ABSTRACT
The MinE protein plays an important role in plastid division. In this study, the MinE gene was isolated from the cassava (Manihot esculenta Crantz) genome. We isolated high quality and quantity protoplasts and succeed in performing the transient expression of the GFP-fused Manihot esculenta MinE (MeMinE) protein in cassava mesophyll protoplasts. The transient expression of MeMinE-GFP in cassava protoplasts showed that the MeMinE protein was located in the chloroplast. Due to the abnormal division of chloroplasts, overexpression of MeMinE proteins in cassava mesophyll protoplasts could result in fewer and smaller chloroplasts. Overexpression of MeMinE proteins also showed abnormal cell division characteristics and minicell occurrence in Escherichia coli caused by aberrant septation events in the cell poles.
Graphical Abstract
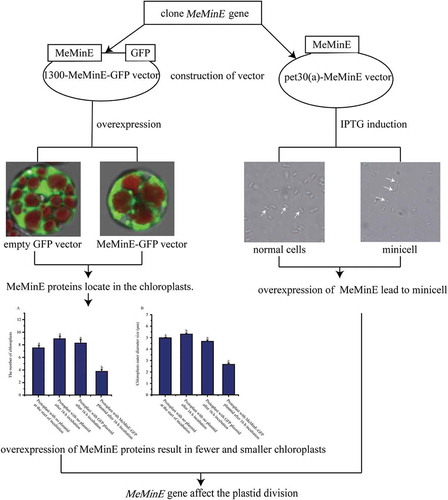
MeMinE proteins function in cassava chloroplasts and Escherichia coli. The MinE protein plays an important role in plastid division.
Cassava is an important tropical crop staple food in subtropical and tropical regions [Citation1]. Cassava is the best raw material for the production of ethanol fuels [Citation2]. Fresh cassava tuberous root contains 30% starch [Citation3]. Therefore, currently, cassava has a good development prospect in agriculture and industry.
The plastids undertake the metabolic activity of key products such as starch, fatty acids, amino acids, nucleic acids, and some soluble proteins [Citation4]. In plants, pre-existing cytosolic plastid binary fission reproduces chloroplasts [Citation5]. Chloroplast division relies on the internal division machinery FtsZ ring [Citation6], the external division machinery (mainly the dynamin-like ARC5 ring) [Citation5,Citation7], and other associated factors. Due to an endosymbiotic event between a photosynthetic prokaryote and a eukaryotic host original plastid, the plastid division mechanism can be dissected by testing the mechanism of bacterial cell division [Citation8]. Recently, details about how cytokinesis works in prokaryotes have been well studied [Citation9]. FtsZ proteins assemble into a Z ring that originally appearing in the cytokinesis of most prokaryotes. A gradient of negative regulators determine the position of the Z ring assembly. In Escherichia coli, the positioning of the Z ring is regulated through a fascinating oscillation causing by the Min system, which is composed of three proteins MinC, MinD, and MinE. The Min system inhibits the formation of the Z ring away from the midcell [Citation9–Citation12]. These three proteins are conserved in most bacteria [Citation13]. MinC with topological specificity acts as an inhibitor of FtsZ polymerization [Citation14,Citation15], while its normal activities require the MinD protein. MinD is an ATPase, which is peripherally associated with the cytoplasmic membrane [Citation16,Citation17]. MinC and MinD cooperate with each other to form an inhibitor of cell division [Citation18,Citation19]. However, the MinCD division inhibitor prevents all septation division sites both central and polar. All of this can be overcome when MinE is present [Citation20]. MinE is a small protein consisting of 88 residues, and it is a dimer with two functional domains [Citation21]. The first domain is present in the N-terminal (residues 1 to 31) domain with anti-MinCD activity which can suppress the MinCD division inhibitory activity. This domain binds to MinD, causing MinD to dissociate from the membrane, thus eliminating the effect of the MinCD on the division site allowing FtsZ to form the Z ring [Citation9]. The second domain is present in the C-terminal (residues 32 to 88), where it spatially restricts the activity of MinCD [Citation9]. Previous studies indicated that the two domains are not completely independent. The C-terminal domain interacts with the N-terminal domain [Citation9,Citation22]. MinC, MinD and MinE are coordinately expressed to control the normal division of cells [Citation15].
