ABSTRACT
Numerous gram-negative bacteria have quorum-sensing systems and produce AHL as a quorum-sensing signal molecule. In this study, we demonstrated that Methylobacterium populi P-1M, an isolate from a pink-pigmented household biofilm, produced two AHLs, C14:1-HSL as a predominant product and 3OHC14-HSL as a minor product. The complete genome sequence of M. populi P-1M revealed the presence of genes that are predicted to encode an AHL synthase (mpoI) and AHL receptor (mpoR). M. populi P-1M formed a pellicle-like biofilm, which had a flat surface and was easily removable. In contrast, biofilms formed by mpoI and/or mpoR deletion mutants had a wavy surface structure and strongly adhered to the glass tube. When C14:1-HSL was added to the mpoI mutant culture, the biofilm structure resembled that of the wild-type strain. These results demonstrated that the structure and adhesion strength of M. populi P-1M biofilms are determined in part by AHL-mediated quorum sensing.
Abbreviations: AHL: N-acyl-l-homoserine lactone; C14:1-HSL: N-tetradecenoyl-l-homoserine lactone; 3OHC14-HSL: N-(3-hydroxytetradecanoyl)-l-homoserine lactone; SAM: S-adenosyl-l-methionine; ACP: acyl-acyl carrier protein; EPS: extracellular polysaccharide; DMSO: dimethyl sulfoxide
Graphical Abstract
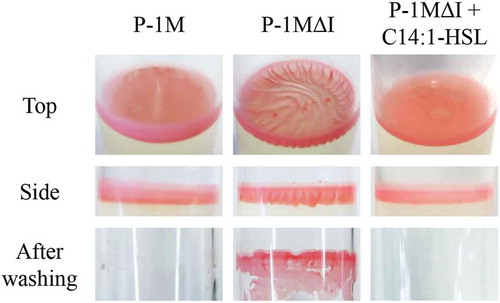
Biofilm formation on the culture surface in glass tubes by M. populi P-1M and quorum-sensing mutants, P-1MΔI, P-1MΔR, and P-1MΔI with C14:1-HSL
Quorum sensing is a bacterial communication mechanism through which bacterial populations coordinate gene expression in response to cell density [Citation1]. In many gram-negative bacteria, the quorum-sensing signal molecule is N-acyl-l-homoserine lactone (AHL) [Citation1,Citation2], and the LuxI/LuxR system is used to control the expression of quorum-sensing regulated genes. LuxI family proteins are AHL synthases that catalyze the acylation of SAM by an ACP or CoA-aryl/acyl moieties to produce AHLs [Citation2,Citation3]. When the concentration of AHL reaches a threshold due to increasing bacterial cell density, LuxR binds to AHL, and the LuxR-AHL complex regulates the expression of numerous genes [Citation2]. AHL-mediated quorum sensing regulates the expression of numerous genes involved in various processes, including bioluminescence, pigment and/or antibiotic production, and virulence [Citation1,Citation2]. In addition, it has been reported that quorum sensing is involved in biofilm development in several gram-negative bacteria [Citation4]. Biofilms are complex bacterial communities that are attached to surfaces by an exopolymeric matrix, and cells within a biofilm interact with each other and their environment [Citation4]. In Pseudomonas aeruginosa, quorum sensing modulates transcription of the genes required for biosynthesis of the EPS that forms the matrix surrounding the cells in a biofilm [Citation5]. In Burkholderia cenocepacia biofilms, surface attachment and stability are modulated by quorum-sensing regulation [Citation6].
