ABSTRACT
Aromatherapy uses plant essential oils and fragrant ingredients for relaxation, sleep assistance, and improvement of restlessness related to dementia. Certain aromatic substances increase the life span and stress tolerance of nematodes. We investigated effects of exposure to linalool, a linear chain monoterpenic alcohol that is present in the essential oils of many plants, and its optical isomer, l-linalool, in Caenorhabditis elegans. Nematodes were repelled by the odor of both linalool and l-linalool; however, linalool odor stimulation decreased fat accumulation and increased motility after thermal stress. Analysis of a gene-deficient mutant revealed that the DAF-16 insulin-signaling pathway, which is involved in heat stress tolerance, was enhanced by linalool treatment. Linalool stimulation increased the expression of downstream genes such as sod-3 and hsp-12.6 via DAF-16. We conclude that linalool odor induces a repelling behavior in nematodes, improves heat stress tolerance through the DAF-16 signaling pathway, and affects fat accumulation.
Graphical Abstract
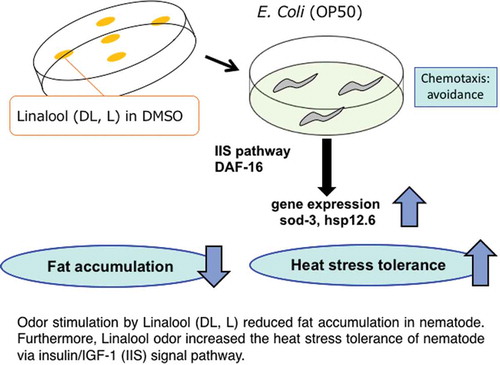
Odor stimulation by Linalool (DL, L) reduced fat accumulation in nematode. Furthermore, Linalool odor increased the heat stress tolerance of nematode via insulin/IGF-1 (IIS) signal pathway.
Aromatherapy is a traditional medicinal practice that uses fragrant plant essential oils. This therapy is applied to support relaxation and sleep [Citation1], as well as to improve dementia-related restlessness [Citation2,Citation3]. However, scientific research on aromatherapy and essential oils is relatively new, and scientific evidence of the physiological actions and functional mechanisms is limited.
Table 1. Sequences of primers used in the gene expression analysis.
The nematode Caenorhabditis elegans was used as an experimental model organism in this study. The hermaphrodite nematode consists of 959 somatic cells, 302 of which are nerve cells. Individual nerve cells have names as well as known cell body position and lineage [Citation4]. There are twelve sensory nerve cilia in the head of the nematode, and two types of sensory cilia in the tail phasmide sensea. Nematodes respond to various chemical substances in the environment using sensory cilia and can sense more than 100 types of chemical substances to which they develop chemotactic behaviors [Citation5]. Three types of olfactory neurons, AWA, AWB, AWC, and the two sensory neurons, ASH and ADL, can sense volatile substances [Citation6,Citation7]. AWA and AWC neurons sense attractive substances; AWB, ASH, and ADL neurons sense repellent substances. Previous studies have identified the molecular mechanisms of olfactory receptors in each nerve [Citation8].
The research on lifespan and stress tolerance in nematodes is extensive. The insulin/IGF-1 signal (IIS) pathway is a representative pathway that controls both features [Citation9]. This transmission pathway is necessary for various biological functions, such as metabolism, development, and lifespan, and is homologous with the IIS signaling pathway in mammals. In the IIS pathway, when an insulin-like peptide ligand binds to the insulin-like peptide receptor DAF-2 on the cell surface, it activates the PDK-1 and AKT-1/AKT-2/SGK-1 kinase cascades. As a result, phosphorylation of the transcription factor DAF-16 inhibits its nuclear translocation. Conversely, when the IIS pathway signal is lost, DAF-16 can translocate into the nucleus, and transcriptional activity increases. Activated DAF-16 controls the expression of various genes involved in lifespan, heat stress tolerance, oxidative stress tolerance, metabolism, and immunity. In addition, the transcription factor HSF-1, which controls the expression of various genes involved in lifespan extension and heat stress tolerance, is negatively regulated by the IIS pathway [Citation10,Citation11].