Transient gene expression can be used to study protein complexes, gene silencing as well as subcellular localization of proteins in vivo. Compared to stable genetic transformation, the transient expression could approach results much quicker and more cost-effective [Citation23]. Predecessors have already developed electroporation-mediated and polyethylene glycol (PEG) protoplast transient transformation strategies [Citation24,Citation25]. By utilizing plant protoplasts transient gene expression, we can quickly identify protein function in vivo. Successful plant protoplasts isolation can be traced back to over 50 years [Citation26]. The study of the isolation of cassava mesophyll protoplasts began in the early 1980s. Anthony obtained high quality mesophyll protoplasts in Manihot esculenta Crantz. cv. M. Thai 8 and other varieties [Citation27]. Wu et al. optimized the transient gene expression and mesophyll protoplast isolation factors in cassava [Citation28].
In this study, we cloned the cassava MinE gene and used bioinformatics software to analyze the function of the cassava MinE gene. In addition, we verified the role of the MinE gene in cassava mesophyll protoplasts. Finally, we introduced the cassava MinE gene into E. coli to verify whether the cassava MinE gene can affect the normal division of E. coli. The purpose of this study was to determine the role of overexpression of the MinE gene in cassava protoplasts, and to provide a theoretical basis for the further study of the MinE gene and the factors that influence plastid division in cassava.
Materials and methods
Plant materials
South China 8 (SC8) cassava variety materials were sampled in the Tropical Crops Genetic Resources Institute, Danzhou, China. Plantlets were cultured on one half of Murashige and Skoog (MS) medium with a photoperiod of 16 h light and 8 h dark at 26°C. The tender 8-week-old leaves were collected for protoplast isolation. Cassava young leaves (the first or second leaves from the top stem), mature leaves (the 7th or 8th leaves from the top stem), stems, fibrous roots, flowers, and fruits were harvested at 225 days for differential gene expression analysis. Samples of root xylems (the location of amyloplast development) were collected at the initial tuber stage (90 days), the expanding tuber stage (135 and 180 days), and the mature tuber stage (225 and 270 days) for the differential expression analysis of the MinE gene during tuber root development. Three biological samples were collected for each analysis. All fresh materials were preserved at −80°C after freezing in liquid nitrogen for subsequent RNA isolation.
Qpcr analysis
Total RNAs were extracted for each biological sample according to the RNAplant Plus reagent (TianGen, Beijing, China) manufacturer’s protocol. We used 2 micrograms of each RNA sample as the template to synthesize first-strand cDNA according to the PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa Biotechnology) manufacturer’s protocol. The specific primers MinE-F (5ʹ-TGTCGCCAGTATCTTTGTATTC-3ʹ) and MinE-R (5ʹ-TTGTCCTCTGAGTCTATTTCCAC-3ʹ) were designed according to the conserved region of the MeMinE (GenBank accession number: JN936177.1). β-tubulin (Phytozome name: 4.1_007598m.g) was amplified as an internal control (Tubulin-F 5ʹ-GTGGAGGAACTGGTTCTGGA-3ʹ Tubulin-R 5ʹ-TGCACTCATCTGCATTCTCC-3ʹ). qPCR was performed on an ABI 7900 Fast Instrument (Applied Biosystems, Foster City, CA, USA) using SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) (TaKaRa Biotechnology). The PCR thermocycler program was as follows: one cycle of 60 s at 95°C, 45 cycles of 5 s at 95°C and 30 s at 60°C, and melting curve analysis 15 s at 95°C, 15 s at 60°C, 15 s at 95°C. We used the 2−DDCt method to express the relative expression data [Citation29]. Three technical replicates were made from each biological sample.
Mine gene cloning and sequence analysis
We isolated total RNA from the leaf samples growing on one half of MS medium according to RNAplant Plus reagent (TianGen, Beijing, China) manufacturer’s protocol. According to the manufacturer’s instructions, cDNA was synthesized using the PrimeScript RT reagent Kit (TaKaRa Biotechnology, Otsu, Japan).