Pink-pigmented biofilms are frequently observed in wet areas within a household [Citation7,Citation8], and many pink-pigmented bacteria have been isolated from these types of environmental biofilms [Citation9,Citation10]. In a previous study, we showed that Methylobacterium is a dominant genus in pink-pigmented household biofilms [Citation8]. Methylobacterium was also reported as the dominant genus in pink-pigmented household biofilms, such as those found on shower curtains [Citation11], in drinking water [Citation12], and bathrooms [Citation7]. AHL production has been detected in many Methylobacterium strains [Citation13–Citation15]. Methylobacterium extorquens AM1 was shown to possess two functional LuxI homologs, which catalyze the production of four AHLs, and AHL-mediated quorum sensing positively regulated EPS production [Citation14,Citation16]. Although EPS production is closely related to biofilm formation, the relationship between biofilm formation and quorum sensing in Methylobacterium has not been completely explained yet. Therefore, we postulated that AHL-mediated quorum sensing regulates the formation of pink-pigmented biofilms by Methylobacterium. Methylobacterium populi P-1M was previously isolated from a pink biofilm formed in a bathroom, and it was shown to be a core bacterium that enhances the formation of a strongly adhered biofilm mixed with another Methylobacterium strain [Citation8]. We reported the complete genome sequence of P-1M, which consists of one chromosome and five plasmids [Citation17]. In this study, we report the identification and characterization of the AHL-synthase and AHL-receptor genes of M. populi P-1M. We also investigated biofilm formation in wild-type and quorum-sensing deletion mutant strains.
Materials and methods
Bacterial strains, compounds, and growth conditions
The bacterial strains used in this study are listed in . Escherichia coli was grown at 37°C in LB medium. The AHL reporter strains C. violaceum CV026 [Citation18] and VIR07 [Citation19], which respond to exogenous AHLs with short and long acyl chain, respectively, were grown at 30°C in LB medium. M. populi P-1M and mutant derivative strains were grown at 30°C in MB medium (10 g/L peptone, 2 g/L yeast extract, 1 g/L MgSO4 · 7H2O, and 1% methanol) [Citation8]. Solid bacterial media were made by adding agar at a final concentration of 1.5%. Antibiotics were added as required, at final concentrations of 100 mg/L for ampicillin, 10 mg/L for chloramphenicol, or 50 mg/L for kanamycin. The AHL standards used in this study were synthesized by a previously described method [Citation20]. AHLs were dissolved in DMSO to prepare 1 mM stock solutions.
Table 1. Bacterial strains and plasmids used in this study.
Extraction, identification, and quantification of AHL molecules
P-1M and deletion mutant strains were inoculated into 4 mL of MB medium and incubated at 30°C for 2 days. Full-grown cultures were inoculated into 50 mL of fresh MB medium and incubated at 30°C for 48 h. AHLs were extracted from the culture supernatants using the previously described method [Citation20]. The presence of AHLs in the sample was checked by AHL reporter strains as previously described method [Citation21]. Identification and quantification of AHL molecules by mass spectrometry were done as described previously [Citation20]. Triplicate samples were averaged and standard deviations were calculated. Means were separated using Tukey’s honest significant difference (HSD) test (at P ≤ 0.01).
Cloning and disruption of the putative quorum-sensing genes in P-1M
The mpoIR coding region in the P-1M genome was amplified with Blend Taq plus DNA polymerase (Toyobo, Osaka, Japan) and the following primer set: 5ʹ-CGGCAGATCAAGAAGGCTGAAGCAGCCCTAAGCCG-3ʹ and 5ʹ-CTAATGAGACTGAGGCTCGGCGCAGCTATGGCTCG-3ʹ. The PCR conditions were as follows: 27 cycles of 94°C for 30 s, 60°C for 30 s, and 74°C for 3 min. Then, the PCR products were cloned into the pGEM-T easy cloning vector (Promega, Tokyo, Japan). To generate a plasmid for deleting the target genes, sequences upstream and downstream of the target gene were amplified using the pGEM-T easy plasmid containing the mpoIR genes as the template with following primers sets: 5ʹ-TCTAGATCTCATAGTCCTGCATGTTCTCAGCGGTC-3ʹ and 5ʹ-TCTAGATCTGCATCACCAGCTTCGTCTCCGAGATG-3ʹ for mpoI deletion and 5ʹ-TCTAGATCTCAGGATATCCTCGCGCGAATGCGCTC-3ʹ and 5ʹ-TCTAGATCTCCGGATGAAGCTCGGATGCATCTCCC-3ʹ for mpoR deletion. The amplified PCR fragments were digested with BglII (sites are underlined in the primers) and self-ligated. Then, the fragment lacking the coding region was digested with EcoRI and inserted into the EcoRI site of the suicide vector pK18mobsacB [Citation22] to create plasmids for gene deletion. The gene-deletion plasmids were conjugated from E. coli S17-1 λpir [Citation23] into P-1M. P-1M recombinants generated by a single-crossover event were selected on MB agar containing kanamycin and chloramphenicol. Deletion mutants of the quorum-sensing target genes were generated by homologous recombination and selection for the loss of sacB, i.e., growth on sucrose. The single-crossover mutants were streaked onto an MB agar plate containing 100 g/L sucrose to select for the second homologous recombinants. The presence of the expected internal gene deletion was confirmed by PCR.
