Abstract
Two cDNAs encoding the minor laccase isozymes (Lac2 and Lac3) of Grifola frondosa were cloned, characterized, and expressed in Pichia pastoris. The recombinant Lac2 (rLac2) was stable at pH 6.0, whereas the recombinant Lac3 (rLac3) was stable in a broad pH range (pH 4.0–8.0). In addition, rLac2 and rLac3 showed the highest catalytic efficiency (kcat/Km) for 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid).
Laccase (EC 1.10.3.2) is a copper-containing ligninolytic enzyme that catalyzes the oxidation of a variety of phenolic compounds.Citation1,2) Fungal laccases have attracted much attention due to their high capacity for oxidizing lignin-related compounds, highly recalcitrant environmental pollutants, and synthetic dyes.Citation3–5) Therefore, they are highly interesting enzymes for various applications such as textile dye decolorization, bioremediation, and effluent detoxification.Citation6,7)
The edible white-rot basidiomycete Grifola frondosa (Dicks.: Fr.) S.F. Gray (maitake) produces several laccase isozymes. A major laccase isozyme (Lac1) of G. frondosa has been purified, characterized,Citation8) and sequenced (DDBJ accession number AB643664) in our laboratory. In order to learn more about the laccases of this basidiomycete, we aimed to characterize the minor laccase isozymes, however, it was difficult to obtain a sufficient amount of homogeneous samples. To overcome this problem, we attempted to prepare sufficient quantities for analysis by producing recombinant laccase isozymes in a heterologous host.Citation9)
In the present study, we describe the isolation and characterization of two cDNAs encoding the minor laccase isozymes (Lac2 and Lac3) of G. frondosa and their expression in the methylotrophic yeast Pichia pastoris. The recombinant proteins were purified, and their kinetic and physicochemical properties were determined.
The total RNA was extracted from 20-day-old G. frondosa vegetative mycelia grown in potato dextrose broth containing 1.5% yeast extract, 0.0002% CuSO4, and 0.006% 2,5-xylidine. The first strand cDNA was synthesized from DNaseI-treated total RNA using PrimeScript Reverse Transcriptase (Takara Bio, Otsu, Japan) with lock-docking oligo-dT primer.Citation10) The primers used in this study are listed in Table S1. A partial cDNA sequence of Lac2 and Lac3 cDNAs was obtained by PCR with the primers designed based on the conserved region sequences of other fungal laccases: Lac2-F and Lac2-R for Lac2, and Lac3-F and Lac3-R for Lac3. The PCR products were cloned and sequenced. To obtain the sequences of the 5′ and 3′ ends of Lac2 and Lac3 cDNAs, 5′ and 3′ rapid amplifications of the cDNA ends (RACEs) were performed using the SMART RACE cDNA Amplification Kit (Clontech, Mountain View, CA, USA) with gene-specific primers based on the above partial cDNA sequences of Lac2 and Lac3. The gene-specific primers used for 5′-RACE were Lac2–5′1 and Lac2–5′2 for Lac2, and Lac3–5′1 and Lac3–5′2 for Lac3. The gene-specific primers used for 3′-RACE were Lac2–3′1 and Lac2–3′2 for Lac2, and Lac3–3′1 and Lac3–3′2 for Lac3. The amplified DNA fragments from 5′- and 3′-RACEs were sequenced. The two cDNAs encoding the minor laccase isozymes (Lac2 and Lac3) of G. frondosa contained open reading frames of 1,557 and 1,551 bp, respectively (DDBJ accession number LC314148 and LC314444, respectively), and encoded proteins with 519 and 517 amino acids, respectively (Fig. S1). The amino acid sequence of Lac2 and Lac3 was 77.4% and 73.9% identical, respectively, to that of the Lac1 of G. frondosa and showed 84.9% sequence identity to each other. The first 21 amino acid residues of Lac2 and Lac3 were predicted to be the signal peptides for secretion. Furthermore, Lac2 and Lac3 contained 8 and 11 putative N-glycosylation sites (Asn-X-Ser/Thr), respectively. The consensus copper-binding domains were also found in both laccases of G. frondosa (Fig. S1).
