ABSTRACT
Phenolic compounds isolated from pepper (Capsicum annum) have been demonstrated to have neuroprotective effects, whereas the physiological properties of Capsicum annuum var. abbreviatum (CAA) have not been studied. Thus, we investigate the chemical composition and neuroprotective activity of CAA extract (CAAE) in HT22 hippocampus cells against H2O2-induced neurotoxicity. CAAE treatment resulted in a significant protection of H2O2-exposed HT22, this protection ultimately occurred through an inhibition of MDA and ROS levels and an induction of SOD activity. Furthermore, CAAE treatment reduced H202-induced apoptosis though decreasing the expression of pro-apoptotic factors (Bax, cytochrome c, and cleaved caspases-3) while increasing the expression of the anti-apoptotic factors (Bcl-2), as well as the accumulation of nucleus-Nrf2-mediated HO-1 signaling. Interestingly, CAAE has a high concentration of unique phenolic compositions (chlrogenic acid, tangeretin, etc.) than other capsicum annum extracts. Altogether, these findings suggest that CAAE can be a useful natural resource for alleviating neurodegenerative diseases.
Graphical Abstract
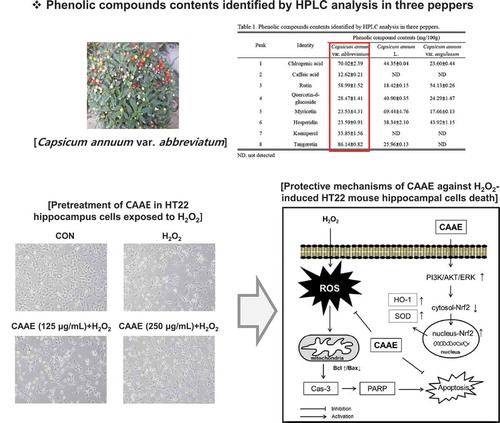
Neuroprotective effect of CAAE against H2O2-induced oxidative stress in HT22 hippocampus cells.
The redox mechanism that balances pro- and anti-oxidants is an important state for the survival of most living organisms [Citation1]. The destruction of the redox system may cause serious injuries to cellular lipids and enzymes due to the increase of the intracellular reactive oxygen species (ROS) such as superoxide anions (·O2−), hydroxyl radicals (·OH), and hydrogen peroxide (H2O2) [Citation2]. Growing evidence suggests that the ROS-induced oxidative stress has been regarded as the main pathological factor in neurodegenerative diseases (NDDs), including Alzheimer’s disease (AD), Huntington’s disease (HD), and Parkinson’s disease (PD) [Citation3].
Here, H2O2 has caused lipid peroxidation, mitochondrial dysfunction, and DNA damage [Citation4]. In the central nervous system (CNS), it has been confirmed that the H2O2 treatment can induce neuronal dysfunction through the overproduction of intracellular ROS and malondialdehyde (MDA). This has resulted in the induction of serious neuronal cell injuries through the activating of mitochondria related apoptotic signals, including the release of cytochrome c, the activation of capase cascade, and the cleavage of poly ADP ribose polymerase (PARP) [Citation5,Citation6].
Recently, phenolic compounds, such as polyphenol and flavonoid, are powerful natural antioxidants that are present in all plants, including fruits and vegetables [Citation7]. Previous studies have shown possible evidence that a (poly) phenol-rich diet may lower the onset rate of neurodegenerative diseases [Citation8]. Most importantly, several phenolic compounds, such as chlorogenic acid, caffeic acid, rutin, keampferol, and tangeretin, have been identified as promising candidates for pre-clinical testing in neurodegeneration [Citation9–Citation13]. For example, caffeic acid, a simple phenylpropanoid that is contained in most plants, was determined to protect neuronal injuries through ameliorating intracellular calcium levels and activating glycogen synthase kinase (GSK)-3β production [Citation11]. In addition, rutin, keampferol, and quercetin have been shown to be involved in eliciting a neuroprotective effect through increasing the anti-apoptotic signals, such as decreasing the level of caspase-3 and apoptosis-inducing factor (AIF), as well as increasing the level of Bcl-2 in various models of neurodegeneration [Citation10,Citation12,Citation14]. Also, tangeretin has been determined to play a protective role in an animal model of PD via regulating the contents of tyrosine hydroxylase positive cells and striatal dopamine [Citation13]. In this regard, natural products containing these compounds are of great interest as candidates for treating neurodegenerative diseases.
