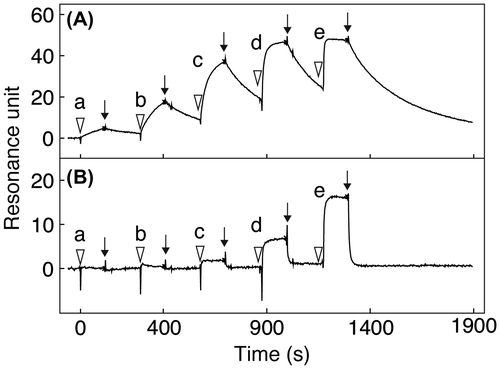
To investigate what degree of the effect that the core-fucosylated N-glycans attached to the mAbs have toward FcγR-binding, we incubated individual mAbs with recombinant human FcγRIIIa-V158 and analyzed their interactions by SPR measurement. The dissociation constants (KD) of individual mAbs in the steady state were determined by a single-cycle kinetic method. The KD values of the mAbs having N-glycans with a homogeneous G2F, G1aF, G1bF or G0F structure were between 3.69 × 10−7 M and 6.37 × 10−7 M, while those of the mAbs having N-glycans with a homogeneous G2, G1a, G1b or G0 structure were between 1.27 × 10−8 M and 2.45 × 10−8 M (Table ). Thus, the KD values of the mAbs having N-glycans with a homogeneous core-fucosylated structure bound to FcγRIIIa-V158 were 10 times higher than those of the mAbs having N-glycans with a homogeneous non-core-fucosylated structure, indicating that the binding of the mAbs with the core-fucosylated N-glycans to FcγRIIIa is attenuated by the presence of a core-fucose residue. When recombinant human FcγRIIIa-V158 of different concentrations (a, 0.0016; b, 0.008; c, 0.04; d, 0.2, and e, 1.0 μM, respectively) freed in solution were injected successively (indicated with each ∇) at 30 μl/min to allow interaction with the mAbs immobilized on the sensor surface for 120 s, followed by injection with plain running buffer (indicated with each ↓) at 30 μl/min to dissociate the complex for 600 s, the FcγRIIIa initially interacted with the sensor chip and was then released gradually from the chip containing mAbs having N-glycans with the G2 structure (Fig. (A)). In contrast, the FcγRIIIa bound was released immediately by injection with plain running buffer from the chip containing mAbs having N-glycans with the G2F structure (Fig. (B)). Almost identical results to the above (Fig. ) were obtained between mAbs having N-glycans with the G1a structure and those having N-glycans with the G1aF structure (Supplement 5A and 5B), mAbs having N-glycans with the G1b structure and those having N-glycans with the G1bF structure (Supplement 5C and 5D), mAbs having N-glycans with the G0 structure and those having N-glycans with the G0F structure (Supplement 5E and 5F), and untreated mAbs as a reference (Supplement 5G). These results indicate that mAbs having core-fucosylated N-glycans can only interact weakly with FcγR when compared with those having non-core-fucosylated N-glycans.
Table 1. Dissociation constants (KD) of mAbs having N-glycans with distinctive structures.
Fig. 4. Sensorgrams obtained by SPR measurement of the affinity between mAbs having N-glycans with the respective structures shown in Fig. and recombinant human FcγRIIIa-V158. (A) mAb having N-glycans with the G2 structure; (B) mAb having N-glycans with the G2F structure. Recombinant human FcγRIIIa-V158 of different concentrations (a, 0.0016; b, 0.008; c, 0.04; d, 0.2, and e, 1.0 μM, respectively) were injected (∇) at 30 μl/min to allow interaction with the mAbs for 120 s, followed by injection with plain running buffer (↓) at 30 μl/min to dissociate the complex for 600 s.