Biofilm formation assay
To prepare biofilm samples, P-1M and the quorum-sensing deletion mutant strains were inoculated into MB medium and cultured with shaking for 48 h at 30°C. Bacterial cells were collected by centrifugation and resuspended in fresh MB medium to an OD600 of 0.01. 4 μL of AHL stock solution was added to the 4 mL of cell suspension at the final concentration of 1 μM. As a control, 4 μL of DMSO was added. Then, a 4-mL cell suspension was transferred to a glass tube and statically incubated for 4 days at 30°C. After incubation, pellicle-like biofilms were observed in the glass tubes at the gas-liquid interface. To estimate the adhesive strength of the biofilm to the glass surface, the culture medium and pellicle were removed from the glass tubes. Then, 5 mL of distilled water was added to each tube and vortexed for 5 seconds at maximum speed. The water was removed, and the remaining biofilm was examined.
Results and discussion
Identification of the quorum-sensing genes in P-1M
We previously reported the complete genome sequence of P-1M (GenBank/ENA/DDBJ accession number AP014809-AP014814) [Citation17]. Here, we searched for the AHL-synthase gene homologues in the complete genome of P-1M by using the BLAST program [Citation24]. Complete genome sequence analysis revealed that P-1M has a single quorum-sensing system (Fig. S1). One predicted open reading frame (MPPM_4994), designated as mpoI, was found on the chromosome of P-1M (Fig. S1). The mpoI gene is predicted to encode a 211 amino acid protein with 96.7% identity to a putative LuxI homolog (MPOP_5028) in the genome of M. populi BJ001 (GenBank accession no. CP001029) and 94.8% identity to MlaI from M. extorquens AM1 [Citation14]. In addition, a putative luxR homolog (MPPM_4995), designated as mpoR, was found upstream of mpoI. The mpoR gene is predicted to encode a 243-amino acid protein with 93.4% identity to a putative LuxR homolog (MPOP_5029) in M. populi BJ001 and 93.8% identity to a putative LuxR homolog in M. extorquens AM1.
The phylogenetic tree was constructed based on the sequences of 16S rRNA from Methylobacterium type strains (Fig. S2). P-1M was phylogenetically similar to M. populi BJ001 and M. extorquens AM1 based on 16S rRNA gene sequences. The complete genome sequences of some Methylobacterium type strains have been reported [Citation25]. The complete genome sequences of the type strain of Methylobacterium zatmanii DSM 5688 and Methylobacterium extorquens DM4 contained the genes encoding the LuxI homolog, which were tagged as B2G69_04550 and METDI5550, respectively. The amino acid sequence of MpoI from P-1M showed high identities (over 95%) to that of B2G69_04550 and METDI5550. In addition, the presence of mpoR gene homolog and spacer sequence between mpoI and mpoR was common among these Methylobacterium strains. M. zatmanii DSM 5688 and M. extorquens DM4 were phylogenetically related to P-1M based on 16S rRNA gene sequences (Fig. S2). These results suggested possibility that M. populi and its closely related Methylobacterium species share mpoIR quorum-sensing system in their genome.