Pepper (Capsicum annum), which belongs to genus Capsicum L. (Solanaceae), is one of the most widely cultivated crops and the most well-known consumed vegetable in the world [Citation15]. Among them, capsicum annuum var. abbreviatum has shown higher levels of total polyphenol and total flavonoid than the other species of peppers (Capsicum annuum var. angulosum and Capsicum annuum. L) [Citation16]. However, the chemical composition and physiological properties of Capsicum annuum var. abbreviatum have not been studied. Therefore, the aim of the present work was to identify the phenolic compound profiles of CAAE as well as investigate the neuroprotective effects of CAAE against the H2O2-induced neuronal cell death.
Materials and methods
Reagents and antibodies
Dulbecco modified eagle medium (DMEM) medium was purchased from Welgene (Namcheon, Gyeongsan, Korea). Fetal bovine serum (FBS) and antibiotics (penicillin & streptomycin, 100 U/mL) were obtained from Gibco BRL (Rockville, MD, USA). Hybond-polyvinylidene difluoride (PVDF) membranes and an electrochemiluminescence (ECL) kit were purchased from Millipore (Merck KGaA, Darmstadt, Germany). H2O2 (30%) and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were acquired from Sigma-Aldrich (St. Louis, MO, USA). A superoxide dismutase (SOD) detection kit was purchased from Dojindo (Rockville, MD, USA). A malondialdehyde (MDA) detection kit was obtained from Oxis Health Products, Inc. (N. Cutter Circle, Portland, OR, USA). A protein extraction solution (NE-PERTM Nuclear and Cytoplasmic Extraction Reagents) was purchased from Thermo Scientific (Carlsbad, CA, USA). The following primary antibodies were used for Western blotting: Anti-Bcl-2, anti-Bax, anti-cytochrome c, anti-pro-caspase 3, anti-cleaved-caspase 3, anti-PARP, anti-Nrf2, anti-lamin B, anti-β-actin, and anti-HO-1 antibodies. All antibodies were obtained from cell signaling technology (Danvers, MA, USA). Horseradish peroxidase (HRP)-conjugated anti-mouse IgG and HRP-conjugated anti-rabbit antibodies were obtained from Calbiochem (San Diego, CA, USA).
Preparation of samples
Three dried peppers (Capsicum annuum var. abbreviatum, Capsicum annum var. angulosum and Capsicum annum L) were extracted with 95% ethanol for 2 days at room temperature and filtered (No.4, Whatman, Kent, UK). Capsicum annuum var. abbreviatum ethanol extract (CAAE), Capsicum annum var. angulosum ethanol extract (CAAM), and Capsicum annum L ethanol extract (CALM) were evaporated and freeze-dried in a vacuum, and then each sample (20 mg) was dissolved by 95% ethanol (1 mL).
High-performance liquid chromatography (HPLC) analysis
To determine the phenolic compound profiles of each sample, we conducted HPLC analysis. An Agilent 1200 HPLC system (Agilent Technologies, Palo Alto, CA, USA) operated by Windows NT based ChemStation software was used. All of the samples were applied to an YMC ODS-AM C18 column (5 μM pore size and length I.D., 250mm × 4.6mm). Separation was conducted at 35°C with a linear gradient of 0.1% acetic acid in DW (solvent A) and 0.1% acetic acid in acetonitrile: DW = 75: 25 (solvent B). Chromatographic separation was performed in 90 min with solvent B: from 22 to 28% in 45 min, 28 to 38% in 60 min, 38 to 48% in 65 min, 68 to 100% in 85 min, and 100 to 12% in 90 min. The flow rate was 1 mL/min and the detection wavelength was set at 285 nm. The standard materials, such as chlorogenic acid, caffeic acid, rutin, quercetin-d-glucoside, myricetin, hesperidin, kaemperol, and tangeretin (Sigma Aldrich), were used to detect the composition of the sample polyphenols and flavonoids.