We designed primers with restriction enzyme sites Sal I and BamH I introduced to the both ends of the MeMinE gene respectively, MinE-F-Sal I (5ʹ-ACGCGGATCCATGGCGATATCGGGAG-3ʹ) and MinE-R-BamH I (5ʹ-CGGGATCCCCGAGTTCTTTCATCTGG-3ʹ) according to the MeMinE (GenBank accession number: JN936177.1) conserved region. The PCR program to amplify the MinE gene using TaKaRa LA Taq was as follows: 180 s at 94°C, and then 35 cycles of amplification (30 s at 94°C, 30 s at 60°C, 45 s at 72°C), finally extended at 72°C for 10 minutes. The PCR products were stored at 4°C and then were confirmed by multiple alignment using DNAman 6.0 software as MeMinE. The sequence alignment was performed with MinE from Manihot esculenta, Hevea brasiliensis, Arabidopsis thaliana, Chlamydomonas reinhardtii, and Escherichia coli using the DNAman 6.0 software. MEGA6 software was used to construct the phylogenetic tree by the neighbor-joining method with 1000 bootstrap samplings. Swiss-Model server (http://swissmodel.expasy.org) was used to assess the 3D structural model of MeMinE protein.
Plasmid construction
The PCR products were inserted between the Cauliflower mosaic virus (CaMV) 35S promoter and the enhanced green fluorescent protein (eGFP) of the expression vector pG1300 (eGFP: pCAMBIA1300), designated as 1300-MeMinE-GFP vector (), and the recombinant plasmid was used for the subcellular localization of the MeMinE protein. The expression vector pet30(a)-MeMinE was made in a similar manner, and the recombinant plasmid was used for the prokaryotic expression.
Protoplast isolation
The isolation of cassava protoplasts and cassava mesophyll protoplasts transformation were conducted as described by Wu et al. [Citation28] with some modifications. The status of leaves was a very crucial factor for extracting protoplasts. The tender 8-week-old leaves of plantlets were selected and were cut into thin strips about 1 mm wide from the red area ()) by using a fresh sharp razor blade in 9% mannitol solution. For each treatment, leaf strips of 0.8 ± 0.1 g were transferred gently into the prepared 10 mL enzyme solution (1.6% cellulase R-10, 0.8% macerozyme R-10, 0.1% BSA, 9% mannitol, 20 mM KCl, 10 mM CaCl2, 20 mM MES, pH 5.8). The enzyme solution was oscillated in the dark (25°C) at 45 rpm for 16 h to hydrolyze the cell wall.
Figure 2. Expression patterns of the MeMinE gene in storage root xylems during storage root developmental stages (A) and in cassava organs and tissues (B).
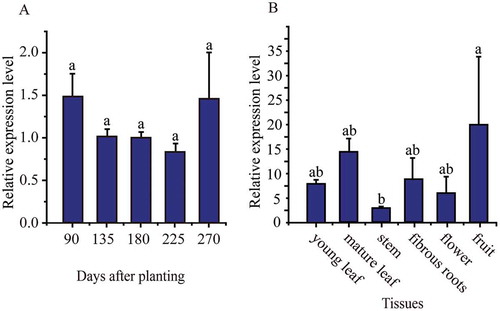
Figure 3. Genome, protein sequences and 3D Structures characterization of Manihot esculenta Crantz MinE. (A) Organization of Manihot esculenta Crantz MinE (JN936177.1) genomic structure. Exons are shown as black boxes and introns are shown as black lines. (B) Manihot esculenta Crantz MinE protein sequence (MeMinE, AFC37489.1) with the corresponding sequences of Hevea brasiliensis (HbMinE, XP_021685230.1), Arabidopsis thaliana (AtMinE, BAB79236), Chlamydomonas reinhardtii (CrMinE, ABK56835), and Escherichia coli (EcMinE, AAB59063). The black underlining indicates the putative signal peptide. The yellow underlining and yellow double-underlining indicate AMD (anti-MinCD domain) and TSD (topological specificity domain) in E. coli MinE, respectively. The N-terminal domain sequence is shown in the blue box, and the C-terminal domain sequence is shown in the red box. Black highlight, sky blue highlight, and magenta highlight indicate that homologous sequences have similarities of 100%, greater than 75%, and greater than 50%, respectively. (C) Predicted three-dimensional (3D) structure model of MeMinE. C1 is the predicted 3D structure models of MeMinE. C2 is the functional domains. The spherical area and rod area indicate AMD (anti-MinCD domain) and TSD (topological specificity domain) in E. coli MinE, respectively. The red area is implicated in MinE-membrane interaction. C3 is the fusion of C1 and C2.