Structural analysis of the ahls produced by P-1M
AHLs produced by P-1M were extracted from 50 mL of culture supernatant with ethyl acetate and concentrated 100-fold. The extracts were tested for AHL production on LB agar plates containing the AHL-reporter strains Chromobacterium violaceum CV026 and VIR07. The results showed that the culture extracts stimulated violacein production by C. violaceum VIR07 but not by C. violaceum CV026, which indicates that P-1M mainly produced AHLs with long acyl chains (Fig. S3). The culture extracts were than subjected to ESI-MS/MS analysis to determine the structures of the AHLs. The ESI-MS/MS results showed that the AHLs were broken down to the same fragment, with a theoretical value of m/z 102. Two MS/MS spectra containing an ion at m/z 102 were detected in the culture extracts. The fragment pattern of the one peak at m/z 328 [M + H]+ matched that of the synthetic N-(3-hydroxytetradecanoyl)-l-homoserine lactone (3OHC14-HSL) standard (). Another peak at m/z 310 was also present in the P-1M extract (). A previous study showed that M. extorquens AM-1 produced a single unsaturated N-tetradecenoyl-l-homoserine lactone (C14:1-HSL), which displayed as a peak at m/z 310 [M + H]+ in the ESI-MS/MS analysis [Citation14]. Therefore, we obtained the fragment pattern of the synthetic C14:1-HSL standard. The results showed that the fragment pattern of the peak at m/z 310 [M + H]+ matched that of C14:1-HSL standard ().
Figure 1. ESI-MS/MS analysis of 3OHC14-HSL extracted from the cell-free supernatant of a M. populi P-1M culture (a) and the synthetic standard (b). All corresponding MS/MS fragment peaks for 3OHC14-HSL (m/z 328) along with the product ion peaks (m/z 102) are marked with arrows.
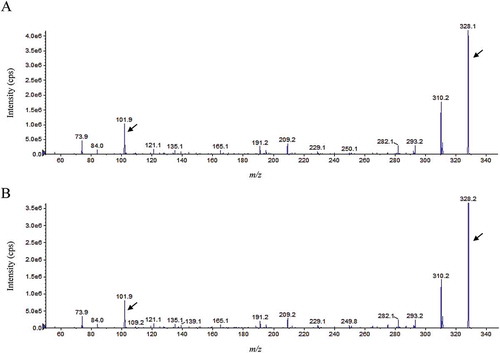
Figure 2. ESI-MS/MS analysis of C14:1-HSL extracted from the cell-free supernatant of a M. populi P-1M culture (a) and the synthetic standard (b). All corresponding MS/MS fragment peaks for C14:1-HSL (m/z 310) along with the product ion peaks (m/z 102) are marked with arrows.
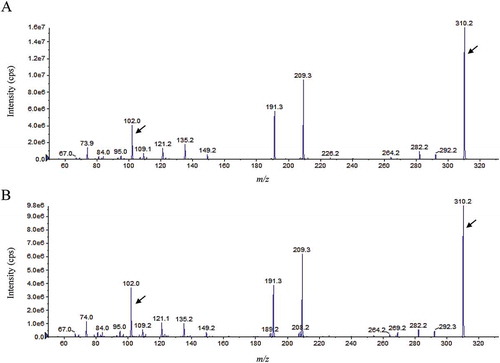
In a previous study, MlaI from M. extorquens AM-1, which shows higher identity to MpoI from P-1M, was shown to catalyze the production of C14:1-HSL [Citation14]. MlaI also catalyzes the biosynthesis of a double unsaturated C14-HSL (C14:2-HSL). However, C14:2-HSL was not detected in the P-1M culture extract. LuxI-type AHL synthase catalyze the biosynthesis of AHLs using SAM and either acyl-ACP and acyl-CoA substrates [Citation2,Citation3]. It is supposed that the structural differences between the AHLs produced by P-1M and AM1 is due to the intracellular composition of fatty acid. The second AHL synthase gene, msaI, is located on the chromosome of M. extorquens AM1 [Citation14]. M. extorquens AM-1 produces the short acyl-chain AHLs N-hexanoyl-l-homoserine lactone (C6-HSL) and N-octanoyl-l-homoserine lactone (C8-HSL) via MsaI. On the other hand, there is no msaI homolog in the P-1M genome, neither C6-HSL nor C8-HSL were detected in P-1M culture extracts. In addition, the tslI gene, which is a truncated luxI homolog, is encoded on an indigenous plasmid of M. extorquens AM1 [Citation16]. TslI controls the transcription of the another AHL synthase gene, msaI, and positively regulates extracellular polysaccharide production [Citation16]. However, no tslI homolog was found on either the chromosome or the five plasmids of P-1M. These results suggested that MpoI is the sole AHL synthase and MpoIR is the only quorum-sensing system in P-1M.