Effect of the core-fucose residue in N-glycans of the mAbs on ADCC activity
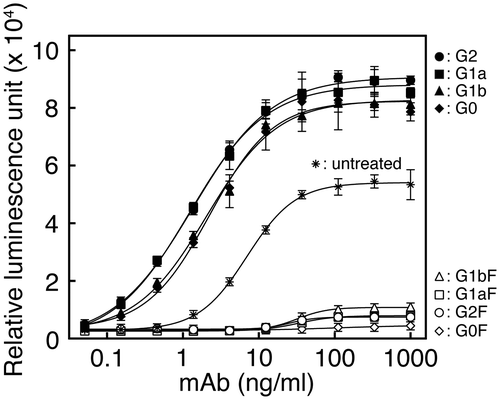
The mAb having N-glycans with the G2F structure (open circle) showed very low relative luminescence units (RLUs) at all concentrations of antibody tested, while that having N-glycans with the G2 structure (closed circle) as a control had higher RLUs which increased as more antibody was added (Fig. ). The mAbs having N-glycans with the G1aF (open square), G1bF (open triangle), or G0F (open diamond) structure showed consistently low RLUs (Fig. ). The mAbs having non-core-fucosylated N-glycans with the G1a (closed square), G1b (closed triangle), or G0 (closed diamond) structure as respective controls had similar or slightly lower RLUs than the mAb having N-glycans with the G2 structure (closed circle) (Fig. ). The mAbs untreated (indicated with asterisk) whose N-glycan composition is described in Supplement 2 showed moderate relative RLUs (Fig. ). These results indicate very weak ADCC activity of mAbs with the core-fucosylated N-glycans.
Discussion
IgG contains two bi-antennary N-glycans attached to each Asn297 of the heavy chain.Citation28) These N-glycans are considered to be involved in maintaining the three-dimensional structure of the Fc-portion of IgG.Citation29) In fact, modification of the N-glycans by digestion with several kinds of exoglycosidases or treatment with synthetic inhibitors caused changes in Fc-mediated functions such as C1q-binding, Fc-receptor-binding, and ADCC activity of IgG.Citation30–33) IgG molecules having N-glycans without a core-fucose residue produced in fucosyltransferase 8-deficient CHO cells had higher ADCC activity than those produced in parent CHO cells,Citation18) indicating that the presence or absence of a core-fucose residue attached to the N-glycan affects the ADCC activity of IgG. We produced anti-HER2 mAbs having two N-glycans with a non-core-fucosylated A2, G2, G0 or M3 structure (Supplement 1) by transglycosylation using mutated Endo S and examined their biological activities.Citation15) The results showed that the absence of a core-fucose residue in the N-glycans enhanced the FcγRIIIa-binding and ADCC activities of the mAbs when compared with controls whose N-glycans are mainly core-fucosylated.
It is well documented that the removal of the core-fucose residue from the N-glycans attached to IgG produced in CHO cells greatly enhances an ADCC activity.Citation18) However, such IgG molecules still contain N-glycans with heterogeneous forms in terms of sialylation, β4-galactosylation, N-acetylglucosaminylation, and mannosylation, and such differences in the glycan structures may also affect Fc-mediated functions. Here, we examined the biological activities of the core-fucose residue on the N-glycans with defined structures using anti-HER2 mAbs. Since the mAbs produced in CHO cells that we used as a starting material contained N-glycans without a core-fucose residue by 14% of the total glycans,Citation15) the mAbs were initially treated with Endo S and Endo D, and then transglycosylated with mutated Endo S using G2-Oxa, G1a-Oxa, G1b-Oxa, or G0-Oxa as a glycan-donor to obtain mAbs having N-glycans with single core-fucosylated structures shown in Fig. .Citation15) To exclude any mAbs having the non-core-fucosylated N-glycans, the mAbs were digested with Endo CC, which hydrolyzes N-glycans without a core-fucose residue.Citation19) This step is quite important for obtaining mAbs having two N-glycan chains with single core-fucosylated structures since mAbs having N-glycans with non-core-fucosylated structures were present in each sample by 4–10% before this treatment. The resultant mAbs having N-glycans with core-fucosylated structures lost activity of binding to FcγRIIIa and lost the ability to induce ADCC regardless of the rest of the N-glycan structures. Recently, α-2,6-sialylation of the core-fucosylated N-glycans attached to the rituximab IgG antibody has been shown to further reduce ADCC activity compared with non-sialylated and core-fucosylated N-glycans,Citation34) suggesting that sialylation of the N-glycan also affects the Fc-mediated biological functions. However, this is the first report to determine the biological activities of anti-HER2 mAbs having two N-glycans with a homogeneous core-fucosylated bi-antennary structure. In this context, Wang and his group prepared an anti-CD20 rituximab mAb having N-glycans with core-fucosylated structures by transglycosylation using mutated Endo F3, but did not determine ADCC activity of the mAb.Citation35) Since the specificity of Endo F3 is higher than that of Endo S and Endo M, our treatment with Endo CC here may not be required to produce mAbs having N-glycans with the core-fucosylated structures; however, this needs further study.
