Abstract
Alzheimer’s disease (AD) is the most common cause of dementia among elderly population. Deranged β-amyloid (Aβ) trafficking across the blood–brain barrier is known to be a critical element in the pathogenesis of AD. In the vascular endothelial cells of hippocampus, Aβ transport is mainly mediated by low-density lipoprotein-associated protein 1 (LRP1) and the receptor for advanced glycation end (RAGE) products; therefore, LRP1 and RAGE endothelial cells are potential therapeutic targets for AD. In this study, we explored the effects of Formononetin (FMN) on learning and memory improvement in APP/PS1 mice and the related mechanisms. We found that FMN significantly improved learning and memory ability by suppressing Aβ production from APP processing, RAGE-dependent inflammatory signaling and promoted LRP1-dependent cerebral Aβ clearance pathway. Moreover, FMN treatment alleviated ultrastructural changes in hippocampal vascular endothelial cells. In conclusion, we believe that FMN may be an efficacious and promising treatment for AD.
Formononetin ameliorates learning and memory impairment in mouse model of Alzheimer’s disease via suppressing inflammatory signaling and promoted Aβ clearance.
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that accounts for 60–80% of all cases of dementia. AD is diagnosed clinically by progressive deterioration of memory and is pathologically characterized by extracellular deposition of amyloid plaques (mainly accumulation of fibrillar β-amyloid [Aβ] peptide), and the neurofibrillary tangles containing hyper-phosphorylated tau [Citation1]. Aβ plays a critical pathogenic role in AD by inducing neuronal and synaptic dysfunction, microglial activation, and up-regulation of pro-inflammatory cytokines [Citation2]. Neuroinflammation is known to be an important element in the pathogenesis of AD [Citation3].
According to the AD neurovascular hypothesis, impaired clearance of Aβ leads to cognitive impairment and induces a cascade of cerebrovascular changes such as, structural changes in the hippocampal vascular endothelium, abnormal protein expression, Aβ accumulation, aggregation, deposition, and clearance abnormalities [Citation4,5]. Increased Aβ level in the brain caused by disruption of the balance between Aβ production and clearance causes neurotoxicity, vascular toxicity, hippocampal dysfunction and cell death [Citation6,7].
Aβ clearance from brain was shown to be mediated by low-density lipoprotein receptor-associated protein 1 (LRP1) [Citation8]. In vitro studies have shown that 7–90% Aβ clearance is mediated by LRP via interaction with its ligands such as apolipoprotein J (Apo J), apolipoprotein E (Apo E) and α2-macroglobulin (α2M) [Citation8–11]. Studies have also shown decreased expression of LRP1 in BBB of patients with AD, which results in dysfunctional Aβ clearance from brain [Citation10,12,13]. Moreover, in a mouse model of AD, LRP1 knock-out was shown to ameliorate cognitive deficit [Citation14–16].
Additionally, clearance of Aβ from brain was also shown to be mediated by receptors for advanced glycation end products (RAGE) [Citation17]. Several lines of evidence have shown the involvement of RAGE in the regulation of Aβ transport from the periphery into the brain [Citation18]. The interaction of Aβ and RAGE was shown to promote neurological inflammatory response by increasing expression of nuclear transcription factor-κB (NF-κB), TNF-α, and interleukin-6 (IL-6). Further, RAGE antagonist treatment was shown to inhibit interaction between Aβ and RAGE and to decrease Aβ level in the brain [Citation19,20].
Formononetin (FMN) extracted from red clover is an O-methylated isoflavonoid and a potent phytoestrogen (Figure (A)). In Chinese traditional medicine, red clover has been widely used in the treatment of post-stroke fatigue (PSF), in neurosurgical patients and in patients with diabetes mellitus [Citation21,22]. FMN has been claimed to have antioxidant and hypolipidemic effects. Studies have shown neuroprotective effects of FMN in rat models of ischemia/reperfusion injury and traumatic brain injury, which is ostensibly mediated via upregulation of the expressions of vascular endothelial growth factor [Citation23], glutathione peroxidase and platelet endothelial cell adhesion molecule, enhanced activity of superoxide dismutase, decreased expressions of TNF-6 and IL-6 and decreased activity of cyclooxygenase-2, thus inhibiting the inflammatory reaction and oxidative stress [Citation21,24,25].