Cell culture
HT22 cells, a mouse hippocampus cell line, were cultured in DMEM media supplemented with 5% FBS and 100 U/mL of penicillin & streptomycin in a CO2 incubator at 37°C with 5% CO2.
Measurement of cell viability
To measure cell viability, we used the MTT assay. HT22 cells were seeded at a density of 8 × 104 cells/well in 48 well-plates. After 12 h, the HT22 cells were pretreated with various concentrations (62.5 to 250 μg/mL) of CAAE, CAAM, and CALM for 1 h. Then they were exposed to H2O2 (600 μM) for 12 h. Next, a MTT solution (final concentration 0.5 mg/mL) was added to each well for 4 h at 37°C. Then each well was treated with DMSO, and the absorbance was measured by a micro-plate reader (BioTek Instruments, Winooski, VT, USA) at 517 nm.
Measurement of intracellular SOD and MDA levels
The HT22 cells were cultivated in 6 well-plates with a density of 1.2 × 107 cells/well for 12 h. Next, the cells were pretreated with CAAE (125 and 250 μg/mL) for 1 h and then exposed to H2O2 (600 μM) for 12 h. The cells were lysed in order to use the freeze-thaw method (−20°C for 20 min, then 37°C bath 10 min, repeat twice) after the cells were centrifuged at 12,000 rpm for 15 min at 4°C. The protein concentrations were determined by using the BCA protein assay (Thermo Scientific, Rockford, IL, USA). The cell lysates were used to determine the intracellular SOD and MDA levels. These levels were confirmed based on the manufacturer’s instructions.
Measurement of intracellular ROS levels
The HT22 cells were cultivated with a density of 1 × 107 cells/well for 12 h. The HT22 cells were pretreated with CAAE (125 and 250 μg/mL) for 1 h and then exposed to H2O2 (600 µM) for 3 h. Next, the cells were washed with PBS and loaded with DCF-DA (final concentration; 5 μM) in a serum-free medium. Finally, images were obtained with a fluorescence microscope at 525nm (IX71, Olympus, Japan).
Western blotting
The HT22 cells were lysed through NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific). Nuclear and cytoplasmic lysates were resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred onto a poly-vinylidene difluoride (PVDF) membrane. The membranes were blocked in 5% skim milk and incubated with the respective primary antibody (1:1000) overnight at 4°C after incubation with an HRP-conjugated secondary antibody (1:4000) for 1 h at room temperature. The proteins were visualized using the ECL advance kit.
Statistical analysis
All experiments were repeated ≥ times. The means and standard deviations for the results were calculated using the Statistical Package for Social Sciences (SPSS) software (SPSS, 10.0, IBM, Chicago, IL, USA). The differences among the mean values were examined by the Student’s two-tailed t-test with significance levels of *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
Measurement of optimum condition of H2O2 in HT22 cells
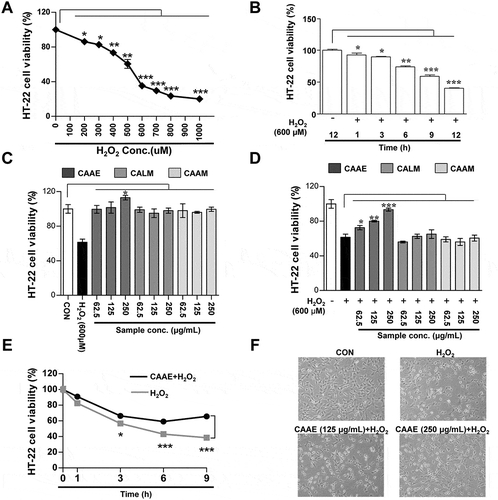
HT22 cells were exposed by H2O2 (200 to 1000 μM) for 12 h, which reduced the cell viability in a dose dependent manner. In particular, H2O2 (600 μM) inhibited an approximate 40% of the HT22 cells’ viability (). Furthermore, the HT22 cells were treated with H2O2 (600 μM) for different times (0 to 12 h). H2O2 treatment for 9 h reduced approximately 50% of the HT22 cells’ viability (). Therefore, we obtained the optimum condition of H2O2 (treatment concentration: 600 μM and exposure time: 9 h), which was used for the following experiments.
