ABSTRACT
We previously found a chemical, designated as NJ15, which inhibited both auxin and brassinosteroid responses in dark-grown Arabidopsis. To study its mode of action, we performed a phenotypic screening of NJ15-low-sensitive lines among mutant pools of Arabidopsis. One line (f127) showed clear NJ15-low-sensitivity in terms of hypocotyl elongation and shoot gravitropism. After further testing, it was determined that DCR, an enzyme involved in cutin polymerization, had lost its function in the mutant, which caused its low sensitivity to NJ15. Fatty acids are the base materials for polymers such as cutin and cuticular wax. We confirmed that NJ15 affects fatty acid biosynthesis, and that it does differently from cafenstrole, a known inhibitor of cuticular wax formation. Based on these results, we propose that the target of NJ15 is likely located within the cutin polymer formation pathway.
Abbreviations: Caf: cafenstrole; DEG: differentially expressed gene; FDR: false discovery rate; FOX: full length cDNA-overexpressor; VLCFA: very-long-chain fatty acid
Graphical Abstract
NJ15 inhibits auxin and brassinosteroid responses in dark-grown Arabidopsis. Its target was supposed to be within the cutin polymer formation pathway.
KEYWORDS:
Auxin and brassinosteroid are both phytohormones that have positive effects on cell elongation and regulate various developmental processes, such as tropism and environmental responses. Several interactions within their signal transduction pathways have been studied. Among these, the cross-regulation point between the two phytohormones at the transcriptional level has been extensively studied [Citation1,Citation2]. For example, an auxin transcriptional activator, auxin response factor 6 (ARF6), was reported to interact directly with the brassinosteroid signaling transcription factor BZR1 and the light/temperature-related transcription factor PIF4 to regulate hypocotyl elongation. The targets for ARF6 interaction overlapped with 51% and 71% of those of BZR1 and PIF4, respectively, indicating that their roles in gene induction are synergistically involved in hypocotyl elongation. However, although some reports in the past provided insights into the crosstalk between these two hormones, their common downstream signaling pathway(s) and interactions at the cellular level are not well understood due to the complexity of these systems.
To elucidate the common downstream pathways of auxin and brassinosteroid signaling, we attempted to find novel chemicals that inhibit both auxin and brassinosteroid signaling simultaneously[Citation3]. We finally obtained a compound, named NJ15, which showed inhibitory effects on the two hormones in Arabidopsis. Using plants with two gain-of-function mutations (bzr1-D and bes1-D) for brassinosteroid signaling, we could conclude that the target of NJ15 was probably downstream from the activities of the BRZ1 and BES1 transcription factors. Further, NJ15 showed an inhibitory effect on shoot negative gravitropism, which was similar to the effect of the auxin transport inhibitor N-1-naphthylphthalamic acid (NPA) except that NJ15 did not affect root gravitropism. Therefore, this chemical was concluded to be likely to inhibit any targets common between both the brassinosteroid and auxin signal transduction pathways.
In this study, we used a forward genetic approach by a screening of NJ15-low-sensitive mutant Arabidopsis plants to identify the target pathways of NJ15. In the end, a mutant line, f127, was obtained, and we showed that the low-sensitivity of f127 to NJ15 was due to loss-of-function of the Defective in Cuticular Ridges (DCR) enzyme. Transcriptome analysis showed that changes in the expression of NJ15-regulated genes were reduced globally in this mutant. The DCR mutation affected the biosynthesis of cutin, as well as the plants’ fatty acid composition. We also demonstrated that changes in fatty acid composition by cafenstrole, a fatty acid elongase inhibitor, attenuated NJ15 sensitivity. We showed that the low-sensitivity of f127 to NJ15 was caused by the loss-of-function of DCR, and thus the target of NJ15 should be located within the cutin polymerization pathway.
Material and methods
Chemicals
N-(1-naphthyl) phthalamic acid (NPA; Tokyo Chemical Industry Co.,Ltd. Tokyo, Japan), as well as brassinazole (Brz), cafenstrole (FUJIFILM Wako Pure Chemicals Ltd., Osaka, Japan) and Ethyl 2-[5-(3,5-dichlorophenyl)-1,2,3,4-tetrazole-2-yl]acetate (NJ15) (NAMIKI SHOJI Co.,Ltd. Tokyo, Japan) were dissolved in dimethyl sulfoxide (DMSO).
Plant materials
Arabidopsis thaliana ecotype Col-0 was used as the wild type (WT) plants in this study. Full-length cDNA over-expressed mutants (FOX-mutants) were developed by the Plant Genome Project of RIKEN PSC[Citation4]. Salk (SALK_128228c, termed dcr-2) T-DNA insertion line was obtained from the Arabidopsis Biological Resource Center. Homogenous lines were checked by using a pair of primers (salk128228_F and salk128228_R/LBb1.3). All primer sequences used in this study are shown in Table S1.