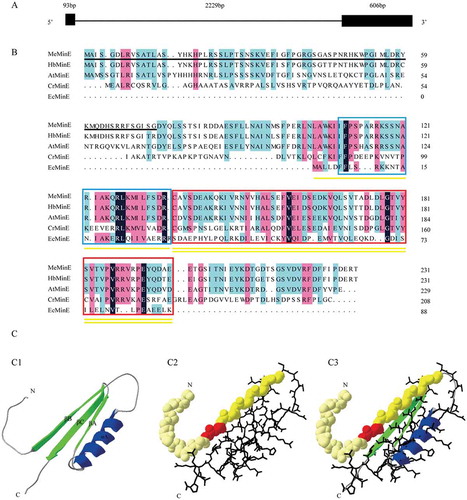
Figure 4. Phylogenetic tree of widespread MinE proteins.
The phylogenetic tree was constructed by the neighbor-joining method. The values shown at the branch nodes are the confidence levels of 1000 replicate bootstrap samplings.
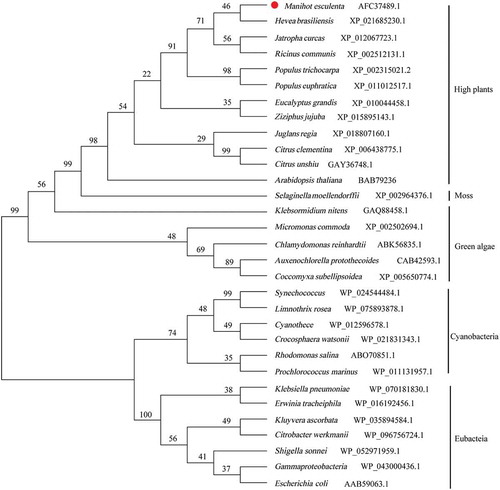
Figure 5. Isolation of protoplasts from cassava green tissue. (A) Representative healthy 8-weeks-old cassava seedling used for protoplasts isolation. Red markers indicate the optimal sections of seedlings yielding protoplasts. (B) Bright field image of protoplasts obtained under an Olympus microscope with a 20× objective. (C) Protoplasts stained with FDA under ultraviolet light obtained under an Olympus microscope with a 20× objective. The bars = 25 µm.
After the hydrolysis, 10 mL of the W5 solution (154 mM NaCl, 5 mM KCl, 125 mM CaCl2, and 2 mM MES, pH 5.7) was added. The digested tissues were filtered through double gauze, then collected by a centrifugation (4°C, 160 g for 7 min). We removed the supernatant as much as possible. Adding 4 mL W5 solution mixed gently, sediment was collected by centrifugation (4°C, 100 g for 4 min). Two mL of W5 solution were added to the protoplast suspension, which was then placed on ice for 30 minutes. The protoplasts were then collected by centrifugation (4°C, 160 g for 6 min). The MMG solution (4 mM MES, 15 mM MgCl2, 9% mannitol at pH 5.7) were added to make the protoplast density 1.0 × 107 per milliliter. The protoplasts were placed on ice throughout the process.
Fluorescein diacetate (FDA) and its derivatives are commonly used to detect bacterial [Citation30], plant cell [Citation31], and mammalian cell [Citation32] viabilities. To test the viability of protoplasts we used FDA (final concentration 0.01%) staining. Protoplast viability (%) determined as follows: (the number of fluorescent protoplasts observed/total number of protoplasts observed) × 100%.
Cassava mesophyll protoplast transformation
Twenty microgram of 1300-MeMinE-GFP plasmid (plasmid concentration reaches 1000 ng μL−1) were mixed gently with 200 μL protoplasts suspension. Then 220 μL freshly prepared PEG4000 solution (25% (W/V) PEG4000, 9% mannitol, and 0.1 M CaCl2) were immediately added. Then the mixture was incubated for 15 min at room temperature. After incubation, 880 μL W5 solution were added slowly and mixed by gentle inversion to stop the transfection process. The supernatant was removed after centrifugation (4°C, 200 g, 7 min). We add 500 μL of WI solution (9% mannitol, 20 mM KCl, 4 mM MES at pH 5.7) to resuspend the protoplasts. Protoplasts were then incubated for 16 hours at room temperature in the dark.