Figure 1. Effect of neuronal cells viability treated with H2O2 or/and CAAE on HT22 hippocampus cells.
Cell viability was analyzed using MTT method. (a, b) Cell viability in HT22 cells by treatment with H2O2 (0 to 1000 μM) for 12 h and by treatment with H2O2 (600 μM) for different times. (c, d) Cell viability in HT22 cells by treatment with three pepper extracts (CAAE, CALM and CAAM; 62.5, 125, 250 μg/mL) for 12 h. (c) Cell viability in HT22 cells by treatment with three pepper extracts (62.5, 125, 250 μg/mL) or H2O2 (600 μM) for 12 h. (d) Cell viability in HT22 cells by pre-treatment with three pepper extracts (62.5, 125, 250 μg/mL) for 1 h after treatment with H2O2 (600 μM) for 12 h. (e) Cell viability by CAAE (250 μg/mL) treatment on H2O2 (600 μM)-exposed HT22 cells for the time indicated. (f) Neuronal cells morphological analysis by pre-treatment with CAAE (125 to 250 μg/mL) for 1 h after treatment with H2O2 (600 μM) for 12 h. All data are expressed as the mean ± SD (n = 3 samples). *p < 0.05, **p < 0.01 and ***p < 0.001. Each experiment was repeated at least 3 times, and similar results were obtained. CAAE: Capsicum annuum var. abbreviatum ethanol extract, CAAM: Capsicum annum var. angulosum ethanol extract, CALM: Capsicum annum L ethanol extract, CON: non-treated cells.
Protective effect of CAAE against H2O2-induced neuronal death
To investigate the cell cytotoxicity of three dried peppers on HT22 cells, the cells were treated with various concentrations (62.5 to 250 μg/mL) of Capsicum annuum var. abbreviatum ethanol extract (CAAE), Capsicum annum var. angulosum ethanol extract (CAAM), and Capsicum annum L ethanol extract (CALM) for 9 h. As shown in , cell cytotoxicity by CAAE, CAAM and CALM treatments were not affected below 250 μg/mL. Interestingly, 250 μg/mL CAAE-treated HT22 cells significantly increased the cell survival. Next, the HT22 cells were pretreated with various doses (62.5 to 250 μg/mL) of three extracts for 1 h, followed by H2O2 (600 μM) for 9 h. The cells’ viability was reduced to about 50% in the H2O2 only group. After CAAM and CALM treatment, H2O2-treated HT22 cells did not affect the cell survival. However, the CAAE-treated groups were protected from the neuronal death induced by H2O2 (). Additionally, to confirm the specific time affecting neuroprotection in HT22 cells, we treated CAAE (250 μg/mL) at different times (0 to 9 h). Compared to the H2O2 only group, cell cytotoxicity began to ameliorate by pretreatment with CAAE (250 μg/mL) after 3 h (). Furthermore, we investigated how pretreatment with CAAE affected the morphology in H2O2-exposed HT22 cells. The control group (non-treated cells) was observed to have a normal HT22 cell shape, whereas the only H2O2-treated group changed its morphology to an abnormal form, such as having an incomplete cellular membrane and cellular swelling. In contrast, the pretreatment with CAAE (125 and 250 μg/mL) prevented morphologic changes induced by H2O2 (). In this regard, the concentrations of 125 and 250 μg/mL CAAE were used for the subsequent experiments.