Previous reports on the effects of isoamyl alcohol and acetic acid on lifespan and stress tolerance of nematodes are examples of the physiological action by odor stimulation [Citation12]. Isoamyl alcohol is perceived by nematode AWC olfactory neuron [Citation5] and can result in increased life span, but acetic acid did not have this effect. In addition, isoamyl alcohol odor stimulation and acetic acid improved thermal stress tolerance in nematodes [Citation12].
Linalool is one of the linear-chain monoterpene alcohols that are found in many plant-based essential oils. Linalool is composed of the optical isomers d-linalool and l-linalool. There are also several industrial synthesis methods for producing linalool isomers, which have the fragrance of lily, lavender, or bergamot; therefore, they are popular as perfume additives. Lavender essential oil and linalool odor stimulation can affect autonomic neurotransmission and decrease blood pressure in rats [Citation13]. Linalool also has an anti-inflammatory action [Citation14]. Lavender essential oil and linalool steam improved autonomic and hormonal imbalance in menopausal female rat models [Citation15]. These studies indicated that linalool can influence a variety of physiological functions; however, studies on how it may affect lifespan and thermal stress resistance have not been conducted.
In this study, we aimed to characterize the physiological effects of linalool and l-linalool on nematode stress tolerance and to define their mechanism of action.
Materials and methods
Nematodes and growth
C. elegans nematodes were raised on nematode growth medium (NGM) plates (OP plates) coated with the E. coli strain OP50. The breeding temperature in a thermostatic chamber was 20°C. Every five days, three worms were transferred to new OP plates to maintain the line. C. elegans wild-type N2 and the daf-16(mgDf50), daf-2(e1370), and odr-3(n1605) mutants were provided by the Caenorhabditis Genetics Center, University of Minnesota, Minneapolis, USA.
Synchronization
Synchronization was performed to align nematode growth stages. Adult worms raised at 20°C were collected in 5 mL of S-basal medium (0.1 M NaCl, 50 mM potassium phosphate buffer pH 6.0). Then, the adult worms were treated with 100% NaClO (Haiter, Kao, Tokyo, Japan) and eggs were collected in a 15-mL tube in S-basal medium. After 18 h, hatched L1 larvae were seeded onto OP plates and used for experiments.
Odor substances and administration
Linalool (referred to as dl-linalool in the text) (Wako Pure Chemical Industries, Osaka, Japan) and l-linalool (Sigma-Aldrich, St. Louis, USA) were diluted in DMSO (Nacalai Tesque, Kyoto, Japan), and 0.1%, 1%, and 10% dl- and l-linalool solutions were prepared. DMSO alone was used for the control treatments in all experiments. Each solution was added to the back of the OP plate lid on which the nematodes were cultured; five 4-μL spots were sufficient to give an odor stimulus ().
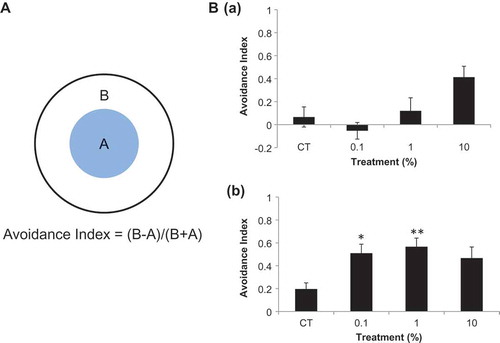
Figure 1. Nematode chemotaxis with odorous substance treatments A: L1 synchronized wild type N2 nematodes were raised on OP plates for 96 h, after which 2 μL of odorous linalool substance was dropped in the center of the plate. The chemotaxis of nematodes was examined 60 min after treatment. B: (a) chemotaxis rate in response to dl-linalool treatment. The graph illustrates the mean ± standard error. N = 300 nematodes, 10%: p = 0.053. (b) Chemotaxis rate of nematodes treated with l-linalool. The vertical axis indicates the repelling index, and the horizontal axis the concentration of the reagent. The graph illustrates the mean ± standard error. N = 180 nematodes, *p < 0.05, ** p < 0.01.