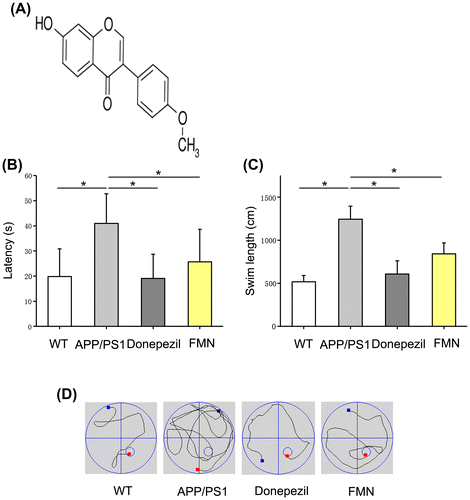
Figure 1. FMN treatment improved spatial learning and memory. APP/PS1 showed significant impairment in spatial learning and memory. Donepezil (positive control) ameliorated the learning and memory deficit. (A) Structure of FMN; (B, C) FMN increased escape latency (F = 4.746, p = 0.012) and swimming length (F = 37.796, p = 0.0001) of APP/PS1 mice measured in MWM; (D) Representative swimming traces of the mice recorded in the maze. Data are presented as mean ± SEM. *p < 0.05 (Bonferroni’s post hoc test for one-way ANOVA).
In this study, we sought to investigate the effects of FMN on memory and learning ability in a mouse model of AD (APP/PS1 mice). We found that FMN treatment promoted Aβ clearance through BBB and ameliorated inflammatory response to Aβ toxicity. Our findings suggest the mechanism of the neuroprotective effect of FMN in AD.
Materials and methods
Drugs, chemicals and kits
Anti-LRP1 (sc-25469), anti-ApoJ (sc-8354), anti-β-actin antibody (sc-47778) were obtained from Santa Cruz; anti-RAGE (Ba-1789) were purchased from Boster; anti-NF-κBp65 (Bs-0465R) antibody was purchased from Beijing Biosynthesis Biotechnology; RNeasy Mini kit was purchased from QIAGEN; PrimeScript RT kit was purchased from Takara Biotechnology (Dalian, China); Western semi-dry transfer film (P0021D), Western wash solution (P0023B), Western wash solution (P0023C), Western secondary antibody dilution (P0023D) was purchased from Beyotime (China). APP-FL, Aβ40, Aβ42, IL-1β, TNF-α, IL-6 ELISA kits were purchased from Nanjing Institute of Bioengineering. Donepezil hydrochloride tablets were purchased from China Chongqing Fuen Pharmaceutical Co., Ltd. The APP / PS1 mice (APPswe / PS1dE9 double transgenic mice) were obtained from Professor Wu Shuliang, Department of Anatomy, Harbin Medical University. The wild-type C57BL/6 mice were obtained from Beijing Vital River Laboratory Animal Surgery Ltd. All experimental animals were housed at suitable temperature and humidity in the Experimental Animal Center at the Heilongjiang University of Chinese Medicine.
Animals and experimental design
Thirty APP/PS1 mice with a C57BL/6J background, as described previously [Citation26], were assigned randomly to three groups (n = 6 for each): APP/PS1 group; positive control group (treated with 1 mg/kg/d Donepezil); and FMN (15 mg/kg/d) group. C57BL/6 mice (WT mice; n = 6) that received saline served as negative controls (WT). All mice underwent intragastric administration for 30 d. All animal experiments were approved by the Animal Care and Use Committee at the Heilongjiang University of Chinese Medicine.
Morris water maze test (MWM)
The water maze is a circular white vinyl chloride pool (100 cm diameter and 30 cm high) [Citation27]. This apparatus was setup to include four blind sides. The tank was filled with water to a height of 12 cm. The water temperature was maintained at 24 °C. The platform was located above the surface of the water at one quadrant of the water maze. To recall the location of the platform, the mice were tested 4 times a day with an interval of 5 min, for 5 consecutive days. On the first day, mice which could not find the platform were guided to the platform and allowed to remain there for 30 s. For mice that failed to find the platform for more than 1 min, the latency was recorded as 1 min. After 30 days treatment, the time to the blind side and the latency time required for the mouse to swim to the platform was recorded.