Protective effect of CAAE against H2O2-induced oxidative stress
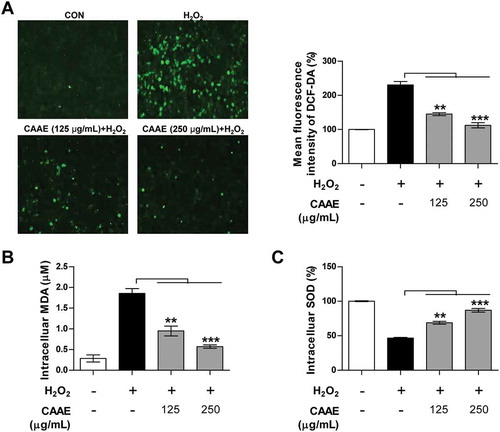
To determine the antioxidant effect of CAAE, we observed the intracellular ROS and oxidative stress indices, such as intracellular MDA and SOD levels, in H2O2-exposed HT22 cells. The H2O2 treatment dramatically increased the intracellular ROS levels in HT22 cells, while the pretreatment with the CAAE in H2O2-exposed HT22 cells was strongly down-regulated in a dose dependent manner (). In addition, the intracellular levels of MDA, which are a major factor of membrane lipid peroxidation and oxidative stress, were greatly ameliorated by the pretreatment with CAAE compared to the H2O2 only group (). As shown in , the H2O2-treated cells attenuated the activity of intracellular SOD, whereas the CAAE pretreatment in H2O2-exposed HT22 cells restored the decline of intracellular SOD levels. Taken together, these results indicate that the CAAE treatment contributes in protecting HT22 cells through the up-regulation of SOD and down-regulation of ROS and MDA.
Figure 2. Effect of CAAE on intracellular ROS, MDA and SOD levels against H2O2-induced oxidative stress in HT22 hippocampus cells.
(a) The cells were incubated with indicated concentrations of CAAE for 1 h, and then stimulated with or without H2O2 (600 μM) for 3 h. ROS generation was measured using a probe; DCF-DA fluorescence (Green fluorescence) was measured by confocal microscopy. Immune-stained cells were chosen by using a fluorescence microscope with 40× objective. (b, c) HT22 cells were incubated with CAAE for 1 h, then were stimulated with or without H2O2 (600 μM) for 9 h. MDA (b) and SOD (c) levels were analyzed using enzymatic kits. All data are expressed as the mean ± SD (n = 3 samples). p < 0.05, **p < 0.01 and ***p < 0.001. Each experiment was repeated at least 3 times, and similar results were obtained. CON: non-treated cells.
Effect of CAAE on the expression of apoptosis related proteins in h2o2-induced HT22 cells
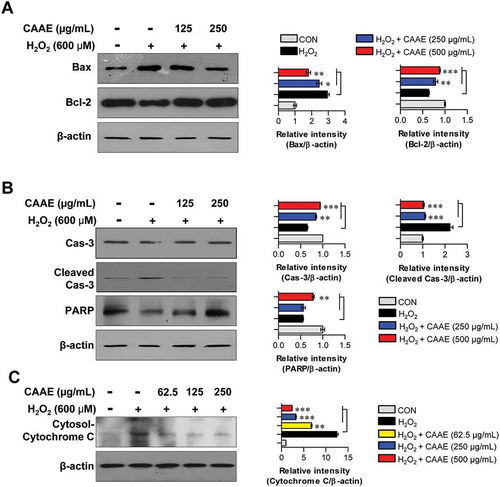
We identified the expression of proteins related to apoptosis such as Bcl-2 and caspase cascade. The H2O2 treatment markedly destroyed the balance of Bcl-2/Bax, while this imbalance was significantly restored by pretreatment with CAAE (). We next investigated how the CAAE pretreatment impacted the expression of caspase cascade and PARP induced by H2O2 treatment. As shown in , compared to the control group (non-treated cells), exposure to H2O2 resulted in an up-regulation of cleaved caspases-3 and cytosolic cytochrome c, as well as a down-regulation of PARP, in HT22 hippocampus cells. In contrast, the H2O2–treated cells in the presence of CAAE pretreatment noticeably reduced the expression of cleaved caspases-3 and cytosolic cytochrome c. They also increased the expression of PARP compared to the case of using H2O2 alone.