Chemotaxis experiment
Synchronized nematodes were bred for 96 h at 20°C. A 3-cm circle was drawn from the center of a 6-cm NGM plate. Five nematodes were transferred to four points on the outer periphery of the circle for a total of 20 animals per plate. A 2-μL volume of each odor substance was added to the center of the NGM plate, and after 60 min, nematodes outside the circle were considered “repelled” by the odor. The repelling index was calculated as follows: avoidance index = ((number of worms outside the circle) – (number of worms inside the circle))/total number of worms.
Fat accumulation quantification
Synchronized nematodes were bred for 72 h and then given odor stimulation for 24 h. Nile red (Wako Pure Chemical Industries, Ltd.) was dissolved in acetone (Kanto Kagaku) to prepare a stock solution of 500 μg/mL, which was then diluted with S-basal to prepare a 1 μg/mL Nile Red solution. The nematodes were treated with 1 μg/mL Nile Red and incubated at room temperature for 30 min with agitation. Then, the worms were fixed in 8% ethanol (Wako Pure Chemical Industries) for 5 min and observed with a fluorescence microscope. The fluorescence intensity was analyzed using ImageJ software.
Pharyngeal pumping motion measurements
The pharyngeal pumping motion of the nematodes cultured on OP plates was measured every 15 s in nematodes stimulated with odor substances after 24 h of exposure.
Nematode survival rate under thermal stress
Nematodes were stimulated for 24 h with odor compounds and then transferred to NGM plates containing 5 mg/mL ampicillin (Wako Pure Chemical Industries, Ltd.) and E. coli OP50, and cultured at 35°C. Survival rates were measured every 2 h starting 10 h after the heat treatment.
Nematode motility after thermal stress
The nematodes were stimulated for 24 h with odor compounds and then collected in S-basal medium and washed. Then, the worms were transferred to sterile NGM plates and incubated at 35°C or 20°C for 4 h. The worms were then transferred to OP plates and the whole-body movement (i.e. thrashing) of the worms in S-basal medium was measured every 15 s after the heat stress treatment. The rate of restoration of mobility was calculated as follows: motion restoration rate = (number of whole-body movements of nematodes at 35°C)/(number of whole-body movements of nematodes at 20°C) × 100.
Gene expression analysis
RNA was extracted from C. elegans treated for 24 h with odor compounds using RNAiso Plus (Takara, Shiga, Japan). Then, cDNA was synthesized by first removing genomic DNA with the PrimeScript™ RT Reagent Kit with gDNA Eraser (Perfect Real Time) (Takara). Quantitative reverse transcription PCR (qRT-PCR) was performed using the THUNDERBIRD SYBR qPCR Mix (Toyobo, Osaka, Japan); the actin gene was used as an internal standard. Primers used in this study are shown in .
Statistical analysis
Statistically significant differences were evaluated using SPSS software (IBM, Armonk, NY). Survival curves were analyzed using a log-rank test, and significant differences between three or more groups were judged by Tukey’s honest significant difference test. Values of p < 0.05 and p < 0.01 are indicated with * and **, respectively.
Results
Nematodes are repelled by dl-linalool and l-linalool
Nematodes respond to external stimuli such as odor, temperature, mechanical stimulation, and light by moving. To evaluate how nematodes behave in response to each odorant, their chemotaxis was observed after exposure to linalool isoforms. Nematodes were repelled by dl-linalool in a concentration-dependent manner; the 10% concentration resulted in the highest repellency index ( (a)). Conversely, l-linalool had the highest repellency index at a 1% concentration, but the nematodes presented evasive behavior at all concentrations ( (b)). These results suggest that nematodes are repelled by linalool odors, but are particularly responsive to l-linalool.