AHL production by P-1M and its mutant strains
To elucidate the relationship between the identified quorum sensing-related genes and AHL production, we constructed gene deletion mutants using a non-marker mutagenesis technique. Single mutants of mpoI and mpoR, and an mpoI and mpoR double mutant were designated as P-1MΔI, P-1MΔR, and P-1MΔIR, respectively. Culture supernatants of P-1M and the abovementioned derived deletion mutants were extracted, and AHLs were quantified by LC-MS/MS (). P-1M wild type mainly produced approximately 19-fold higher concentration of C14:1-HSL than that of 3OHC14-HSL. In contrast, AHLs were not detected in the culture extracts of P-1MΔI and P-1MΔIR. These results indicate that MpoI catalyzes the biosynthesis of both two AHLs, C14:1-HSL, as a predominant product, and 3-OH-C14-HSL, as a minor product. Interestingly, compared to the biosynthesis of AHLs of P-1M wild type, that of an mpoR deletion mutant significantly decreased. It has been reported that the gene coding for AHL-synthase is positively autoregulated by the AHL itself in some bacteria [Citation26]. In Vibrio fisheri, the AHL synthase gene luxI is upregulated by LuxR-AHL and this autoinduction of LuxI exponentially increases AHL levels [Citation27]. It is possible that in P-1M, MpoI produces a basal level of AHL, and the AHL-MpoR complex activates the transcription of the mpoI gene, along with a number of genes, according to cell density.
Table 2. Quantification of AHLs produced by P-1M and its mutant strains.
Structure of the pellicle-like biofilm is affected by AHL-mediated quorum sensing
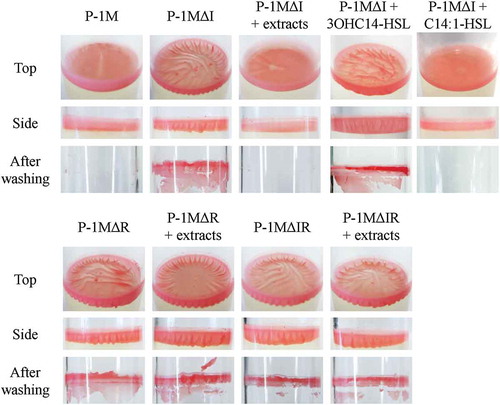
In a previous study, we demonstrated that P-1M forms a pellicle-like biofilm at the liquid-gas interface of MB medium in a glass tube [Citation8]. When the biofilms are washed with sterilized water, the P-1M biofilm is easily washed from the glass surface [Citation8]. To investigate the relationship between biofilm formation and AHL-mediated quorum sensing in P-1M, biofilms of P-1M and the mpoI and mpoR deletion mutant strains were formed on MB liquid medium with or without AHLs extracted from a P-1M culture (). When the preculture of P-1M and its mutant strains were prepared with shaking for 48 h, morphological differences and cell aggregation were not observed in shaking culture (data not shown). After a static incubation for 4 days, the pellicle-like biofilm of P-1M had a flat surface and uniform thickness. In contrast, the biofilm of P-1MΔI had wrinkles and a sparse structure compared with the wild-type P-1M biofilm. A side view of the P-1MΔI biofilm showed a wavy structure, like a curtain. When AHL extracted from P-1M was added to the P-1MΔI culture, the appearance of the biofilm changed to that of wild-type P-1M. The appearance of the P-1MΔR biofilm was very similar to that of the P-1MΔI biofilm. Although the mpoR deletion mutation caused a reduction in AHL production, the structure of the P-1MΔR biofilm did not change following the addition of AHL extracted from P-1M. The appearance of the P-1MΔIR biofilm was also similar to that of the P-1MΔI and P-1MΔR biofilms, both with and without P-1M AHL extract. These results suggested that in P-1M, biofilm structure is regulated by a quorum-sensing system that is activated by the MpoR-AHL complex. After washing with water by vortexing for 5 s, the P-1M biofilm