The expression vectors pLac2 and pLac3 for the expression of Lac2 and Lac3 were constructed. The Lac2 and Lac3 cDNA coding regions, including their original signal peptide sequences, were amplified by PCR using the first strand cDNA as template with the primers pairs of Lac2-EcoRI and Lac2-SacII for Lac2, and Lac3-XhoI and Lac3-ApaI for Lac3. The amplified DNA fragments were digested with restriction enzymes and ligated to the P. pastoris expression vector pPICZA (Invitrogen, Carlsbad, CA, USA). The resulting expression vectors, pLac2 and pLac3, were verified by restriction enzyme analysis and sequencing. The pLac2 and pLac3 vectors were linearized with NsiI and BglII, respectively, and transformed into P. pastoris GS115 by electroporation using the Gene Pulser Xcell Electroporation System (Bio-Rad, Hercules, CA, USA) at 2.0 kV, 25 μF, and 200 Ω. The transformants were screened on YPDS (1% yeast extract, 2% peptone, 2% dextrose, 1 M sorbitol, 2% agar) plates containing Zeocin at a final concentration of 100 μg mL−1. Cultivation of P. pastoris transformants were carried out according to the instructions of EasySelect Pichia Expression Kit (Invitrogen). In this experiment, CuSO4 (0.2 mM) was added to the medium, as laccase is a copper containing enzyme.
Laccase activity was determined using 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) as a substrate unless otherwise stated. The assay mixture contained 1 mM ABTS, 100 mM sodium tartrate buffer (pH 2.0), and enzyme solution in a total volume of 1 mL. The oxidation of ABTS was monitored by following the increase in the absorbance at 420 nm (ε420 = 36,000 M−1 cm−1). One unit of enzyme was defined as the amount of enzyme required to oxidize 1 μmol of substrate per min at 30 °C. Protein concentration was determined by the Bradford method with bovine serum albumin (BSA) as the standard.Citation11)
For both transformants, the laccase activity was detected from the first day after inoculation and gradually increased until 19 days. Therefore, the recombinant laccases were purified from the 19-day growth culture fluid of each transformant. Purification steps of the recombinant laccases (rLac2 and rLac3) were shown in Table S2A and S2B. Enzyme purity was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Based on gel filtration and SDS-PAGE analyses, rLac2 and rLac3 were monomers with molecular masses of 93 and 96 kDa, respectively. After the removal of N-linked carbohydrates with PNGaseF (New England BioLabs, Beverly, MA, USA), their molecular masses were reduced to about 60 kDa (Fig. (A)). This result suggested that rLac2 and rLac3 were glycosylated with N-linked carbohydrates.
Fig. 1. Properties of purified rLac2 and rLac3.
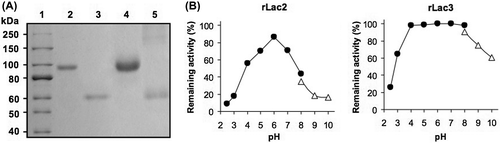
The effect of temperature on enzyme stability was investigated by measuring the remaining activity after incubating the purified laccases in buffer A containing 50 μg mL−1 BSA at various temperatures (30–90 °C). BSA was added to avoid adsorption of the enzyme to the tube. The rLac2 and rLac3 proteins were stable up to 30 and 40 °C, respectively. The effect of pH on the stability of each laccase isozyme was found to be distinctly different. As shown in Fig. (B), rLac2 was stable in the narrow pH range (around pH 6.0), whereas rLac3 was stable in a broad pH range (pH 4.0–8.0). The production of laccase isozymes having different pH stability profiles by a fungal strain has also been reported for other fungal laccases.Citation1,12)
To study the catalytic properties of rLac2 and rLac3, assays were performed using ABTS, 2,6-dimethoxyphenol (DMP), and guaiacol as substrates. As shown in Table , the optimum pH varied among the isozymes and substrates. For all isozymes, pH 2.0 (the lowest tested pH) was the optimum pH for ABTS. On the other hand, the optimum pH was higher for 2,6-DMP (pH 3.0 for rLac2 and pH 4.0 for rLac3) and guaiacol (pH 4.0 for rLac2 and pH 4.0 for rLac3). The optimum pH values of these laccases were in the acidic pH range, which are in agreement with those of other fungal laccases reported thus far.Citation1) The variation in the optimum pH for different substrates has also been reported for several fungal laccases in other studiesCitation1) and has been ascribed to varying degrees of substrate protonation under different pH conditions.
Table 1. Substrate specificity and apparent kinetic constants of Lac1, rLac2, and rLac3.