Screening of chemical-low-sensitivity mutants using FOX-mutant pool
Full-length cDNA over-expressed mutant (FOX-mutant) seeds were kindly provided by RIKEN Bioresource center. 414 seed pools (one seed pool contained approx. 400 seeds from 50 lines) were subjected to screening. These pools contained mixture of homozygous and heterozygous FOX-mutant seeds. Hygromycin was used for selection, but some mutants showed the gene silencing for surviving with Hygromycin. Approximately 50 surface-sterilized seeds per seed pool were plated on 1/2 MS medium (1.5% sucrose added) containing 10 μM NJ15. The plates were then incubated at 4°C for 3 days in the dark. After this, the plates were exposed to fluorescent light at 22°C for 3 h to stimulate germination before again being transferred back to the dark. Plants were then grown in a horizontal position at 22°C for 4 days in the dark. FOX-mutants, which showed negative gravitropic responses and long hypocotyls, were selected as candidates for further study. For dcr-3 (f127) mutant, homozygous lines were generated by PCR-based genotyping using primers gDNA-dcr_F, gDNA-dcr_R, and LB100.
In planta assay (1, hypocotyl elongation): Surface-sterilized seeds were plated on 1/2 MS medium containing 0.1% DMSO (mock substance) or a specific concentration of chemicals indicated in each experiment. The plates were then incubated at 4°C for 2 days in the dark. For a photomorphogenesis assay, seedlings were placed vertically at 22°C for 4 days, or for 5 days in the dark after cold treatment at 4°C. Hypocotyls of 12–20 seedlings were photographed, and their lengths were measured using ImageJ software (National Institutes of Health; http://rsb.info.nih.gov/ij). All experiments were repeated three times.
In planta assay (2, shoot gravitropism): Surface- sterilized seeds were plated on 1/2 MS medium containing 0.1% DMSO, 5 μM NPA, 0.3 μM Brz, or a specific concentration of chemicals indicated in each experiment, and then stratified at 4°C for 2 days. Plants were then grown in a vertical position at 22°C for 4 days in the dark. These plates were then turned 90°, and the seedlings grew in their new horizontal orientation for 24 h in the dark. Using ImageJ, the angle of shoot growth was measured relative to the horizontal plane. These experiments were independently repeated three times.
Quantitative real-time PCR
Total RNA was isolated from seedlings using a Total RNA Extraction Kit Mini for Plant (RBC Bioscience, New Taipei City, Taiwan). Reverse transcription was carried out on DNase-treated RNA using a PrimeScriptTM RT reagent Kit with gDNA Eraser (Perfect Real Time; TaKaRa Bio Inc., Shiga, Japan) according to the manufacturer’s instructions. For quantitative polymerase chain reaction (PCR), reactions containing the diluted cDNA were prepared using a KAPA SYBR® Fast One-Step Master mix (Kapa Biosystems). qRT-PCR was conducted in a Thermal Cycler DiceTM Real Time System (TP800; TaKaRa Bio Inc.). PCR conditions were 3 min at 95°C, then 40 cycles of 3 s at 95°C, and 30 s at 60°C. Reactions were carried out in triplicate for each biological sample. Raw values were normalized relative to the amount of ACT7, an internal reference standard.
Thermal asymmetric interlaced-polymerase chain reaction (TAIL-PCR)
To map genomic sequences flanking regions of inserted T-DNA in FOX127 mutants, a modified TAIL-PCR method[Citation5] was used. Genomic DNA was extracted using a Genomic DNA Extraction Kit Mini (Plant) (RBC Bioscience, New Taipei City, Taiwan). The primer pairs used in the TAIL-PCR method are shown in Table S1. Primary TAIL-PCR was done by utilization of a T-DNA-specific primer (LB1) as the first nested primer, and then using arbitrary degenerate (AD) primers AD1, AD2, AD3, AD5, and AD6, respectively. The primary amplification (TAIL1) method was as follows: (1) 93°C for 1 min, 95°C for 1 min; (2) 4 cycles of 94°C for 45 s, 62°C for 1 min, 72°C for 2.5 min; (3) one cycle of 94°C for 45 s, 25°C for 3 s; (4) ramped up to 72°C within 3 min and held at 72°C for 2.5 min; (5) 14 cycles of 94°C for 20 s, 68°C for 1 min, 72°C for 2.5 min, 94°C for 20 s, 68°C for 1 min, 72°C for 2.5 min, 94°C for 20 s, 44°C for 1 min, 72°C for 2.5 min; and then (6) 72°C for 5 min.