Dl-linalool odor stimulation reduces fat accumulation in nematodes
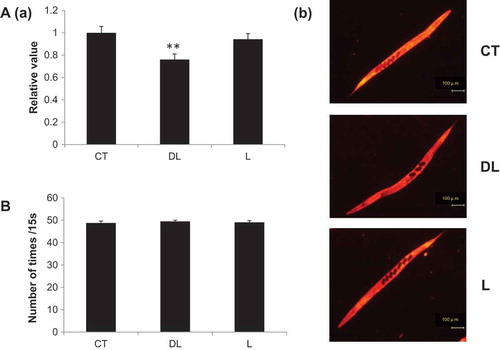
To investigate the effect of linalool exposure on fat accumulation, nematodes were treated with each isoform for 24 h before body fat was stained with Nile Red reagent. Body fat was observed using a fluorescence microscope and analyzed with ImageJ software. We observed that fat accumulation decreased in nematodes treated with dl-linalool, whereas no change was observed in nematodes treated with l-linalool (). In addition, we evaluated changes in feeding movements due to the decrease in fat accumulation by measuring the pumping motion of nematodes treated with odor stimulation for 24 h. Neither dl-linalool nor l-linalool affected the pumping rate in treated compared to control nematodes (). In previous studies, odor stimulation with isoamyl alcohol lengthened the lifespan of nematodes [Citation12]; therefore, the effect of linalool on the lifespan of nematodes was analyzed. However, we did not observe any changes in lifespan after linalool treatment (data not shown).
Figure 2. Fat accumulation and eating movement in nematodes treated with linalool The synchronized nematodes were treated with linalool odor stimulation for 24 h. A: (a) Nematodes were stained with a Nile Red solution, fixed with 8% ethanol, and observed with a fluorescence microscope. CT: DMSO, DL: 1% dl-linalool, L: 1% l-linalool. N = 92, 91, 101, in order from the left, **p < 0.01, (b) Nematodes were observed by fluorescence microscopy; the scale bars indicate 100 μm. B: The pharyngeal pumping motion of worms was measured for 15 s. The vertical axis shows the number of pumps per 15 s, and the horizontal axis shows the respective odor treatments. The graph shows the mean ± standard error. CT: DMSO, DL: 1% dl-linalool, L: 1% l-linalool. N = 10.
Dl-linalool or l-linalool odor stimulation improves nematode motility after heat stress
Next, the effect of linalool odor stimulation on motility after thermal stress was examined. When nematodes were cultured at 35°C for 4 h, whole-body movement decreased significantly, but could be recovered after 3–24 h. However, nematodes stimulated with dl-linalool and l-linalool had higher motility and better restoration after 12 h than the control worms ( (a)).
Figure 3. Nematode motility after thermal stress Synchronized nematodes were given linalool odor stimulation for 24 h. (a) N2 wild type, (b) daf-16 mutant, (c) daf-2 mutant, and (d) odr-3 mutant. The nematodes were cultured at 35°C for 4 h, then returned to 20°C before whole-body movement was measured at 12 and 24 h (or at 0, 3, 6 h). The number of whole-body movements per 15 s was measured. The vertical axis shows the ratio of the amount of exercise at 35°C to the number of movements at 20°C. The horizontal axis shows the elapsed time after thermal stress. The graphs represent the mean ± standard error. CT: DMSO, DL: 1% dl-linalool, L: 1% l-linalool, N = 10, *p < 0.05, ** p < 0.01.