Overexpression of the memine in E. coli
The plasmid pet30(a)-MeMinE was transformed into E. coli BL21(DE3). To study the influence of the overexpression of the MinE proteins in E. coli, after overnight growth at 37°C, 400 μL of the cultures were added in 40 mL of the same medium. After about 4 h of growth, E. coli cells were in the exponential growth stage. The optical density (600 nm) values at this time was between 0.5 and 1.0. Isopropyl-1-thio-β-D-galactopyranoside (IPTG) was added to a final concentration of 4 mM at 37°C grown for 5 h.
Confocal laser scanning microscopy
We used Leica confocal laser scanning Microscope SP8 that was provided by Hainan Key Laboratory for Sustainable Utilization of Tropical Bioresource to detect the fluorescence in cassava protoplasts. All samples were commissioned by Hainan University professional operators to observe. For confocal microscopy, GFP and chlorophyll were excited at 488 nm and 512 nm, respectively, and the intensity was show as 26.59%. The pictures were detected by four different channels. The first channel emission wavelength was 500 nm to 540 nm, and the corresponding detector was PMT. The second channel emission wavelength was 649 nm to 700 nm, and the corresponding detector was PMT. The third channel emission wavelength was 792 nm to 800 nm, and the corresponding detector was HPD. The transmission channel detector was TLD. Protoplasts were magnified 4000 times by objective (HCX PL APO CS 40×/0.70 DRY) and eyepiece (Leica TCS SP8 10×) lenses. The confocal settings were: scan mode value of XYZ; scan direction X was unidirectional; scan speed value was 400 Hz; Z position value was 2925.4 µm.
Results
Differential expression analysis of the mine in cassava organs and tissues
Quantitative polymerase chain reaction (qPCR) was used to examine the expression of the MeMinE gene in cassava storage root xylems at 90, 135, 180, 225, and 270 days after planting ()). The MinE gene had relatively lower expression levels at expanding stage (135, 180, and 225 days), and relatively higher expression level at mature stage (270 days), whereas there was no significant difference between the expression levels at 90, 135, 180, 225, and 270 days after planting.
qPCR was used to examine the expression of the MeMinE gene in young and mature leaves, stems, fibrous roots, flowers, and fruits ()). The MeMinE gene was expressed in all tissues. A relatively higher expression level was observed in fruits, and a relatively lower expression level was observed in stem.
Structural analysis of memine
We aligned the MeMinE gene cDNA sequences with the cassava genomic sequence (http://www.phytozome.net/cassava). The results reveal that the MeMinE gene has two exons and one intron ()).
The other MinE amino acid sequences were compared with the amino acid sequences of the MeMinE ()). Signal P 4.1 Server analysis showed that at the N-terminal amino acids 1 to 73 MeMinE contains a putative signal peptide. The E. coli MinE protein has two functional domains: the C-terminal topological specificity domain (TSD) and the N-termnal anti-MinD domain (AMD) [Citation9,Citation20,Citation33]. The results showed that the MeMinE shared 90.9%, 60.8%, 21.2%, and 9.4% identity with MinE from Hevea brasiliensis, Arabidopsis thaliana, hlamydomonas reinhardtii, and Escherichia coli. respectively. Several high similarity sequences of MinE protein indicate that the MinE protein remains functionally conserved during the evolution of chloroplasts.
We used a known three-dimensional (3D) structure at 2kxo.1 (Swiss model template library identification number) of the MinE protein from Neisseria gonorrhoeae (shared 33% sequence identity with MeMinE) as a template through the Swiss model server to get a reasonable theoretical structure of the MeMinE. A SPDBV program was used for the late modification of the 3D models. The N-terminus sequence formed a β-sheet, and the C-terminus sequence formed one β-sheet and two α-helices ()).
Phylogenetic analysis of the memine protein
The phylogenetic relationship of MinE was compared using the MEGA6 program based on the amino acid sequences. We analyzed the amino acid sequences of MinE from eubacteria, cyanobacteria, green algae, moss, and high plants to construct a phylogenetic tree (). The phylogenetic relationship tree reveals a close relationship between the MeMinE protein and the MinE proteins of others. The MeMinE protein was shown to have a close relationship with the MinE protein from Manihot esculenta and Hevea brasiliensis that belong to the Euphorbiaceae family.