was completely removed from the surface of the glass tube. However, biofilms of P-1MΔI, P-1MΔI, and P-1MΔIR were strongly adhered to the surface of the glass tube and could not be removed by vortexing with water. The addition of AHL extracts made the P-1MΔI biofilm easily removable, but not the other deletion mutant biofilms. Next, two AHLs produced by P-1M, 3OHC14-HSL and C14:1-HSL, were individually added to the culture of P-1MΔI. The addition of C14:1-HSL made the P-1MΔI biofilm easily removable, but 3OHC14-HSL did not. In M. extorquens AM-1, AHL-mediated quorum-sensing positively regulates the production of EPS, which is one of the major matrix components of biofilms [Citation16]. In P-1M and the quorum-sensing deletion mutant strains, pellicle-like biofilm were formed at the liquid-gas interface of MB medium in a glass tube, and there was no difference in the area and thickness of biofilm production (). These results suggested MpoR form the complex with C14:1-HSL and regulate structural changes in the pellicle-like biofilm, but not significant change of EPS production.
Figure 3. Biofilm formation on the culture surface in glass tubes by M. populi P-1M and quorum-sensing deletion mutant strains, P-1MΔI, P-1MΔR, and P-1MΔIR. Bacterial strains were inoculated into 4 mL of MB medium in glass tubes with or without extracts from the wild-type P-1M strain. For P-1MΔI, C14:1-HSL or 3OHC14-HSL were also added to the medium at the final concentration of 1 μM. After incubation for 3 days at 30°C, pellicle-like biofilms were observed at the gas-liquid interface. The visual appearance of biofilms was shown as top and side views. The remaining biofilms after washing with distilled water were also shown.
In conclusion, our study demonstrated that quorum sensing affects the adhesive strength of biofilms formed on the glass tube in M. populi P-1M. Pink biofilms, which frequently contain Methylobacterium species, are rapidly generated and are difficult to remove under dry conditions [Citation7]. In addition, Methylobacterium species exhibit higher stress-tolerance under nutrient limitation conditions [Citation7]. The results of our study suggest that there is a possibility to convert a difficult to remove biofilm to an easily removable biofilm by activating quorum sensing in Methylobacterium.
Author contributions
T.M. and T.I. conceived and designed the experiments. T.M. and X.X. performed the experiments and wrote the manuscript. All the authors have read and approved the final manuscript.
Supplement_Figure.pdf
Download PDF (474.1 KB)Acknowledgments
We thank Mr. Ryota Moro from Utsunomiya University for technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346.
- Parsek MR, Greenberg EP. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc Natl Acad Sci USA. 2000 Aug 1;97(16):8789–8793.
- Dong SH, Frane ND, Christensen QH, et al. Molecular basis for the substrate specificity of quorum signal synthases. Proc Natl Acad Sci USA. 2017 Aug 22;114(34):9092–9097.
- Davey ME, O’Toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev. 2000;64(4):847–867.
- De Kievit T. Quorum sensing in Pseudomonas aeruginosa biofilms. Environ Microbiol. 2009;11(2):279–288.
- Tomlin KL, Malott RJ, Ramage G, et al Quorum-sensing mutations affect attachment and stability of Burkholderia cenocepacia biofilms. Appl Environ Microbiol. 2005;71(9):5208–5218.