PCR products from TAIL1 were subjected to secondary amplification with the same AD primers and a T-DNA-specific second nested primer (LB2). The secondary amplification (TAIL2) method was as follows: (1) 11 cycles of 94°C for 20 s, 64°C for 1 min, 72°C for 2.5 min, 94°C for 20 s, 64°C for 1 min, 72°C for 2.5 min, 94°C for 20 s, 44°C for 1 min, 72°C for 2.5 min; and then (2) 72°C for 5 min. After TAIL2, PCR products were examined with gel electrophoresis on 1% (w/w) agarose gel (Nacalai Tesque, Japan). AD1 primers that yielded different DNA bands of sizes between those of WT Arabidopsis and FOX mutant samples were selected and used in tertiary and quaternary amplifications. In the tertiary amplification (TAIL3), the TAIL2 PCR product was amplified using a selected AD1 primer and a T-DNA-specific third nested primer (LB-180) with the following PCR method: (1) 20 cycles of 94°C for 38 s, 44°C for 1 min, 72°C for 2.5 min; and then (2) 72°C for 5 min. The TAIL3 PCR products were then separated by gel electrophoresis on 1% (w/w) agarose gel. DNA fragments were purified from the gel using the HiYield™ Gel/PCR DNA Fragments Extraction Kit (RBC Bioscience, New Taipei City, Taiwan). For the quaternary amplification (TAIL4), each DNA fragment was used as the template for 4 PCR reactions using different primers: (1) PCR components with only the AD1 primer; (2) PCR components with only the third nested primer (LB-180); (3) PCR components with an AD1 and LB-180 primer pair; and (4) PCR components with an AD1 and fourth nested primer (LB-100) pair. The amplification profile was set following the standard PCR cycling conditions for TaKaRa ExTaq® (TAKARA BIO, Japan). PCR products from TAIL4 were examined again with gel electrophoresis on 1% (w/w) agarose gel. DNA fragments, which were detected only from quaternary reactions 3 and 4, were purified and sequenced with the primer LB-100. Sequences were then subjected to a nucleotide-nucleotide BLAST search in the TAIR database (https://www.arabidopsis.org/Blast/).
Cuticular permeability assays by toluidine-blue (TB) test
Hypocotyls were stained using toluidine-blue as described by Tanaka et al [Citation6]. with little modification. A solution of 0.03% (w/v) TB (Wako Pure Chemical Industries, Japan) was applied directly onto etiolated seedlings that had grown on the 1/2 MS medium plate until the seedlings were submerged. After 5 min, the TB solution was removed, and seedlings were rinsed gently with water before imaging.
Determination of total fatty acid profiles
Total fatty acid analysis was carried out based on a direct acid-catalyzed transmethylation protocol[Citation7]. First, 30–50 mg fresh weigh of 5-day-old etiolated seedlings was catalyzed, and then methyl ester derivatives of the total fatty acids (FAMEs) were extracted and analyzed by gas chromatography coupled to mass spectrometry (GC-MS) (Shimadzu, Japan) in a DB-1 capillary column (15 m x 0.25 mm x 0.25 μm film thickness) with helium as the carrier gas. The initial temperature of 50°C was held for 2 min. The column temperature was programmed to increase from 50°C to 200°C at 40°C min−1 and then held at 200°C for 1 min, then was raised from 200°C to 320°C at 3°C min−1, and finally held for 10 min at 320°C. Quantification of total fatty acids was based on flame ionization detector peak areas and the internal standard (margaric acid). The total amount of fatty acid was calculated per mg fresh weight of seedlings. Significant differences were tested for with a Student’s t-test. Fatty acids were identified based on their fragmentation patterns (Table S2).
Rna-seq and statistical analyses
For transcriptome analysis by RNA-seq, WT and dcr-3 (f127) plants were grown vertically as a shoot gravitropism response assay for 4 days on 1/2 MS plates in dark conditions at 22°C. Then, plants were sprayed with either mock (DMSO) or 10 μM NJ15, and then gravistimulated by turning them 90° relative to their initial orientation. After 3 h, plants were harvested and total RNA was extracted with a Total RNA Extraction Kit Mini for Plant (RBC Bioscience).
For RNA seq analysis, the quality of total RNA was evaluated using an Agilent 2100 Bioanalyzer (Agilent, USA). From each sample, 2 μg of total RNA was used to construct separate libraries using the TruSeq RNA and TruSeq DNA Sample Prep kits, according to the manufacturer’s instructions (Illumina, USA). The quality of each library was assessed using an Agilent 2100 Bioanalyzer (Agilent) and then sequenced using the Illumina HiSeqTM 2500 Sequencer (paired-end sequencing, 100 bp). The data sets from this study have been deposited in the DDBJ database (accession numbers: DRA006685 for WT NJ15 and mock substance, DRA006686 for dcr-3 (f127)). The experiment was performed three times for both WT and dcr-3 mutants using several independently prepared Arabidopsis seedlings. Differential gene expression analysis was performed using the R package EdgeR version 3.18.1[Citation8]. Benjamin-Hochberg false discovery rate (FDR) were calculated to identify significantly upregulated and downregulated genes in the NJ15-treated WT (WT-NJ15) plants versus those in the WT-mock [WT-NJ15/WT-mock], [(dcr-3)-NJ15/(dcr-3)-mock], and [(dcr-3)-mock/WT-mock] plants. Gene-annotation enrichment analysis of DEGs was performed using the R package goProfiles based on Gene Ontology (GO) annotation from the TAIR database.