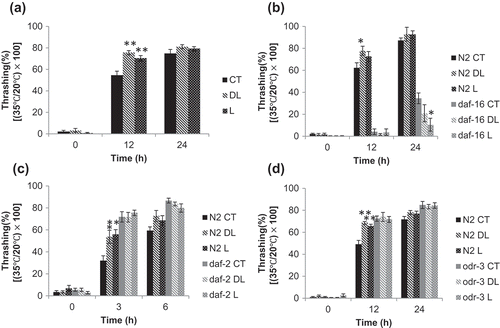
Previous studies have shown that DAF-16 is involved in motility restoration after thermal stress [Citation16]. Therefore, a daf-16(mgDf50) mutant was used to evaluate the association between DAF-16 and motility improvement with odor stimulation. Wild-type N2 worms treated with each odor stimulus showed higher motility restoration after 12 h of heat stress than the control worms ( (b)). In the daf-16 mutant, even when odor stimulus was given, it had the same or lower motility than the control ( (b)). Therefore, DAF-16 is involved in improving motility after linalool odor stimulation. DAF-16 is mainly regulated by insulin/IGF-1 signaling, and the insulin-like peptide receptor DAF-2 is involved in this regulation [Citation17]. Therefore, the daf-2(e1370) mutant was used. In wild-type N2 nematodes, the restoration of motility rafter 3 h was greater than that in the control ( (c)); however, the motility was not recovered in the daf-2 mutant after treatment ( (c)). Therefore, DAF-2 is involved in improving motility after heat stress with linalool odor stimulation.
Odorous substances are first recognized by G-protein-coupled receptors expressed on neurons, which open downstream channels via Gα protein activity, and allow signals to be transmitted. We focused on ODR-3, which is a G03B1 protein. ODR-3 is expressed in five types of neurons; it is expressed in the olfactory neurons AWA, AWB, and AWC, and the sensory neurons ASH and ADF [Citation18]. Therefore, we analyzed the odr-3(n1605) mutant. In wild-type N2 nematodes, motility was significantly recovered at 12 h after heat stress by odor stimulation ( (d)). However, in the odr-3 mutant, there was no restoration of motility with either odor stimulus ( (d)). From these results, we suggest that the ODR-3 protein is involved in odor-dependent restoration of motility after thermal stress.
We also tested the effect of linalool odor treatment on the nematode survival rate under thermal stress conditions. The survival rate of nematodes treated with l-linalool odor stimulation was lower than that of the control nematodes (). In addition, although there was no significant difference when we used dl-linalool, the survival rate was lower than that of the control nematodes ().
Figure 4. Nematode survival rate and gene expression during thermal stress The synchronized nematodes were given odor stimulation for 24 h. A: The nematodes were cultured at 35°C and the survival rate was measured 10 h after heat treatment. The vertical axis indicates the percent survival rate, and the horizontal axis indicates time. CT: DMSO, DL: 1% dl-linalool, L: 1% l-linalool, N = 40, *p < 0.05. B: Gene expression was examined using quantitative PCR. The vertical axis represents the mRNA expression level relative to the internal actin control, and the horizontal axis represents each gene analyzed. The graph represents the mean ± standard error. CT: DMSO, DL: 1% dl-linalool, L: 1% l-linalool, N = 3, *p < 0.05, **p < 0.01.Odor stimulation by Linalool (DL, L) reduced fat accumulation in nematode. Furthermore, Linalool odor increased the heat stress tolerance of nematode via insulin/IGF-1 (IIS) signal pathway.