mAbs are now widely used for treatment of patients with cancers. A variety of mechanisms have been proposed to be involved in anti-tumor activities of mAbs. For examples, blocking of the binding of growth factors to their receptors at cell surfaces, shutdown of active cellular signals required for tumor growth, induction of apoptosis into cells with signals mediated by cross-linking of cell surface antigens, complement-dependent cytotoxicity, and ADCC. Therefore, not only medical scientists but also physicians pay attention to their high therapeutic potentials for cancer patients (reviewed in Citation36)).
Since commercially available therapeutic mAbs such as trastuzumab and rituximab contain N-glycans with a variety of forms including a core-fucose residue,Citation25,37) these mAbs are not effective to induce ADCC activity. Accordingly, mAbs for therapeutic purposes should have N-glycans without a core-fucose residue. To this end, fucosyltransferase 8-deficient CHO cells are proposed to be an ideal host cell to produce mAbs with high ADCC activity.Citation18)
Supplemental data
Supplemental data for this article can be accessed at https://doi.org/10.1080/09168451.2017.1394813.
Disclosure statement
No potential conflict of interest was reported by the authors.
Author contributions
Conceived and designed the study: WT, MK, AM, and TS. Performed the experiments: WT, MK, MM, and KO. Analyzed the data: WT, MK, AM, KF, and TS. Contributed materials: KT. Wrote the paper: WT, MK, MM, KO, KF, and TS.
Supplement_5.pdf
Download PDF (222.8 KB)Supplement_4.pdf
Download PDF (298.6 KB)Supplement_3.pdf
Download PDF (52.9 KB)Supplement_2.pdf
Download PDF (750 KB)Supplement_1.pdf
Download PDF (528.7 KB)Acknowledgments
We thank Dr. T. Kinoshita and Mr. M. Dozaki at Fushimi Pharmaceutical Co., Ltd., for their kind gifts of Endo CC and hen egg yolk sialylglycopeptides, respectively.
Notes
Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; CHO, Chinese hamster ovary; Endo, endo-β-N-acetylglucosaminidase; Fc, fragment crystallizable; FcγRIIIa, Fcγ receptor type IIIa; HPLC, high-performance liquid chromatography; IgG, immunoglobulin G; KD, dissociation constants; LC-MS/MS, liquid chromatography-tandem mass spectrometry; mAb, monoclonal antibody; MALDI-TOF MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; Oxa, oxazoline; RLU, relative luminescence unit; SPR, surface plasmon resonance.
References
- Knight DM, Trinh H, Le J, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993;30:1443–1453.10.1016/0161-5890(93)90106-L
- Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–445.
- Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992;89:4285–4289.10.1073/pnas.89.10.4285
- Ishii T, Ishida T, Utsunomiya A, et al. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult t-cell leukemia/lymphoma. Clin Cancer Res. 2010;16:1520–1531.10.1158/1078-0432.CCR-09-2697
- Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs. 2015;7:9–14.
- Jefferis R. Glycosylation of recombinant antibody therapeutics. Biotechnol Prog. 2005;21:11–16.
- Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47.10.1038/nri2206
- Huber R, Deisenhofer J, Colman PM, et al. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976;264:415–420.10.1038/264415a0
- Wormald MR, Rudd PM, Harvey DJ, et al. Variations in oligosaccharide-protein interactions in immunoglobulin G determine the site-specific glycosylation profiles and modulate the dynamic Motion of the Fc oligosaccharides. Biochemistry. 1997;36:1370–1380.10.1021/bi9621472
- Krapp S, Mimura Y, Jefferis R, et al. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol. 2003;325:979–989.10.1016/S0022-2836(02)01250-0
- Kobata A. The N-linked sugar chains of human immunoglobulin G: Their unique pattern, and their functional roles. Biochim Biophys Acta. 2008;1780:472–478.10.1016/j.bbagen.2007.06.012
- Umana P, Jean-Mairet J, Moudry R, et al. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol. 1999;17:176–180.