Results
F127 is an nj15-low-sensitive mutant
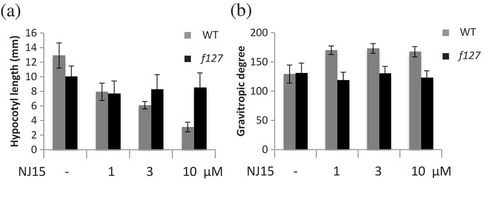
Among Arabidopsis seedlings grown in the dark, NJ15 clearly repressed elongation and negative gravitropism of their hypocotyls (Fig. S1). To elucidate the target(s) of this chemical’s actions, we performed a screening to identify mutants with NJ15-low-sensitivity from the full-length cDNA-overexpressor (FOX) Arabidopsis library[Citation4]. For this screening, seedlings were grown in the dark horizontally on 1/2 MS medium containing NJ15. Most of these seedlings showed inhibition of both the hypocotyl elongation and negative gravitropism of seedlings in the presence of 10 µM NJ15. Out of 20,496 lines, a line named f127 showed distinct low-sensitivity to NJ15 ().
Figure 1. f127 showed lower sensitivity to NJ15 than WT Arabidopsis.
Seeds of WT and f127 plants were grown vertically in 1/2 MS medium containing NJ15 at the concentrations indicated in the figure. After four days, these plates were turned 90° from their initial orientation and allowed to grow for one day. Then, (a) hypocotyl length and (b) gravitropic response of the shoots were measured. Data are means ± SD (n = 12). Experiments were repeated three times independently, and similar results were obtained.
In our previous report[Citation3], we showed that the brassinosteroid biosynthesis inhibitor brassinazole (Brz) decreased the hypocotyl length but not the gravitropic response of etiolated seedlings. Conversely, treatment with the auxin transport inhibitor NPA inhibited only the gravitropic response of etiolated seedlings, but not their hypocotyl length. To confirm the sensitivity of f127 to these known auxin or brassinosteroid inhibitors, the inhibitory effect of Brz or NPA on f127 was examined (Fig. S2b-c). Similar to results observed for WT plants, Brz and NPA inhibited hypocotyl elongation and gravitropism of f127. These results indicated that the low-sensitivity of f127 to NJ15 might be due to an unknown mechanism, different from those in known brassinosteroid- or auxin-signaling mutants.
NJ15-low-sensitivity of f127 is caused by loss-of-function of DCR
To confirm whether the low-sensitivity observed in f127 is due to over-expression of a gene, identification of inserted genes with T-DNA was carried out. Full-length cDNA of the At4g11100 gene, which has no known annotation, was inserted in f127. However, the At4g11100-overexpressed transgenic lines we generated did not show any low-sensitivity to NJ15 (Fig. S3a-c). To study this further, the flanking region of T-DNA inserted in f127 was investigated by thermal asymmetric interlaced-PCR (TAIL-PCR)[Citation5]. The insertion of T-DNA in f127 was verified to be inserted in the Defective in Cuticular Ridges (DCR) gene (At5g23940) (located 2,947 bp downstream of ATG) (). DCR encodes an HXXXD-type acyl-transferase family protein that localizes in the cytoplasm[Citation9], and there is a report that this enzyme has diacylglycerol acyltransferase activity in vitro[Citation10]. Although DCR has been hypothesized to contribute to cutin polymerization[Citation9], its function in vivo is still unclear. Sequence analysis showed that the insertion in f127 was located in the second exon of DCR. Therefore, DCR expression might be altered in this mutant. Quantitative PCR showed that although the expression of At4g11100 was increased in f127, DCR expression was strikingly decreased (). To ascertain whether the low-sensitivity of f127 to NJ15 resulted from deficiencies of the DCR gene, the previously reported DCR deficient mutant (dcr-2) [Citation9] was assessed. Mutant dcr-2 plants raised in the dark showed low-sensitivity to NJ15, similar to the f127 mutants (Fig. S3d-e). From the above-mentioned report[Citation9], the insertion of T-DNA in dcr-2 was located in the second exon (2,904 bp downstream of ATG), and this mutant also has reduced DCR transcription levels. Although At4g11100 gene was overexpressed in f127, the phenotype of f127 was very similar to dcr-2. Thus, f127 has a new allele for the dcr mutation, and we renamed it dcr-3.
Figure 2. Low-sensitivity of f127 to NJ15 is caused by loss-of-function of DCR.
(a) With TAIL-PCR analysis of f127 mutants, insertion of T-DNA was detected in the second exon of the DCR gene. (b) Expression analysis of DCR (left) and At4g11100 (right). (c) & (d) Cuticle permeability stained with toluidine blue-O (TB). Etiolated seedlings (c) grown in the negative control, and (d) in the medium containing NJ15 (10 µM) were used. Staining was repeated three times with similar results. Scale bars are 5 mm.
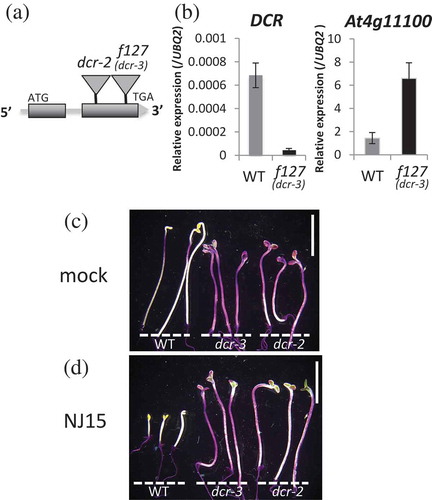
As a known DCR-deficient mutant was reported to show increased permeability of the cuticle, the cuticle permeability of etiolated dcr-3 was visualized by toluidine blue (TB) staining[Citation6]. Consistent with the previous report[Citation9]), dcr-3 displayed stained hypocotyls, which indicated the presence of epidermal cuticle defects in this mutant (-). To examine the effect of NJ15 on cuticle permeability, WT and dcr-3 seedlings were grown on media containing NJ15 in the dark and subjected to TB staining. However, NJ15 showed no effect on the cuticle permeability of both plant types (). This suggested that NJ15 did not negatively affect cuticle permeability, nor did it restore the impaired cuticle. Taken together, these results led us to assume that defects in DCR might cause low-sensitivity to NJ15 indirectly due to lack of cuticle integrity via some unknown mechanism.