Protoplast isolation and subcellular localization of the GFP-fused MeMinE protein in cassava mesophyll protoplasts
Materials incubated on one-half of MS medium with a photoperiod of 16 h light and 8 h dark at 26°C for 8 weeks, were used for protoplast isolation ()). After 16 h plasmolysis, high quantity and quality protoplasts of which more than 90% vitality of cassava mesophyll protoplasts was obtained in our study (,)). Protoplasts with high concentration and viability can be used for plasmid transfection.
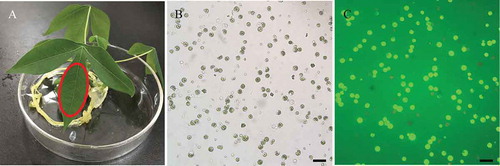
We performed the transient expression of the GFP-fused MeMinE proteins in cassava mesophyll protoplasts to test the feasibility subcellular localization of protein in cassava cells and the location of the MeMinE protein. After 16 hours of transfection, brilliant green fluorescence was observed. No-fused GFP proteins were distributed throughout the nucleus and cytoplasm, while GFP-fused MeMinE proteins were specifically detected as a single spot at one end of chloroplasts (,,). The white arrows indicated abnormal cleavage of the chloroplast after overexpression of the MinE proteins, with a small chloroplast appearing (,,)). We rarely found “mini cells” in the protoplasts (marked with white arrows) ()), similar to the small cells caused by the abnormal division of E. coli (). In addition, in order to better illustrate the repeatability of our experiments and the localization pattern of MinE protein on chloroplast, we have placed five sets of non-fused GFP and MinE-GFP plasmid protoplasts pictures in the supplementary materials. From these pictures, we could see that the number and size of chloroplasts were distinctively different in protoplasts that have been transformed with MeMinE-GFP plasmid and protoplasts that have been transformed with no-fused GFP plasmid.
Figure 6. Subcellular localization of the MeMinE protein in cassava mesophyll protoplasts.
Samples were visualized under a confocal microscope. A to D show images of no-fused GFP plasmid protoplasts used as negative control. E to H and I to L show images of MeMinE-GFP plasmid protoplasts. Bright field images of protoplasts, chlorophyll autofluorescence (Chl), and GFP signals as well as merged images of GFP are shown. Scale bar represents 10 µm.
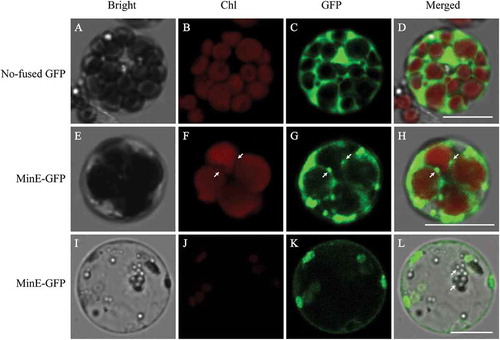
Figure 7. Comparison of the number and the size (outer diameters) of chloroplasts in protoplasts that were transfected with plasmid at the start of incubation and after 16 h of incubation, protoplasts that were transfected with no-fused GFP plasmid and MeMinE-GFP plasmid at start of incubation and after 16 h of incubation.
Different chloroplasts from different protoplasts were averaged and the statistical significance of differences between treatments and control was analyzed using the Student’s t-test. Same letters indicate no statistical difference at P > 0.05 among samples. n = 279 chloroplasts in 39 protoplasts that were not transfected with plasmid at the start of incubation, 300 chloroplasts in 39 protoplasts that were not transfected with plasmid after 16 h of incubation, 168 chloroplasts in 25 protoplasts that were transfected with no-fused GFP plasmid after 16 h of incubation, 70 chloroplasts in 27 protoplasts that were transfected with MeMinE-GFP plasmid after 16 h of incubation. Bars indicate the mean ± SE.
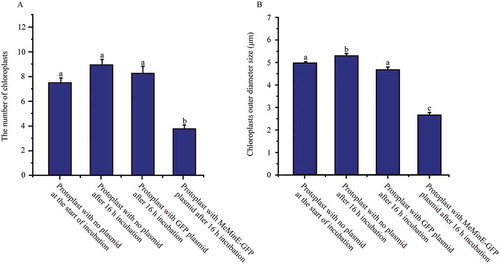
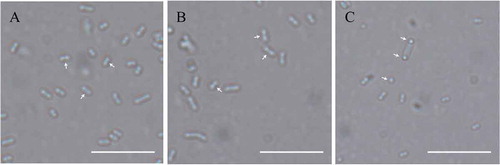
Figure 8. Overexpression of MeMinE proteins affect the normal division of E. coli. (A) Wildtype, BL21(DE3) at 0 μM IPTG. (B) Wildtype at 4 mM IPTG. (C) Cells transformed the plasmid pet30(a)-MeMinE at 4 mM IPTG. Scale bar represents 10 µm.