Elisa
Protein expressions in the hippocampal tissues were determined using ELISA kits according to the manufacturer’s instructions. For Aβ1-40, Aβ1-42, and APP measurement, the hippocampus of each mouse was separated, homogenized in RIPA buffer, and diluted with 1% protease inhibitor (04693116001, Roche). The levels of total Aβ1-40 and Aβ1-42 were quantified using Aβ ELISA kits. Expressions of cytokines (TNF-α, IL-1β, and IL-6) in the hippocampal tissues were also assessed using ELISA kits.
Western blot
Hippocampus tissue was homogenized in RIPA buffer and diluted with 1% protease inhibitor in ice and centrifuged at 13,000 ×g for 15 min. Equal amounts of the total protein (30 μg) were analyzed with SDS–PAGE gel electrophoresis. Subsequently, proteins on gel were transferred to PVDF membranes and blotted with specific primary antibodies. Proteins were detected after incubation with HRP-conjugated secondary antibodies and results were analyzed with high-definition color medical image analysis system.
RT-PCR determination of mRNA level
Trizol reagent was used for extraction of total RNA from fresh hippocampal tissues, and cDNA reverse transcription and PCR amplification were performed. Primer sequences were synthesized for LRP1 (forward 5′-CCG ACT GGC GAA CAA ATA CAC-3′ and reverse 5′-ATC GGC TTT GTT GCA CGT G-3′); ApoJ (forward 5′-TGA CCC CAT CAC AGT GGT GTT-3′ and reverse 5′-GCT TTT CCT GCG GTA TTC CTG-3′); RAGE (forward 5′-AAA ACG ACA ACC CAG GCG T-3′and reverse 5′-ATT CTC TGG CAT CTC CGC TTC-3′); NF-κBp65 (forward 5′-TGT GCG ACA AGG TGC AGA AA-3′, reverse 5′-ACA ATG GCC ACT TGC CGA T-3′); and β-actin (forward 5′-CGT GCG TGA CAT CAA AGA GAA -3′ and reverse 5′-AAC CGC TCG TTG CCA ATA GT-3′), from Shanghai Generay Biotech Co. Ltd (China). PCR reaction system parameters were as follows: 50 °C for 2 min followed by 95 °C for 10 min, 95 °C for 15 s, 60 °C for 1 min, for 40 cycles. Amplification products were analyzed by high-definition color medical image analysis system; the ΔΔCT method was used to calculate relative expression.
Transmission electron microscopy
Hippocampus was isolated and 500 μm thick transverse sections prepared. Ultra-thin sections were incubated with 20 μL of 3% uranyl acetate-alcohol saturated solution in a dish with dye liquor for 30 min; the sections were then washed with water 3 times for 10 min each, dried, incubated with 6% lead citrate dye for 5 min, washed with water 3 times (10 min each), and dried at room temperature. Transmission electron microscope (JEM-1200EX, Japan) was used to observe the ultrastructure of neurons.
Statistical analysis
The data are presented as mean ± standard deviation (SD). Statistical analysis was performed using SPSS 19.0 software. For comparison across multiple groups, Bonferroni’ s post hoc test for two-way ANOVA was used. The results were considered as statistically significant when p < 0.05.
Results
FMN ameliorates learning and memory deficit in APP/PS1 mice
To explore whether FMN leads to functional improvement, cognitive ability of mice was measured by the MWM. MWM analysis is a reliable behavioral test to determine learning ability and memory of animals and it has been widely used in studies on AD mouse models [Citation26]. Previous studies have shown that APP/PS1 mice have a high β-amyloid deposition in brain from 7 months of age onwards, especially in hippocampal and cortical area; therefore, we used 7-month-old APP/PS1 mice to receive the drug treatment. In this study, these mice were tested at 32 weeks of age when the cognitive impairment and pathological alterations were evident.