Transcriptome analysis with NJ15 application and dcr-3 (f127)
To clarify how a deficiency in the DCR gene could affect the sensitivity of plants to NJ15, we performed transcriptome analysis with dcr-3 mutant exposed to NJ15. Four-day-old etiolated seedlings of the dcr-3 and WT plants were treated with 10 μM NJ15 or a mock solvent (DMSO). Plants were then stimulated gravitropically by turning them 90° relative to their initial plane of orientation for 3 h before harvesting them. A total of 334,201,047 reads were generated from 12 samples using the Illumina HiSeqTM 2500 Sequencer. For data analysis, the transcript levels were calculated and normalized to RPKM (reads per kilobase of transcript per million mapped reads), which were then used to identify differentially expressed genes (DEGs) between NJ15-treated WT (WT-NJ15) vs. WT-mock [WT-NJ15/WT-mock] plants; between mock-treated dcr-3 vs. mock-treated WT [(dcr-3)-mock/WT-mock] plants; and between NJ15-treated dcr-3 vs. mock-treated dcr-3 [(dcr-3)-NJ15/(dcr-3)-mock] plants. DEGs were identified by calculating Benjamin-Hochberg false discovery rate (FDR) of each gene, and filtered if they had FDR ≦ 0.05. Comparative analysis of DEGs between the treatments mentioned above was conducted. As a result, there were 5,695 genes differentially expressed in mock-treated dcr-3 vs. mock-treated WT. Among these, 139 genes were also differentially expressed in NJ15-treated WT vs. mock-treated WT, and did not respond to NJ15 treatment. Furthermore, almost half of the DEGs in NJ15-treated WT vs. mock-treated WT were shared with those in mock-treated dcr-3 vs. mock-treated WT. Also, there were only 106 genes differentially expressed between NJ15-treated dcr-3 and mock-treated dcr-3. These results indicated that NJ15-regulated gene expression changes are reduced globally in dcr-3 mutants ().
Figure 3. The numbers of NJ15-responsive genes are decreased in dcr-3.
(a) The Venn diagram shows the overlap between the sets of differently expressed genes (DEGs) in NJ15-treated WT (WT-NJ15) vs. those in WT-mock [WT-NJ15/WT-mock], [(dcr-3)-NJ15/(dcr-3)-mock], and [(dcr-3)-mock/WT-mock] plants. Four-day-old seedlings grown in the dark were treated with 10 μM of NJ15 or mock substance (DMSO). Then, they were subjected to gravitropic stimulus for 3 h before RNA extraction. (b) Comparison of Gene Ontology (GO) profiles of upregulated and downregulated DEGs in a set of common 139 genes between WT-NJ15/WT-mock and (dcr-3)-NJ15/(dcr-3)-mock plants. Terms were adopted at the second level of biological process ontology. Y-axis bar is the frequency of each enriched GO term.
![Figure 3. The numbers of NJ15-responsive genes are decreased in dcr-3.(a) The Venn diagram shows the overlap between the sets of differently expressed genes (DEGs) in NJ15-treated WT (WT-NJ15) vs. those in WT-mock [WT-NJ15/WT-mock], [(dcr-3)-NJ15/(dcr-3)-mock], and [(dcr-3)-mock/WT-mock] plants. Four-day-old seedlings grown in the dark were treated with 10 μM of NJ15 or mock substance (DMSO). Then, they were subjected to gravitropic stimulus for 3 h before RNA extraction. (b) Comparison of Gene Ontology (GO) profiles of upregulated and downregulated DEGs in a set of common 139 genes between WT-NJ15/WT-mock and (dcr-3)-NJ15/(dcr-3)-mock plants. Terms were adopted at the second level of biological process ontology. Y-axis bar is the frequency of each enriched GO term.](/cms/asset/65ef8fd1-c978-4837-ad9c-8ae9d92f7ba2/tbbb_a_1484278_f0003_b.gif)
We then focused on the 139 genes shared between NJ15-treated WT vs. mock-treated WT and between mock-treated dcr-3 vs. mock-treated WT, because we assumed that the expression of genes related to NJ15-low-sensitivity might be intrinsically altered in dcr-3 and therefore it should not be altered in a big way by the application of NJ15 in dcr-3, whereas it should be clearly responsive to NJ15 in WT. The identities of the top 100 common DEGs are shown in Table S3. The functions of these common genes were characterized by gene ontology (GO) analysis (Fig. S4a-c). Among biological process classes, most of the enriched GO terms of common genes were in the cellular processes, single-organism processes and response to stimuli categories (). The mutant dcr-3 was reported to be impaired in cuticle formation. Therefore, to identify more DEGs related to cuticle formation, we assigned DEGs of mock-treated dcr-3 vs. mock-treated WT to reference pathways in the Kyoto Encyclopedia of Genes and Genomes (KEGG) database using the “Pathview” software package in R. Interestingly, in addition to genes involved in cutin, suberin, and wax biosynthesis, genes involved in biosynthesis and elongation of fatty acids were also strikingly changed in dcr-3 mutants (Fig. S5a-c, Table S4, Table S5). These genes expression were also confirmed by qRT-PCR (Fig. S6a-c). These transcriptome analysis results of dcr-3 were consistent with those of previous studies that showed that overexpression or mutations in genes involved in cuticle integrity had pleiotropic effects on plants [Citation9,Citation11]. Thus, the deficiency in dcr-3 could affect the expression of many genes, which is likely related to the target(s) of NJ15.