To test whether the MeMinE proteins overexpression could lead to changes in the number and size (outer diameters) of chloroplasts, we counted the number and size of chloroplasts at different times in protoplasts that were not transformed with plasmid, and protoplasts that were transformed with no-fused GFP plasmid and MeMinE-GFP plasmid. Statistical analysis revealed no significant changes in the number and size of chloroplasts in protoplasts that were not transformed with plasmid at the start of incubation, those that were not transformed with plasmid after 16 h of incubation, and protoplasts that were transformed with no-fused GFP plasmid after 16 h of incubation. In protoplasts that were transformed with MeMinE-GFP plasmid, we found that the number and size of chloroplasts significantly decreased after 16 h of incubation ().
Overexpression of memine proteins in E. coli
The image of E. coli cells was obtained under an Olympus microscope with a 100× objective. It is seen from the results that wild-type BL21(DE3) at 0 mM and 4 mM IPTG presented the correct placement of the division site at the middle (marked with the white arrows) (,)). Compared to the former cells transformed, the plasmid pet30(a)-MeMinE at 4 mM IPTG showed abnormal cell division characteristics leading to minicells (marked with the white arrows) ()) which caused by the aberrant septation events adjacent to the cell poles.
Discussion
The studies of the expression pattern of MeMinE provide a basis for understanding the MeMinE function. We found MeMinE expressed in all tested tissues and organs and highly expressed in rich chloroplasts and amyloplasts organs and tissues. One type of plastid division produces chloroplasts and amyloplasts [Citation5,Citation7]. The plastid carries important metabolic activity products [Citation4]. Therefore, changing the expression of the MeMinE gene may affect the division of plastids and affect the photosynthetic and starch accumulation of cassava.
Recalcitrant cell walls and thick cuticle may cause incomplete enzymatic hydrolysis [Citation34]. Therefore, we chose plantlets for protoplast isolation. The release of protoplasts is critically affected by source tissue, and the concentration of enzymes [Citation20,Citation28,Citation35,Citation36]. Wu et al. have successfully isolated high-quality cassava protoplasts and successfully used these for transient expression [Citation28]. We also isolated high quality and quantity protoplasts and succeed in performing the transient expression of GFP-fused MeMinE protein in cassava mesophyll protoplasts.
Our experiments show that the MinE protein is located on the chloroplast as a single spot at one end of chloroplasts. After our extensive observations, the reason that the detected signal intensity and range of GFP fused to MinE protein differs in different chloroplasts may be due to the uneven distribution of the GFP protein caused by the back and forth oscillation of the MinE protein in the chloroplast. Maple et al. report that AtMinE is a dynamic protein due to the different localization patterns of AtMinE in Arabidopsis mesophyll cells [Citation37]. This localization pattern is similar to the localization dynamics of the MinE protein in bacteria, where the MinE ring is a dynamic structure that moves from one cell pole to the opposite pole [Citation38]. We rarely found “mini cells” in protoplasts, similar to the small cells that result from abnormal division of E. coli (). These “mini cells” may be caused by the abnormal division of chloroplasts due to overexpression of MinE proteins. This “mimi cell” are considered not viable and rapidly degrade [Citation39]. This may be the reason why “mimi cells” are not easily observed. However, how these small cells are formed and decomposed requires further study.