Figure 3. Effect of CAAE on the expression of apoptotic related proteins against H2O2-induced apoptosis in HT22 hippocampus cells.
The cells were incubated with indicated concentrations of CAAE for 1 h, and then stimulated with or without H2O2 (600 μM) for 9 h. (a, b, c) Cells lysates were subjected to SDS-PAGE and western blot analysis was performed using each specific antibody to Bax, Bcl-2, caspase-3, cleaved-caspase-3, PARP and cytochrome C. Relative band intensity of each protein was expressed as a percentage when compared to the value of controls (β-actin). All data are expressed as the mean ± SD (n = 3 samples). *p < 0.05, **p < 0.01 and ***p < 0.001. Each experiment was repeated at least 3 times, and similar results were obtained.
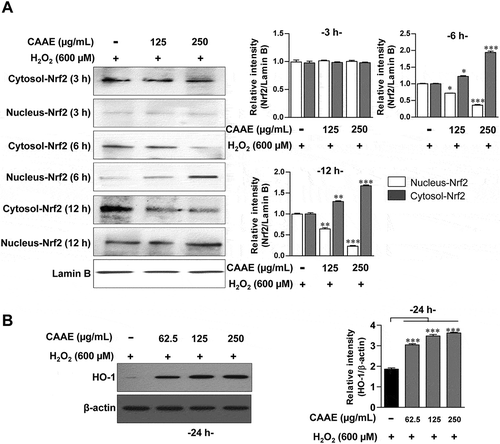
Effect of CAAE on the expression of Nrf2/HO-1 pathways in HT22 cells
To investigate the neuroprotective effect of CAAE, we confirmed the expression of Nrf2 and HO-1. The expression levels of Nrf2 significantly increased the transposition of Nrf2 into the nucleus in a time dependent manner (). We next investigated whether the treatment with CAAE potentially affects the production of HO-1, which results in the CAAE treatment significantly enhancing the levels of HO-1 ().
Figure 4. Effect of CAAE on the expression of Nrf2/HO-1 against H2O2-induced oxidative stress in HT22 hippocampus cells.
(a, b) The cells were incubated with CAAE (indicated concentrations) for the time indicated, and then stimulated with or without H2O2 (600 μM) for 9 h. Cells lysates were subjected to SDS-PAGE and western blot analysis was performed using each specific antibody to Nrf2, and HO-1. Relative band intensity of each protein was expressed as a percentage when compared to the value of controls (Lamin B or β-actin). All data are expressed as the mean ± SD (n = 3 samples). *p < 0.05, **p < 0.01 and ***p < 0.001 for the treatments compared with nucleus- or cytosol-Nrf2 in H2O2-treated cells. Each experiment was repeated at least 3 times, and similar results were obtained.
Chemical characterization of three peppers using HPLC
Chlorogenic acid, caffeic acid, rutin, quercetin-d-glucoside, myricetin, hesperidin, kaemperol, and tangertin were separated and identified by HPLC analysis (Supplementary Figure. 1). . specifies the amounts of the eight aforementioned substances for three peppers. All of the samples revealed similar phenolic compound profiles, while the CAAE mainly contained five phenolic compounds, including chlorogenic acid, caffeic acid, rutin, kaempferol, and tangeretin compared with other extracts.
Table 1. Phenolic compounds contents identified by HPLC analysis in three peppers.