- Yano T, Kubota H, Hanai J, et al Stress tolerance of Methylobacterium biofilms in bathrooms. Microbes Environ. 2013;28(1):87–95.
- Xu FF, Morohoshi T, Wang WZ, et al Evaluation of intraspecies interactions in biofilm formation by Methylobacterium species isolated from pink-pigmented household biofilms. Microbes Environ. 2014;29(4):388–392.
- Furuhata K, Kato Y, Goto K, et al Identification of pink-pigmented bacteria isolated from environmental water samples and their biofilm formation abilities. Biocontrol Sci. 2008 Jun;13(2):33–39.
- Furuhata K, Goto K, Kato Y, et al Characteristics of a pink-pigmented bacterium isolated from biofilm in a cooling tower in Tokyo, Japan. Microbiol Immunol. 2007;51(6):637–641.
- Kelley ST, Theisen U, Angenent LT, et al Molecular analysis of shower curtain biofilm microbes. Appl Environ Microbiol. 2004;70(7):4187–4192.
- Simões LC, Simoes M, Vieira MJ. Biofilm interactions between distinct bacterial genera isolated from drinking water. Appl Environ Microbiol. 2007;73(19):6192–6200.
- Poonguzhali S, Madhaiyan M, Sa T. Production of acyl-homoserine lactone quorum-sensing signals is wide-spread in gram-negative Methylobacterium. J Microbiol Biotechnol. 2007;17(2):226.
- Nieto Penalver CG, Morin D, Cantet F, et al Methylobacterium extorquens AM1 produces a novel type of acyl-homoserine lactone with a double unsaturated side chain under methylotrophic growth conditions. FEBS Lett. 2006;580(2):561–567.
- Pomini AM, Cruz PL, Gai C, et al Long-chain acyl-homoserine lactones from Methylobacterium mesophilicum: synthesis and absolute configuration. J Nat Prod. 2009;72(12):2125–2129.
- Penalver CGN, Cantet F, Morin D, et al A plasmid-borne truncated luxI homolog controls quorum-sensing systems and extracellular carbohydrate production in Methylobacterium extorquens AM1. J Bacteriol. 2006;188(20):7321–7324.
- Morohoshi T, Ikeda T. Complete genome sequence of Methylobacterium populi P-1M, isolated from pink-pigmented household biofilm. Genome Announc. 2016;4(3):e00458–e00416.
- McClean KH, Winson MK, Fish L, et al. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997;143:3703–3711.
- Morohoshi T, Kato M, Fukamachi K, et al N-acylhomoserine lactone regulates violacein production in Chromobacterium violaceum type strain ATCC 12472. FEMS Microbiol Lett. 2008 Feb;279(1):124–130.
- Morohoshi T, Yamaguchi T, Xie X, et al. Complete genome sequence of Pseudomonas chlororaphis subsp. aurantiaca reveals a triplicate quorum-sensing mechanism for regulation of phenazine paroduction. Microbes Environ. 2017 Mar 31;32(1):47–53.
- Okutsu N, Morohoshi T, Xie X, et al Characterization of N-acylhomoserine lactones produced by bacteria isolated from industrial cooling water systems. Sensors. 2015;16(1):44.
- Schafer A, Tauch A, Jager W, et al. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994 Jul 22;145(1):69–73.
- Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat Biotechnol. 1983;1(9):784–791.
- Altschul SF, Gish W, Miller W, et al. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410.
- Vuilleumier S, Chistoserdova L, Lee MC, et al Methylobacterium genome sequences: a reference blueprint to investigate microbial metabolism of C1 compounds from natural and industrial sources. PLoS One. 2009;4(5):e5584.
- Scholz RL, Greenberg EP. Positive autoregulation of an acyl-homoserine lactone quorum-sensing circuit synchronizes the population response. mBio. 2017 Jul 25;8:4.
- Nealson KH, Platt T, Hastings JW. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970;104(1):313–322.