The results showed no significant preference for platform exhibited by vehicle treated APP/PS1 mice, while the positive control showed a significant improvement in spatial learning and memory with increased swimming length and decreased latency. Notably, the results of FMN treatment were similar to those observed with donepezil, and were characterized by improved learning and memory ability in APP/PS1 mice (Figure (B–D)). Overall, these results suggest that FMN exerts robust neuroprotective effects against pathological alterations and cognitive dysfunction in Alzheimer’s disease mouse model.
FMN reduces Aβ levels in the brain of APP/PS1 mice
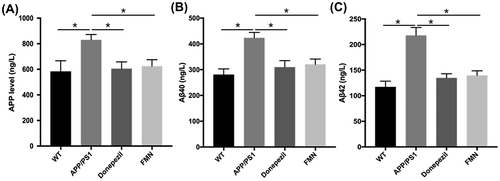
To elucidate the mechanism, whereby FMN treatment reduces Aβ production in APP/PS1 mice, the expression levels of Aβ1-40, Aβ1-42 full-length APP (FL-APP) were determined by ELISA. The results showed significant difference in the expressions of Aβ1-40 and Aβ1-42 full-length APP (FL-APP) between APP/PS1 mice with or without FMN treatment. Aβ is derived from proteolytic cleavage of APP by the amyloidogenic β-secretases and γ-secretases. The results suggest that FMN reduces Aβ burden by decreasing APP synthesis in the brain of mice and by inhibiting amyloidogenic cleavage of APP processing (Figure ).
Figure 2. FMN reduces brain Aβ production in APP/PS1 mice as measured by ELISA. (A) FMN treatment decreased levels of APP, F = 3.556, p = 0.033. (B) FMN treatment decreased levels of Aβ40, F = 7.355, p = 002. (C) FMN treatment decreased levels of Aβ42 in APP/PS1 mice, F = 15.068, p = 0001. Data are presented as mean ± SEM. *p < 0.05 (Bonferroni’s post hoc test for one-way ANOVA).
FMN promoted LRP1-dependent Aβ clearance pathway in the brain of APP/PS1 mice
Although significantly increased production of Aβ occurs in familial AD, several reports suggest that in patients with late-onset AD, Aβ accumulation and deposition is related to its impaired clearance from the brain. LRP1 is a large multi-functional receptor that regulates the endocytosis of diverse ligands and might also affect extracellular degradation of Aβ via modulation of the degrading enzymes. ApoJ-dependent Aβ clearance is another well-known pathway for cerebral Aβ removal via enhancement of Aβ phagocytosis by microglial cells and macrophages and other clearance pathways.
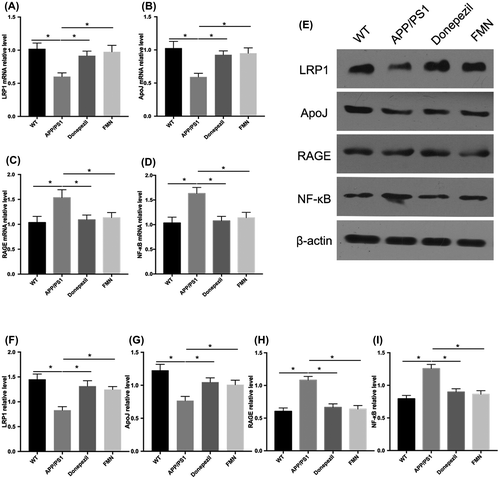
To identify the role of FMN in Aβ clearance, we examined the levels of LRP1 and ApoJ in the brain after 30 days treatment. Results of Western blot revealed that FMN increased LRP1 and ApoJ levels. Moreover, RT-PCR analysis also showed upregulated mRNA expressions of LRP1 and ApoJ (Figure (A–D)). These findings suggested that FMN may promote cerebral Aβ clearance by regulating LRP1 and ApoJ.
Figure 3. FMN promotes LRP1-ApoJ pathway, but inhibits RAGE-NF-κB pathway. Representative RT-PCR (A–D), Western blots (E) and quantitative results (F–I) show that FMN increased expression of LRP1and ApoJ pathway, but decreased expression of RAGE and NF-κBp65 in mouse brain homogenates (n = 6 for each genotype) at the age of 8 months. Data are presented as mean ± SEM. *p < 0.05 (Bonferroni’s post hoc test for one-way ANOVA).