Discussion
The mitochondria play an essential role in the cell progression and also apoptosis [Citation17]. Bcl-2 families are located on the mitochondria membranes, which modulate the permeability of membranes [Citation18]. Among the various kinds of Bcl-2 family proteins, the pro-apoptotic factor of the Bax located on the inner membrane might impact the transfer cytochrome c in the cytoplasm, while the anti-apoptotic factor of Bc1-2 that resides on the outer membrane could block the translocation of cytochrome c onto the cytoplasm [Citation19]. Thus, the ratio of Bax/Bcl-2 is a very important regulator in mitochondrial depolarization. In oxidative stress, the imbalance ratio of Bax/Bcl-2 can lead to a release of cytochrome c, which results in the activation of caspase-9 [Citation20]. It has been demonstrated that the activation of the caspase-9 formed the apoptosome, which is a complex of cytochrome c, apoptotic protease-activating factor 1 (APAF-1), and capase-9 [Citation21]. This complex progresses mitochondrial-related apoptosis by promoting the activation of caspase-3, caspase-6, and caspase-7. In particular, the activation of the caspase-3 accelerates apoptotic signals and aggravates the DNA denaturation [Citation22]. In the present study, the pretreatment with CAAE regulated the mitochondrial permeability by maintaining the ratio of Bcl-2/Bax in H2O2-exposed hippocampus cells. Additionally, the CAAE blocked the release of cytosolic cytochrome c and increased the expression of PARP. In this context, it is believed that CAAE can protect neuronal cells by preventing mitochondrial-related apoptosis signaling.
In previous studies, the Nrf2/ARE pathway has been reported as an essential antioxidant defense system for preventing oxidative stress [Citation23]. The Nrf2 binds the antioxidant response elements’ (AREs) promoter region in chromatin and acts as a key transcription factor for anti-oxidative enzyme genes such as heme oxygenase-1 (HO-1), NADH quinone oxidoreductase 1 (NQO1), and glutamate-cysteine ligase catalytic (GCLc) [Citation24]. Among these, HO-1 catalyzes heme to biliverdin, which results in carbon monoxide (CO) and free iron (Fe2) [Citation25]. These products contribute to antioxidant activities by reducing the superoxide anions and MDA [Citation25]. Additionally, the HO-1 promoter exists in most of the AREs. Therefore, the HO-1 promoter is regarded as a highly effective therapeutic target for protection against oxidative stress [Citation26]. In our findings, it is likely that CAAE protects neuronal cells against oxidative stress via the Nrf2/HO-1 pathway.
In conclusion, CAAE possesses a strong neuroprotective activity by restraining mitochondrial-related apoptosis signaling, including the modulation of bcl-2 families, inhibition of caspase cascades, and activation of the Nrf2/HO-1 signaling pathway. These findings might be closely related with the phenolic compound profiles of CAAE. Thus, we carried out HPLC analysis, and the chromatograms of CAAE exhibited eight phenolic compounds, including chlorogenic acid, caffeic acid, rutin, quercetin-d-glucoside, myricetin, hesperidin, kaemperol, and tangertin. Among these, in particular, five phenolic compounds, such as chlorogenic acid, caffeic acid, rutin, kaemperol and tangertin, showed higher content than CALM and CAAM. In this regard, these results support the belief that CAAE has the potential to become a preventing agent of neurodegenerative diseases in the functional foods and medical industry.
Supplementary_Figure_1.pdf
Download PDF (238.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Supplementary data for this article can be accessed here.
Additional information
Funding
References
- Pyo YH, Seong KS. Effects of Monascus-fermented grain extracts on plasma antioxidant status and tissue levels of ubiquinones and alpha-tocopherol in hyperlipidemic rats. Food Chem. 2013;141(1):428–435.
- Wei YH, Lu CY, Wei CY, et al Oxidative stress in human aging and mitochondrial disease-consequences of defective mitochondrial respiration and impaired antioxidant enzyme system. Chin J Physiol. 2001;44(1):1–11.
- Butterfield DA, Howard B, Yatin S, et al Elevated oxidative stress in models of normal brain aging and Alzheimer’s disease. Life Sci. 1999;65(18–19):1883–1892.