Cafenstrole reverts phenotype by NJ15 application
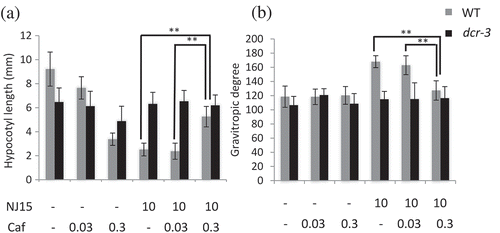
From the transcriptome analysis, biosynthesis and elongation of fatty acids appeared to contribute to NJ15 sensitivity. To gain further insight into the relation of these pathways to the effects of NJ15, we attempted to modify the fatty acid elongation pathway using cafenstrole (Caf), a potent inhibitor of very-long-chain fatty acid (VLCFA) biosynthesis[Citation12]. When etiolated WT seedlings were grown on a medium containing this inhibitor, hypocotyl length was decreased in a concentration-dependent manner. However, shoot negative gravitropism of WT seedlings was not altered by Caf (-). Surprisingly, when 10 μM NJ15 and 0.3 µM Caf were co-applied to etiolated WT seedlings, the resulting hypocotyl lengths were greater than those in both individual treatments of NJ15 or Caf at the same concentrations. Also, shoot negative gravitropism, which was inhibited by NJ15, was also restored by co-application of NJ15 with Caf at 0.3 µM (). These results implied that changes in fatty acid biosynthesis and elongation occurred due to both defects in DCR and inhibition of VLCFA elongases, which could lead to low-sensitivity to NJ15. On the other hand, Caf also showed a slightly inhibitory effect on hypocotyl elongation of dcr-3, while no change in shoot gravitropism was observed whether Caf was applied on its own or co-applied with NJ15 (Fig. S7). Based on this result, we proposed a hypothesis that the inhibitory effect of NJ15 was abolished by Caf via a different mechanism from that responsible for the low-sensitivity of dcr-3 to NJ15.
Figure 4. Effects of NJ15 and Cafenstrole on hypocotyl length and gravitropic response of seedlings grown in the dark.
Seedlings were grown vertically on 1/2 MS medium containing the indicated chemicals. After 4 days, plates were turned 90° from the initial orientation and then continued growing for one day. Then, (a) the hypocotyl length and (b) gravitropic response of seedlings were measured. Data are means ± SD (n = 20). Experiments were repeated three times independently, and similar results were obtained. Asterisks indicate significant differences (*, 0.01 < p < 0.05; **, p < 0.01; Student’s t-test).
NJ15 and cafenstrole independently regulate fatty acid composition
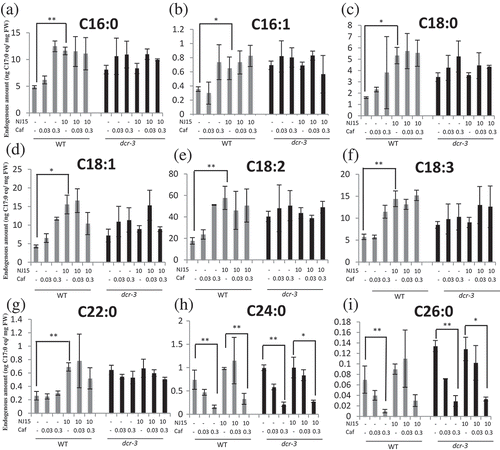
To confirm the above hypothesis, we analyzed the fatty acids of WT and dcr-3 etiolated seedlings grown on NJ15 and Caf media with gas chromatography-mass spectrometry (GC/MS) (). Caf obviously decreased the amount of C24:0 and C26:0 fatty acids in WT plants, which confirmed its function as a VLCFA biosynthesis inhibitor (-). Moreover, Caf seemed to increase the amount of fatty acids produced that had shorter carbon chains (C < 22). In contrast, NJ15 treatment showed no effect on the amounts of C24:0 and C26:0, but increased the amounts of other fatty acids with C ≦ 22 (-). This result indicated that NJ15 and Caf likely have a different mode of action. Also, when NJ15 was co-applied with Caf to WT plants, the amounts of C24:0 and C26:0 fatty acids were higher than those in WT plants treated solely with Caf (-). In etiolated dcr-3 mutants, the amount of fatty acids was higher than in WT plants (Fig. S8). NJ15 failed to increase the amount of any fatty acid in dcr-3, but decreased amounts of C24:0 and C26:0 fatty acids by Caf were still observed in this mutant. These results implied that the effect of NJ15 treatment appeared to increase the amount of endogenous fatty acids with C ≦ 22, whereas the dcr-3 mutant might have low-sensitivity to NJ15 due to its originally high amount of endogenous fatty acids. However, Caf showed decreased amounts of C24:0 and C26:0 fatty acids in dcr-3 plants, which correlated with its inhibitory effect on dcr-3 hypocotyl length. Based on total fatty acid analysis, we concluded that low-sensitivity to NJ15 in the dcr-3 mutants and attenuated effect of NJ15 by Caf are attributable to different mechanisms, although both of these resulted in alteration of fatty acid components and restored the effect of NJ15 on seedlings.