FMN inhibited RAGE-NF-κB pathway in APP/PS1 mouse brain
RAGE is regarded as a multi-ligand receptor in the immunoglobulin family that is expressed at basal levels in a range of physiological cell types, and may have an impact on neurodegenerative diseases. Up-regulated RAGE expression was noted in the activated microglial cells and astrocytes. It has been suggested that the binding and interaction of RAGE to the ligand’s Aβ activates the cellular oxidative stress cascades and, subsequently, the NF-κB pathway. RAGE/NF-κB axis activation is detrimental to the neuronal activity and function in AD.
To further investigate the mechanism underlying FMN-mediated downregulation of amyloidosis, we analyzed the effects of FMN on the RAGE/NF-κB signal in vivo. Western blot and RT-PCR assays showed decreased protein expressions of RAGE and NF-κB p65 following FMN treatment (Figure (B) and (C)). These results suggest that FMN prevented the vicious cycle induced by RAGE/NF-κB signaling pathway, hence resulting in decreased oxidative stress injury, inflammatory response and neuronal cell death.
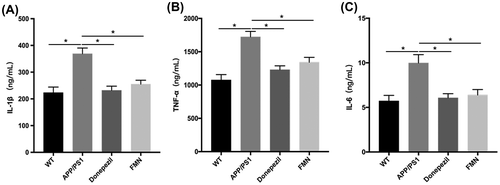
FMN reduces IL-6 and TNF-α levels in the brain of APP/PS1 mice
The accumulation of Aβ and activation of NF-κB in AD trigger expression of pro-inflammatory cytokines, which can cause neuronal toxicity. Aβ induced upregulation of IL-6 and TNF-α was shown to be associated with cognitive impairment and other neuronal dysfunction in AD [Citation27]. To investigate the anti-inflammatory and neuroprotective effects of FMN, the mRNA expressions of these pro-inflammatory cytokines were detected using real time PCR, and cytokines in the brain were measured using ELISA. Treatment with FMN induced a significant increase in IL-6 and TNF-α; consistent with FMN-induced reduction in protein level, decreased mRNA expressions of IL-6 and TNF-α were also observed (Figure ). These findings suggested that FMN could inhibit Aβ-induced neuroinflammation, and had a protective effect on Aβ-induced brain damage.
Figure 4. FMN reduces IL-1β, IL-6, and TNF-α levels in the brain of APP/PS1 mice. APP/PS1 showed significantly increased levels of IL-1β (A), TNF‑α (B) and IL-6 (C). FMN treatment decreased levels of IL-1β (F = 13.311, p < 0.0001), TNF‑α (F = 13.575, p < 0.0001), and IL-6 (F = 8.338, p = 0.001) in APP/PS1 mice, as measured by ELISA. Data are presented as mean ± SEM. *p < 0.05 (Bonferroni’s post hoc test for one-way ANOVA).
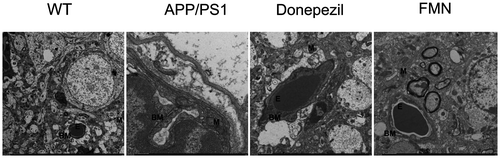
FMN protects hippocampus and BBB against Alzheimer’s like pathology
It has been reported that AD leads to diverse morphological changes in the hippocampus such as vascular impairment, with resulting cognitive symptoms. The effect of FMN on hippocampal structure was examined by transmission electron microscopy (TEM). In WT mice, the structure of neurons and capillary was regular with uniform chromatin and distinct cytoplasm outline. However, in APP/PS1 mice, the morphology of neurons was irregular and many vacuoles were observed. The capillary endothelial cells were intact with swollen mitochondria and few organelles. However, in FMN-treated group, the damage of neuronal and capillary morphology was milder than that in the AD model group. The structure of neurons and capillary endothelial cells was relatively preserved with a small amount of dissolved cytoplasm and some slight chromatin aggregates (Figure ). The results suggest that pretreatment with FMN protected the hippocampus in the CA1 region.