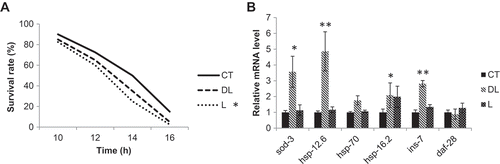
Dl-linalool odor stimulation increases the expression of genes regulated by DAF-16
From the above results, it was clear that dl-linalool odor stimulation reduces fat accumulation in nematodes and improves their motility after thermal stress, which is dependent on DAF-16 function. Therefore, the expression of the genes sod-3 and hsp-12.6, which are regulated by DAF-16 [Citation19,Citation20], was examined using real-time PCR. The expression levels of sod-3 increased approximately three-fold and the expression level of hsp-12.6 increased approximately five-fold upon dl-linalool treatment (). Conversely, with l-linalool treatment, we measured no significant change in the expression levels of sod-3 and hsp-12.6. Next, we focused on the expression of the transcription factor HSF-1, which is involved in heat stress tolerance [Citation11,Citation21]. When the expression levels of the HSF-1-regulated genes hsp-16.2 and hsp-70 were examined, hsp-70 was not changed by dl-linalool treatment, but hsp-16.2 expression was increased (). With l-linalool treatment, neither of the genes showed expression level changes compared to the DMSO-treated control (). There are a number of insulin-like peptides, but we focused on INS-7 and DAF-28, which are agonists that activate DAF-2 [Citation22]. We found that dl-linalool treatment did not change the expression level of daf-28, while it did increase the expression level of ins-7 (). l-Linalool treatment did not induce a significant change in gene expression levels ().
Discussion
This study revealed that odor stimulation with dl-linalool decreased fat accumulation in nematodes, while l-linalool had no such effect. Furthermore, pumping motion in the nematodes did not change, suggesting that this odor stimulation does not affect nematode feeding. However, previous studies in rats reported that lavender oil and linalool could inhibit lipolysis through a histaminergic response and promote appetite and weight gain [Citation23].
dl-Linalool and l-linalool odor stimulation improved motility in nematodes after heat stress. When odor stimulation was applied to daf-16 and daf-2 mutants, motility was restored to the similar extent as that observed in the control, suggesting the involvement of DAF-16 and DAF-2. In addition, ODR-3 is involved in the heat stress tolerance by odor stimulation, as motility was not recovered in the odr-3 mutant with or without linalool treatment. dl-Linalool treatment increased the expression of ins-7, but L-linalool did not. This result contradicts the involvement of DAF-16, but there are approximately 40 insulin-like peptides in C. elegans [Citation24] and odor stimulation may affect the expression of other agonists and antagonists. Furthermore, dl-linalool increased the expression of sod-3 and hsp-12.6, but both dl-linalool and l-linalool lowered the survival rate of nematodes under thermal stress, which may be due to changes in the expression of other genes involved in heat stress tolerance as well as tissue-specific DAF-16 expression. Indeed, in nematodes, the activation of DAF-16 in the intestine is important for lifespan extension [Citation25].
In this paper, we newly described physiological effects of dl-linalool and l-linalool odor stimulation in nematodes. In addition, we showed that the physiological actions of dl-linalool and l-linalool partly differ. DAF-16 is a factor related to aging and heat stress tolerance and is a homologous protein of the forkhead type transcription factor FOXO [Citation26]. Moreover, the IIS pathway that controls DAF-16 function has homology between nematodes and humans [Citation27]. In this study, nematodes showed repelling behavior against both DL-linalool and L-linalool (). However, it is unknown whether there is a clear association between physiological action by each odors and chemotaxis. Previous studies using isoamyl alcohol and acetic acid have shown that there is no particular relationship between chemotaxis and lifespan or stress resistance due to odor stimulation [Citation12].
The results of this study have opened up possibilities for application to mammals and humans.
Author contribution
NH and KS conceived and designed experiments; NH performed all experiments, KS provided every tools and reagents, NH and KS analyzed data, NH and KS wrote the paper. NH and KS made manuscript revisions. KS supervised the study as a principal investigator. All authors read and approved the final manuscript.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research and Education from University of Tsukuba, Japan. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Hwang E, Shin S. The effects of aromatherapy on sleep improvement: A systematic literature review and meta-analysis. J Altern Complement Med. 2015;21:61–68.
- Ballard CG, O’Brien JT, Reichelt K, et al Aromatherapy as a safe and effective treatment for the management of agitation in severe dementia: the results of a double-blind, placebo-controlled trial with melissa. J Clin Psychiatry. 2002;63:553–558.