- Hodoniczky J, Zheng YZ, James DC. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol Prog. 2005;21:1644–1652.10.1021/bp050228w
- Huang W, Giddens J, Fan SQ, et al. Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions. J Am Chem Soc. 2012;134:12308–12318.10.1021/ja3051266
- Kurogochi M, Mori M, Osumi K, et al. Glycoengineered monoclonal antibodies with homogeneous glycan (M3, G0, G2, and A2) using a chemoenzymatic approach have different affinities for FcγRIIIa and variable antibody-dependent cellular cytotoxicity activities. PLoS One. 2015;10:e0132848.10.1371/journal.pone.0132848
- Shields RL, Lai J, Keck R, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740.10.1074/jbc.M202069200
- Shinkawa T, Nakamura K, Yamane N, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–3473.10.1074/jbc.M210665200
- Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004;87:614–622.10.1002/(ISSN)1097-0290
- Eshima Y, Higuchi Y, Kinoshita T, et al. Transglycosylation activity of glycosynthase mutants of Endo-β-N-acetylglucosaminidase from coprinopsis cinerea. PLoS One. 2015;10:e0132859.10.1371/journal.pone.0132859
- Collin M, Olsen A. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 2001;20:3046–3055.10.1093/emboj/20.12.3046
- Noguchi M, Tanaka T, Gyakushi H, et al. Efficient synthesis of sugar oxazolines from unprotected N-Acetyl-2-amino sugars by using chloroformamidinium reagent in water. J Org Chem. 2009;74:2210–2212.10.1021/jo8024708
- Wang S, Ionescu R, Peekhaus N, et al. Separation of post-translational modifications in monoclonal antibodies by exploiting subtle conformational changes under mildly acidic conditions. J Chromatogr A. 2010;1217:6496–6502.10.1016/j.chroma.2010.08.044
- Kurogochi M, Amano J. Relative quantitation of glycopeptides based on stable isotope labeling using MALDI-TOF MS. Molecules. 2014;19:9944–9961.10.3390/molecules19079944
- Parekh BS, Berger E, Sibley S, et al. Development and validation of an antibody-dependent cell-mediated cytotoxicity-reporter gene assay. mAbs. 2012;4:310–318.10.4161/mabs.19873
- Junttila TT, Parsons K, Olsson C, et al. Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer Res. 2010;70:4481–4489.10.1158/0008-5472.CAN-09-3704
- Fridman WH. Fc receptors and immunoglobulin binding factors. FASEB J. 1991;5:2684–2690.
- Iannello A, Ahmad A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev. 2005;24:487–499.10.1007/s10555-005-6192-2
- Arnold JN, Wormald MR, Sim RB, et al. The Impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50.10.1146/annurev.immunol.25.022106.141702
- Kobata A. Function and pathology of the sugar chains of human immunoglobulin G. Glycobiology. 1990;1:5–8.10.1093/glycob/1.1.5
- Koide N, Nose M, Muramatsu T. Recognition of IgG by Fc receptor and complement: Effects of glycosidase digestion. Biochem Biophys Res Commun. 1977;75:838–844.10.1016/0006-291X(77)91458-9
- Tsuchiya N, Endo T, Matsuta K, et al. Effects of galactose depletion from oligosaccharide chains on immunological activities of human IgG. J Rheumatol. 1989;16:285–290.
- Ferrara C, Grau S, Jager C, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci USA. 2011;108:12669–12674.10.1073/pnas.1108455108
- Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8:226–234.10.1038/nrd2804
- Li T, DiLillo DJ, Bournazos S, et al. Modulating IgG effector function by Fc glycan engineering. Proc Natl Acad Sci USA. 2017;114:3485–3490.10.1073/pnas.1702173114
- Giddens JP, Lomino JV, Amin MN, et al. Endo-F3 glycosynthase mutants enable chemoenzymatic synthesis of core-fucosylated triantennary complex type glycopeptides and glycoproteins. J Biol Chem. 2016;291:9356–9370.10.1074/jbc.M116.721597
- Weiner GJ. Monoclonal antibody mechanisms of action in cancer. Immunol Res. 2007;39:271–278.10.1007/s12026-007-0073-4
- Li C, Rossomando A, Wu SL, et al. Comparability analysis of anti-CD20 commercial (rituximab) and RNAi-mediated fucosylated antibodies by two LC-MS approaches. mAbs. 2013;5:565–575.10.4161/mabs.24814