Figure 5. FMN protects hippocampus and BBB against Alzheimer’s like pathology. Pathomorphological changes of hippocampal structure in WT, APP/PS1, donepezil-treated, and FMN-treated mice were examined by transmission electron microscopy (×13000 magnification. BM, basement membrane; E, erythrocyte; m, mitochondrion).
Discussion
AD is one of the most common degenerative diseases which is characterized by the accumulation of Aβ and morphological changes of vascular endothelial cells in BBB [Citation28]. In the present study, we used APP/PS1 mice as experimental animals and found that FMN improved spatial learning and memory in AD mouse model by suppressing RAGE-dependent inflammatory signaling, and promoted LRP1-dependent cerebral Aβ clearance pathway in vivo.
Firstly, we determined the effects of FMN on cognitive ability of APP/PS1 mice. In the water Morris test, APP/PS1 mice showed significantly decreased latency and swimming distance as compared to that in the WT group (p < 0.05), which indicates impaired learning ability and memory of APP/PS1 mice. After 30 days of treatment with FMN, the latency and swimming distance were significantly improved (p < 0.05). These results suggest that FMN may reverse learning and memory deficit in APP/PS1 mice, which is consistent with previous reports that red clover improved learning and memory ability in AD rats.
Aβ accumulation and deposition are a pathological marker of AD and prevention of Aβ production and deposition is an important topic in AD research. Trafficking of Aβ from brain to the periphery across BBB is an important way to reduce Aβ in the nervous system. LRP1 in BBB serves a critical role in promoting Aβ trafficking from brain to the periphery [Citation29,30]. Our findings indicate that FMN could promote Aβ clearance by increasing expressions of LRP1 and ApoJ in the BBB. In the AD transgenic mice, up-regulation of LRP1 expression in the cerebrovascular endothelial cells increased the Aβ level and exacerbated the cognitive deficit. Moreover, the interaction of LRP1 and ApoJ significantly enhanced the rate of clearance of Aβ. The binding of ApoJ and Aβ promoted Aβ clearance by 83% in the hippocampus, which indicates that ApoJ is an important therapeutic target for treatment of AD.
Additionally, in AD, Aβ accumulation, and deposition induces significant inflammatory response which is associated with increased levels of TNF-α, IL-1β, and IL-6; further, anti-inflammatory drugs have been shown to significantly reduce the levels of cytokines and the risk of AD and improve cognitive symptoms [Citation31,32]. In our study, FMN treatment attenuated the inflammatory response in hippocampus of APP/ PS1 mice. RAGE in BBB promotes Aβ transport from the blood to the brain and RAGE activation can cause activation of NF-κB and increase expressions of IL-1β, TNF-α, and IL-6 [Citation8]. In the present study, FMN decreased RAGE expression in brain, which prevented activation of NF-κB as well as the consequent upregulation of cytokines, or directly decreased the release of cytokines. Our findings show that effective regulation of RAGE-NF-κB inflammatory pathway is particularly important for treatment of AD.
Neuropathology in the hippocampal CA3 region and related vascular changes were shown to be closely related to the learning and memory deficit in AD [Citation33]. In our study, TEM of hippocampal tissues of AD mice showed irregular capillary morphology and decreased number of mitochondria in the endothelial cells, which led to increased permeability of the BBB. Cerebral capillary structural abnormalities may result from Aβ toxicity and inflammatory response, which contribute to dysfunctional hippocampus and memory impairment. After FMN treatment for 30 days, the capillary morphology in the hippocampal CA3 region was more homogenous, and the endothelial cells were clearly visible with abundant cytoplasm and organelles; these findings indicate that FMN could improve capillary structure in hippocampal CA3 region of AD mice, which is probably associated with FMN-mediated reduction in expressions of cytokines and Aβ.
In conclusion, FMN has protective effects on APP/PS1 mice’s hippocampal vascular endothelial cells and its potential mechanism may include the regulation of LRP1-mediated pathway and down-regulation of RAGE-mediated pathway to remove Aβ. This study provides valuable insights that may inform potential interventions for prevention and treatment of AD.