- Yang MH, Lin LC, Wu SC, et al. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement Altern Med. 2015;15: 93-015-0612-9.
- Hobert O. Neurogenesis in the nematode Caenorhabditis elegans. Bethesda (MD): Wormbook; 2010. p. 1–24.
- Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527.
- Mori I, Ohshima Y. Molecular neurogenetics of chemotaxis and thermotaxis in the nematode Caenorhabditis elegans. Bioessays. 1997;19:1055–1064.
- Starich TA, Herman RK, Kari CK, et al Riddle, Mutations affecting the chemosensory neurons of Caenorhabditis elegans. Genetics. 1995;139:171–188.
- Bargmann CI. Wormbook. Chemosensation in C. elegans. Bethesda (MD): Wormbook; 2006. p. 1–29.
- Murphy CT, Hu PJ. Wormbook. Insulin/insulin-like growth factor signaling in C. elegans. Bethesda (MD): Wormbook; 2013. p. 1–43
- Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15:657–664.
- Furuhashi T, Sakamoto K. Heat shock factor 1 prevents the reduction in thrashing due to heat shock in Caenorhabditis elegans. Biochem Biophys Res Commun. 2015;462:190–194.
- Kurino C, Furuhashi T, Sudoh K, et al Isoamyl alcohol odor promotes longevity and stress tolerance via DAF-16 in Caenorhabditis elegans. Biochem Biophys Res Commun. 2017;485:395–399.
- Tanida M, Niijima A, Shen J, et al Olfactory stimulation with scent of lavender oil affects autonomic neurotransmission and blood pressure in rats. Neurosci Lett. 2006;398:155–160.
- Peana AT, D’Aquila PS, Panin F, et al Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine. 2002;9:721–726.
- Yamada K, Mimaki Y, Sashida Y. Effects of inhaling the vapor of Lavandula burnatii super-derived essential oil and linalool on plasma adrenocorticotropic hormone (ACTH), catecholamine and gonadotropin levels in experimental menopausal female rats. Biol Pharm Bull. 2005;28:378–379.
- Furuhashi T, Sakamoto K. FoxO/Daf-16 restored thrashing movement reduced by heat stress in Caenorhabditis elegans. Comp Biochem Physiol B Biochem Mol Biol. 2014;170:26–32.
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351.
- Roayaie K, Crump JG, Sagasti A, et al The G alpha protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. Elegans Olfactory Neurons. Neuron. 1998;20:55–67.
- Henderson ST, Bonafe M, Johnson TE. Daf-16 protects the nematode Caenorhabditis elegans during food deprivation. J Gerontol A Biol Sci Med Sci. 2006;61:444–460.
- Hesp K, Smant G, Kammenga JE. Caenorhabditis elegans DAF-16/FOXO transcription factor and its mammalian homologs associate with age-related disease. Exp Gerontol. 2015;72:1–7.
- Kourtis N, Nikoletopoulou V, Tavernarakis N. Small heat-shock proteins protect from heat-stroke-associated neurodegeneration. Nature. 2012;490:213–218.
- Chen Y, Baugh LR. Ins-4 and daf-28 function redundantly to regulate C. elegans L1 arrest. Dev Biol. 2014;394:314–326.
- Shen J, Niijima A, Tanida M, et al Olfactory stimulation with scent of lavender oil affects autonomic nerves, lipolysis and appetite in rats. Neurosci Lett. 2005;383:188–193.
- Kaletsky R, Murphy CT. The role of insulin/IGF-like signaling in C. Elegans Longevity and Aging. Dis Model Mech. 2010;3:415–419.
- Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502.
- Ogg S, Paradis S, Gottlieb S, et al The fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. Elegans. Nature. 1997;389:994–999.
- Kimura KD, Tissenbaum HA, Liu Y, et al Daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946.