Figure 5. Effects of NJ15 and cafenstrole on an endogenous fatty acid fluctuation in etiolated seedlings.
Five-day-old seedlings grown on 1/2 MS medium containing the indicated chemicals were subjected to the quantification of several kinds of fatty acids with GC/MS. Relative contents of (a) Palmitic acid (C16:0), (b) palmitoleic acid (C16:1), (c) stearic acid (C18:0), (d) oleic acid (C18:1), (e) linoleic acid (C18:2), (f) alpha-linolenic acid (C18:3), (g) behenic acid (C22:0), (h) lignoceric acid (C24:0), and (i) cerotic acid (C26:0) were quantified with exogenously applied margaric acid (C17:0) as an internal standard. Data are means ± SD (n = 3). Asterisks indicate significant differences (*, 0.01 < p < 0.05; **, p < 0.01; Student’s t-test).
Discussion
In this study, we attempted to elucidate the target of NJ15 within biosynthetic pathways by employing a low-sensitivity mutant screening approach. An NJ15-resistant mutant line, dcr-3, was identified that showed increased cuticle permeability. Transcriptome analysis by RNA-seq allowed us to further identify that alteration in fatty acid elongation and cuticle and wax biosynthesis could lead to attenuate inhibitory effect of NJ15. Further, based on the quantification of fatty acids in dcr-3 and WT plants after Caf and/or NJ15 application, we were able to observe the resultant changes in fatty acid composition.
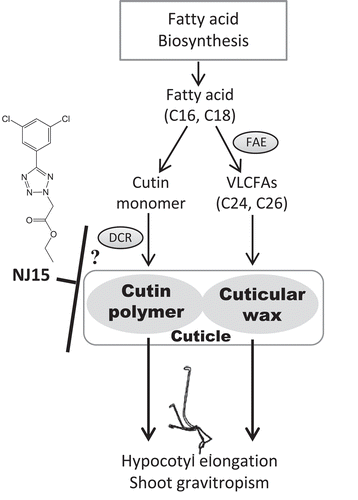
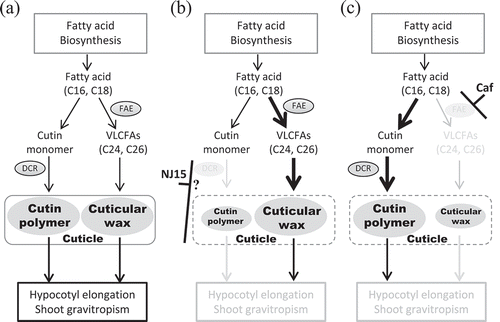
Based on our results, we speculated that the mechanism by which dcr-3 exhibits low sensitivity to NJ15 is similar to one of those shown in . Cutin and cuticular wax are necessary for organ growth, including hypocotyl elongation, and also for shoot gravitropism. Both cutin and cuticular wax are biosynthesized from common types of fatty acids (C16, C18) (). Cutin polymerization has been hypothesized to be catalyzed by DCR, whereas cuticular wax is synthesized from VLCFAs, the synthesis of which is blocked by Caf. In dcr-3 mutants, levels of VLCFAs as well as C16 and C18 fatty acids were increased compared to those in the WT. This correlates with the fact that amounts of cutin monomers and wax might be higher in mutants than in WT plants, because loss-of-function of DCR should lead to deficient cutin polymerization (). In this situation, the loss-of-function of DCR makes NJ15 powerless to exert an effect on this process. Moreover, NJ15 had no effect on levels of VLCFAs as well as C16 and C18 fatty acids in dcr-3 (-). Therefore, the target of this compound should be located around the point of cutin polymerization or cuticle formation (). In WT plants treated with Caf, the amounts of VLCFAs were decreased due to inhibition by Caf. In contrast, the amounts of C16-C18 fatty acids were elevated. These results indicated that decreasing the amount of VLCFAs is likely to affect the accumulation of shorter fatty acids and promote pathways for the synthesis of other long-chain fatty acids (LCFAs) (). On the other hand, when WT plants were co-treated with Caf and NJ15, hypocotyl length was greater than in the sole treatment of either inhibitor (). We postulate that the optimal inhibitory effect of the two inhibitors should balance the fluctuations of fatty acid composition and lead to restoration of hypocotyl elongation.