The kinetic parameters of each isozyme were determined at the optimum pH for each substrate (Table ). All isozymes had the highest catalytic efficiency (kcat/Km) for ABTS with the lowest Km and highest kcat values. The kcat/Km values of the three isozymes were higher for the dimethoxylated substrate (2,6-DMP) compared with the monomethoxylated substrate (guaiacol); a possible explanation for this is the strong electron-donating effect of the two methoxyl substituents and the favorable redox potential of 2,6-DMP.Citation13) Among the three isozymes, the rLac3 isozyme exhibited the lowest affinity (Km) for all substrates and the lowest turnover number (kcat) for the phenolic substrates (2,6-DMP and guaiacol); however, its kcat value for the nonphenolic substrate (ABTS) was the highest, suggesting each enzyme may have the different physiological function.Citation14)
In conclusion, we have cloned and characterized two cDNAs encoding the minor laccase isozymes of G. frondosa and successfully obtained sufficient amounts of recombinant laccase isozymes for characterization using the heterologous P. pastoris expression system. We are now conducting further studies on the decolorization of synthetic dyes and biodegradation of some recalcitrant environmental pollutants.
Author contributions
T.N., A.W., and Y.A. designed the experiments and discussed the results. T.N. and A.W. prepared manuscript.
Supplemental data
Supplemental data for this article can be accessed at https://doi.org/10.1080/09168451.2017.1394814.
Disclosure statement
No potential conflict of interest was reported by the authors.
Fig.S1_v2.doc
Download MS Word (392 KB)Table_S2_v2.doc
Download MS Word (208 KB)Table_S1_v2.doc
Download MS Word (253 KB)References
- Baldrian P. Fungal laccases–occurrence and properties. FEMS Microbiol Rev. 2006;30:215–242.10.1111/j.1574-4976.2005.00010.x
- Singh Arora D, Kumar Sharma R. Ligninolytic fungal laccases and their biotechnological applications. Appl Biochem Biotechnol. 2010;160:1760–1788.10.1007/s12010-009-8676-y
- Nitheranont T, Watanabe A, Suzuki T, et al. Decolorization of synthetic dyes and biodegradation of bisphenol a by laccase from the edible mushroom. Grifola frondosa. Biosci Biotechnol Biochem. 2011;75:1845–1847.10.1271/bbb.110329
- Daâssi D, Prieto A, Zouari-Mechichi H, et al. Degradation of bisphenol A by different fungal laccases and identification of its degradation products. Int Biodeter Biodegr. 2016;110:181–188.10.1016/j.ibiod.2016.03.017
- Zheng F, An Q, Meng G, et al. A novel laccase from white rot fungus Trametes orientalis: Purification, characterization, and application. Int J Biol Macromol. 2017;102:758–770.10.1016/j.ijbiomac.2017.04.089
- Rodríguez Couto S, Toca Herrera JL. Industrial and biotechnological applications of laccases: A review. Biotechnol Adv. 2006;24:500–513.10.1016/j.biotechadv.2006.04.003
- Viswanath B, Rajesh B, Janardhan A, et al. Fungal laccases and their applications in bioremediation. Enzyme Res. 2014;2014:1–21.10.1155/2014/163242
- Nitheranont T, Watanabe A, Asada Y. Extracellular laccase produced by an edible basidiomycetous mushroom, Grifola frondosa: Purification and characterization. Biosci Biotechnol Biochem. 2011;75:538–543.10.1271/bbb.100790
- Mate DM, Alcalde M. Laccase engineering: From rational design to directed evolution. Biotechnol Adv. 2015;33:25–40.10.1016/j.biotechadv.2014.12.007
- Borson ND, Salo WL, Drewes LR. A lock-docking oligo(dT) primer for 5′ and 3′ RACE PCR. PCR Methods Appl. 1992;2:144–148.10.1101/gr.2.2.144
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254.10.1016/0003-2697(76)90527-3
- Yuan X, Tian G, Zhao Y, et al. Biochemical characteristics of three laccase isoforms from the basidiomycete Pleurotus nebrodensis. Molecules. 2016;21:203.10.3390/molecules21020203
- Xu F, Shin W, Brown SH, et al. A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim Biophys Acta. 1996;1292:303–311.10.1016/0167-4838(95)00210-3
- Park M, Kim M, Kim S, et al. Differential expression of laccase genes in pleurotus ostreatus and biochemical characterization of laccase isozymes produced in Pichia pastoris. Mycobiology. 2015;43:280–287.10.5941/MYCO.2015.43.3.280