- Kwon SH, Hong SI, Ma SX, et al 3ʹ,4ʹ,7-Trihydroxyflavone prevents apoptotic cell death in neuronal cells from hydrogen peroxide-induced oxidative stress. Food Chem Toxicol. 2015;80:41–51.
- Han SM, Kim JM, Park KK, et al Neuroprotective effects of melittin on hydrogen peroxide-induced apoptotic cell death in neuroblastoma SH-SY5Y cells. BMC Complement Altern Med. 2014;14:286.
- Hu XL, Niu YX, Zhang Q, et al Neuroprotective effects of Kukoamine B against hydrogen peroxide-induced apoptosis and potential mechanisms in SH-SY5Y cells. Environ Toxicol Pharmacol. 2015;40(1):230–240.
- Castrejón ADR, Eichholz I, Rohn S, et al Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008;109(3):564–572.
- Solanki I, Parihar P, Mansuri ML, et al Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv Nutr. 2015;6(1):64–72.
- Taram F, Winter AN, Linseman DA. Neuroprotection comparison of chlorogenic acid and its metabolites against mechanistically distinct cell death-inducing agents in cultured cerebellar granule neurons. Brain Res. 2016;1648(Pt A):69–80.
- Ola MS, Ahmed MM, Ahmad R, et al Neuroprotective effects of rutin in Streptozotocin-induced diabetic rat retina. J Mol Neurosci. 2015;56(2):440–448.
- Sul D, Kim HS, Lee D, et al Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci. 2009;84(9–10):257–262.
- Yang EJ, Kim GS, Jun M, et al Kaempferol attenuates the glutamate-induced oxidative stress in mouse-derived hippocampal neuronal HT22 cells. Food Funct. 2014;5(7):1395–1402.
- Datla KP, Christidou M, Widmer WW, et al Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson’s disease. Neuroreport. 2001;12(17):3871–3875.
- Magalingam KB, Radhakrishnan A, Ramdas P, et al Quercetin glycosides induced neuroprotection by changes in the gene expression in a cellular model of Parkinson’s disease. J Mol Neurosci. 2015;55(3):609–617.
- Yang HJ, Jang D-J, Hwang J-T. Anti-diabetic effects of Korean red pepper via AMPK and PPAR-γ activation in C2C12 myotubes. J Funct Foods. 2012;4(2):552–558.
- Byun E-B, Park W-Y, Ahn D-H, et al Comparison study of three varieties of red peppers in terms of total polyphenol, total flavonoid contents, and antioxidant activities. J Korean Soc Food Sci Nutr. 2016;45(5):765–770.
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58(4):495–505.
- Fong CS, Wu RM, Shieh JC, et al Pesticide exposure on southwestern Taiwanese with MnSOD and NQO1 polymorphisms is associated with increased risk of Parkinson’s disease. Clin Chim Acta. 2007;378(1–2):136–141.
- Borner C. The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol. 2003;39(11):615–647.
- Luo P, Chen T, Zhao Y, et al Protective effect of Homer 1a against hydrogen peroxide-induced oxidative stress in PC12 cells. Free Radic Res. 2012;46(6):766–776.
- Kim CS, Kawada T, Kim BS, et al Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell Signal. 2003;15(3):299–306.
- Grutter MG. Caspases: key players in programmed cell death. Curr Opin Struct Biol. 2000;10(6):649–655.
- Buendia I, Michalska P, Navarro E, et al Nrf2-ARE pathway: an emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther. 2016;157:84–104.
- Jang HJ, Hong EM, Kim M, et al Simvastatin induces heme oxygenase-1 via NF-E2-related factor 2 (Nrf2) activation through ERK and PI3K/Akt pathway in colon cancer. Oncotarget. 2016;7(29):46219–46229.
- Adin CA, Croker BP, Agarwal A. Protective effects of exogenous bilirubin on ischemia-reperfusion injury in the isolated, perfused rat kidney. Am J Physiol Renal Physiol. 2005;288(4):F778–784.
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116.