Figure 6. A possible mechanism for low sensitivity to NJ15 in dcr-3.
(a) In WT Arabidopsis, fatty acid (FA) biosynthesis contributes to hypocotyl elongation and shoot gravitropism via at least two routes. One is the formation of cutin polymer from monomers in the cuticle (left route), catalyzed by DCR. The other (right route) is the formation of cuticular wax in the cuticle from very-long-chain FAs (VLCFAs). (b) The dcr-3 mutant lacks functional DCR, which affects the formation of cutin polymer. As a result, the contribution of the left route for hypocotyl elongation and shoot gravitropism should decrease in dcr-3, which leads to low sensitivity to NJ15. We proposed that the target of NJ15 activity is likely around the formation of cutin polymer because NJ15 had no effect on the amount of VLCFAs as well as those of C16 and C18 fatty acids in dcr-3. (c) Cafenstrole targets the right route, which affects the formation of cuticular wax.
Although the reason why changes in fatty acid composition suppress the effect of NJ15 on hypocotyl elongation and negative gravitropism in etiolated seedlings is still unclear, we did demonstrate that a combination of chemicals and genetic screening could be a useful way to uncover the downstream components of the brassinosteroid- and auxin-signaling pathways. Epidermal VLCFAs and cutin monomers are known to act as signaling molecules that regulate plant growth and defense [Citation13,Citation14]. Thus, changes in the amount of these fatty acids and their derivatives might influence the target pathway of NJ15. Further studies will be required to clarify the exact molecule of fatty acids that lead to low-sensitivity to NJ15. Once this is identified, its role in the regulation of cell elongation and shoot gravitropism should be investigated.
Author contributions
N.J-T. and M.N. planed the research. N.J-T. performed mutant screening, TAIL-PCR, and genes expression experiments. A.H., K.T., and S.It. carried out transcriptome analysis. N.J-T. and S.M. worked out RNA-seq data analysis. S.Iu. and M.K. contributed to FOX mutant preparation and verification of inserted genes. T.N. supervised for TAIL-PCR. N.J-T., T.A., and M.N. wrote the manuscript.
Supplemental Material
Download PDF (1,001.7 KB)Acknowledgments
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI [grant no. 15H04492 and 18H02142 (to MN)], [grant no. 26440132 (to TA)]; by Wada Kunko-kai research foundation (to MN) and by MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2013–2017 (S1311017) from the Ministry of Education, Culture, Sports, Science and Technology of Japan for RNA sequencing experiment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Oh E, Zhu JY, Bai MY, et al Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. Elife. 2014;27:3.
- Cho H, Ryu H, Rho S, et al A secreted peptide acts on BIN2-mediated phosphorylation of ARFs to potentiate auxin response during lateral root development. Nat Cell Biol. 2014;16:66–76.
- Jaroensanti N, Yoon JM, Nakai Y, et al. Does Brassinosteroid Signal Pathway Photomorphogenesis Overlap with Gravitropic Response Caused by Auxin? Biosci Biotechnol Biochem. 2014;78:1839–1849.
- Ichikawa T, Nakazawa M, Kawashima M, et al The FOX hunting system: an alternative gain-of-function gene hunting technique. Plant J. 2006;48:974–985.
- Liu Y-G, Mitsukawa N, Oosumi T, et al Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 1995;8:457–463.
- Tanaka T, Tanaka H, Machida C, et al A new method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. Plant J. 2004;37:139–146.
- Li-Beisson Y, Shorrosh B, Beisson F, et al. Acyl-lipid metabolism. The Arabidopsis Book. 2010;8:e0133.
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140.
- Panikashvili D, Shi JX, Schreiber L, et al The Arabidopsis DCR encoding a soluble BAHD acyltransferase is required for cutin polyester formation and seed hydration properties. Plant Physiol. 2009;151:1773–1789.
- Rani SH, Krishna TH, Saha S, et al Defective in cuticular ridges (DCR) of Arabidopsis thaliana, a gene associated with surface cutin formation, encodes a soluble diacylglycerol acyltransferase. J Biol Chem. 2010;285:38337–38347.
- Xu L, Zeisler V, Schreiber L, et al Overexpression of the Novel Arabidopsis Gene At5g02890 Alters Inflorescence Stem Wax Composition and Affects Phytohormone Homeostasis. Front Plant Sci. 2017;8:68.
- Trenkamp S, Martin W, Tietjen K. Specific and differential inhibition of very-long-chain fatty acid elongases from Arabidopsis thaliana by different herbicides. Proc Natl Acad Sci U S A. 2004;101:11903–11908.
- Nobusawa T, Okushima Y, Nagata N, et al Synthesis of very-long-chain fatty acids in the epidermis controls plant organ growth by restricting cell proliferation. PLoS Biol. 2013;11:e1001531.
- Serrano M, Coluccia F, Torres M, et al The cuticle and plant defense to pathogens. Front Plant Sci. 2014;5:274.