Author contribution
HXF and YBZ designed the study. HXF, YBZ, TL, XJZ, and SLW collected and analysed the data. HXF, YBZ, and TL contributed samples collection and intellectual input. HXF, YBZ, and TL drafted and wrote the manuscript. XJZ and SLW revised the manuscript critically for intellectual content. All authors gave intellectual input to the study and approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Heilongjiang Qiqihar Medical College Doctor Scientific Research Fund [No. QY2016B-26], [No. QY2016B-21]; Medical College Fund [No. QY2016B-26]; Heilongjiang Qiqihar Technology Office Fund [No. SFGG-201630]; Heilongjiang Provincial Department of Education Project [No. 11521323], [No. 12531788]; and National Natural Science Foundation of China [No. 81373777], [No. 81173599].
Acknowledgments
We acknowledge Professor Shu-Liang Wu, Department of Anatomy, Harbin Medical University for kindly providing the APP/PS1 mice.
References
- Kugaevskaya EV. Angiotensin converting enzyme and Alzheimer’s disease. Biochem (Moscow) Suppl Series B: Biomed Chem. 2012;6(1):11–22.
- Zhang L, Ma Q, Yang W, et al. Recombinant DNA vaccine against neurite outgrowth inhibitors attenuates behavioral deficits and decreases Abeta in an Alzheimer’s disease mouse model. Neuropharmacology. 2013;70:200–210.10.1016/j.neuropharm.2012.10.023
- Bettens K, Sleegers K, Van Broeckhoven C. Genetic insights in Alzheimer’s disease. Lancet Neurol. 2013;12(1):92–104.10.1016/S1474-4422(12)70259-4
- Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28(4):202–208.10.1016/j.tins.2005.02.001
- Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer’s Abeta peptide: the many roads to perdition. Neuron. 2004;43(5):605–608.
- Zlokovic BV. Neurodegeneration and the neurovascular unit. Nat Med. 2010;16(12):1370–1371.10.1038/nm1210-1370
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–738.
- Deane R, Wu Z, Zlokovic BV. RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke. 2004;35(11_Suppl_1):2628–2631.10.1161/01.STR.0000143452.85382.d1
- Wang D, Di X, Fu L, et al. Analysis of serum beta-amyloid peptides, alpha2-macroglobulin, complement factor H, and clusterin levels in APP/PS1 transgenic mice during progression of Alzheimer’s disease. NeuroReport. 2016;27(15):1114–1119.10.1097/WNR.0000000000000661
- Donahue JE, Flaherty SL, Johanson CE, et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006;112(4):405–415.10.1007/s00401-006-0115-3
- Tokuda T, Calero M, Matsubara E, et al. Lipidation of apolipoprotein E influences its isoform-specific interaction with Alzheimer’s amyloid beta peptides. Biochem J. 2000;348(Pt 2):359–365.
- Qosa H, Abuasal BS, Romero IA, et al. Differences in amyloid-beta clearance across mouse and human blood-brain barrier models: kinetic analysis and mechanistic modeling. Neuropharmacology. 2014;79:668–678.10.1016/j.neuropharm.2014.01.023
- Qosa H, Mohamed LA, Al Rihani SB, et al. High-throughput screening for identification of blood-brain barrier integrity enhancers: a drug repurposing opportunity to rectify vascular amyloid toxicity. J Alzheimers Dis. 2016;53(4):1499–1516.10.3233/JAD-151179
- Kafa H, Wang JT, Rubio N, et al. Translocation of LRP1 targeted carbon nanotubes of different diameters across the blood-brain barrier in vitro and in vivo. J Control Release. 2016;225:217–229.10.1016/j.jconrel.2016.01.031
- Kanekiyo T, Cirrito JR, Liu CC, et al. Neuronal clearance of amyloid-beta by endocytic receptor LRP1. J Neurosci. 2013;33(49):19276–19283.10.1523/JNEUROSCI.3487-13.2013
- Zlokovic BV, Yamada S, Holtzman D, et al. Clearance of amyloid beta-peptide from brain: transport or metabolism? Nat Med. 2000;6(7):718–718.10.1038/77397
- Cai Z, Liu N, Wang C, et al. Role of RAGE in Alzheimer’s disease. Cell Mol Neurobiol. 2016;36(4):483–495.10.1007/s10571-015-0233-3
- Meneghini V, Bortolotto V, Francese MT, et al. High-mobility group box-1 protein and beta-amyloid oligomers promote neuronal differentiation of adult hippocampal neural progenitors via receptor for advanced glycation end products/nuclear factor-kappa B axis: relevance for Alzheimer’s disease. J Neurosci. 2013;33(14):6047–6059.10.1523/JNEUROSCI.2052-12.2013
- Tan X, Gu J, Zhao B, et al. Ginseng improves cognitive deficit via the RAGE/NF-kappaB pathway in advanced glycation end product-induced rats. J Ginseng Res. 2015;39(2):116–124.10.1016/j.jgr.2014.09.002
- Lee YS, Kim H, Kim YH, et al. Synthesis and structure-activity relationships of tri-substituted thiazoles as RAGE antagonists for the treatment of Alzheimer’s disease. Bioorg Med Chem Lett. 2012;22(24):7555–7561.10.1016/j.bmcl.2012.10.022
- Liu CH, Tsai CH, Li TC, et al. Effects of the traditional Chinese herb Astragalus membranaceus in patients with poststroke fatigue: A double-blind, randomized, controlled preliminary study. J Ethnopharmacol. 2016;194:954–962.10.1016/j.jep.2016.10.058
- Zhu H, Zou L, Tian J, et al. Protective effects of sulphonated formononetin in a rat model of cerebral ischemia and reperfusion injury. Planta Med. 2014;80(4):262–268.
- Liang K, Ye Y, Wang Y, et al. Formononetin mediates neuroprotection against cerebral ischemia/reperfusion in rats via downregulation of the Bax/Bcl-2 ratio and upregulation PI3 K/Akt signaling pathway. J Neurol Sci. 2014;344(1–2):100–104.10.1016/j.jns.2014.06.033
- Li WZ, Wu WY, Huang DK, et al. Protective effects of astragalosides on dexamethasone and Abeta25-35 induced learning and memory impairments due to decrease amyloid precursor protein expression in 12-month male rats. Food Chem Toxicol. 2012;50(6):1883–1890.10.1016/j.fct.2012.03.064
- Li Z, Dong X, Zhang J, et al. Formononetin protects TBI rats against neurological lesions and the underlying mechanism. J Neurol Sci. 2014;338(1–2):112–117.10.1016/j.jns.2013.12.027
- Wu J, Fu B, Lei H, et al. Gender differences of peripheral plasma and liver metabolic profiling in APP/PS1 transgenic AD mice. Neuroscience. 2016;332:160–169.10.1016/j.neuroscience.2016.06.049
- Jurgens HA, Amancherla K, Johnson RW. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J Neurosci. 2012;32(12):3958–3968.10.1523/JNEUROSCI.6389-11.2012
- Gupta V, Gupta VB, Chitranshi N, et al. One protein, multiple pathologies: multifaceted involvement of amyloid beta in neurodegenerative disorders of the brain and retina. Cell Mol Life Sci. 2016;73(22):4279–4297.10.1007/s00018-016-2295-x
- Storck SE, Meister S, Nahrath J, et al. Endothelial LRP1 transports amyloid-beta(1-42) across the blood-brain barrier. J Clin Invest. 2016;126(1):123–136.
- Kim DK, Park JD, Choi BS. Mercury-induced amyloid-beta (Abeta) accumulation in the brain is mediated by disruption of Abeta transport. J Toxicol Sci. 2014;39(4):625–635.10.2131/jts.39.625
- McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007;28(5):639–647.10.1016/j.neurobiolaging.2006.03.013
- Lehrer S. Nasal NSAIDs for Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2014;29(5):401–403.10.1177/1533317513518658
- Morin JP, Cerón-Solano G, Velázquez-Campos G, et al. Spatial memory impairment is associated with intraneural amyloid-beta immunoreactivity and dysfunctional arc expression in the hippocampal-CA3 region of a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2016;51(1):69–79.10.3233/JAD-150975