To ensure the normal life activities of cells, the number and size of chloroplasts are controlled by nuclear genes [Citation40]. Therefore, the number and size of chloroplasts in normal cells should remain relatively stable. For protoplasts that were not transfected with plasmid at the start of incubation and after 16 h of incubation, protoplasts that were transformed with the no-fused GFP plasmid, we also found no significant changes in the number and size of chloroplasts. However, this changed when the MinE gene was overexpressed. For protoplasts that were transformed with the MeMinE-GFP plasmid, the number and size of chloroplasts significantly decreased after 16 h of incubation (). This is consistent with the results of previous studies in Arabidopsis caused by the abnormal division of chloroplast [Citation39,Citation41]. Itoh et al. showed that overexpression of Arabidopsis MinE has an inhibitory effect on chloroplast division [Citation42]. The authors suggested that this type of inhibition would be the result of the proteins behaving as physical obstacle, rather than by disruption of the chloroplast division machinery [Citation42]. However, our results show that in cassava it is not a large aggregate of GFP proteins imported into chloroplasts behaving as a physical obstacle (). In cassava, it is the overexpression of MinE proteins that results in fewer and smaller chloroplasts caused by the abnormal division of chloroplasts. Previous research shows that an appropriate chloroplast population and size in leaf cells are important to assure the photosynthetic capacity of the leaf [Citation43]. Therefore, whether overexpression of MinE proteins in cassava could causes an effect on cassava photosynthesis and growth requires further study.
Although the amino acid sequences of the MinE genes are quite different between cassava and E. coli, our results suggest that the MeMinE protein still acts and recognizes the division site of E. coli. Previous studies have shown that in the absence of EcMinE (Escherichia coli MinE) leads to the appearance of long filamentous cells; cells that divide from the middle into two normal cells under normal EcMinE conditions; overexpression of EcMinE leads to the classical minicell phenotype caused by the loss of topological specificity [Citation44]. Our research also found that overexpression of MeMinE in E. coli leads to minicells (). Wang et al. obtained similar results when they returned the Chlamydomonas reinhardtii MinE gene to an E. coli cell [Citation33]. The unicellular algae Chlamydomonas contains a single chloroplast. The Chlamydomonas cell division model is similar to that of higher plants because it grows alternately in the dark and light. Wang et al. thought that using Chlamydomonas as the model organism can provide many unique opinions of chloroplast division [Citation33,Citation45].
Predecessors have identified homologues of MinD and MinE proteins in the plant nuclear genome [Citation37,Citation46–Citation48], while no MinC homologs have been reported in plants to date. Therefore, a non-typical MinC-like protein may make up for this loss. Maple et al. have shown that the ARC3 protein interacts with several stromal division proteins and abnormal ARC3 levels lead to FtsZ-ring misplacement. Their research shows that the ARC3 protein is a bona fide stromal plastid division component and may replace the function of MinC protein in plants [Citation5].
Conclusion
In summary, the cassava MinE gene was isolated and characterized in this study. MeMinE was expressed in all tissues and organs and is highly expressed in rich chloroplasts and amyloplasts organs and tissues. The MinE protein remains functionally conserved during the evolution of chloroplasts. We isolated high quantity and quality protoplasts and used these for plasmid transfection. The MeMinE protein locates in the chloroplast, and overexpression of MeMinE proteins in cassava mesophyll protoplast can result in fewer and smaller chloroplasts. This indicates that this is not due to a large aggregation of GFP proteins imported into chloroplasts behaving as a physical obstacle. The MeMinE protein still acts and recognizes the division site of E. coli, leading to “minicells”. In conclusion, overexpression of the MeMinE gene could lead to changes in plastid division, which might affect the cassava photosynthetic capacity and storage root development. This provides new insight for the study of cassava photosynthesis capacity and molecular breeding.
Author contributions
Cong-Cong Wang and Lei Ke were responsible for all aspects of the research, including experimental design, data acquisition and analysis. Cong-Cong Wang was responsibility for the writing of manuscript; Liang-Jing Cao, Yuan Yao, Meng-Ting Geng worked on RNA extraction, differential expression; Ying Wang, Yu Xiao, Wu Huang, Xiao-Han Liu, Peng Cao participated in experimental procedures; Jian-Chun Guo and Yi Min were responsibility for the programs and all experiments, critically revised the manuscript and provided the final approval of the article.
Supplementary_materials.pdf
Download PDF (253.1 KB)Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 31160061, 31600196, 31671767, 31601359), the Natural Science Foundation of Hainan (No. 20153047); Scientific Research Fund of Young Teachers in Hainan University (No: hdkyxj201714); National Modern Industrial System of Cassava (CARS-HNGJC); Scientific Research Fund of Hainan University (QNJS-2011-12); National Nonprofit Institute Research Grant of CATAS-ITBB; First-class Discipline Construction Project of Biology in Hainan University.
Disclosure statement
The authors declare no conflict of interest.
Supplemental data
Supplemental data can be accessed here.
Additional information
Funding
References
- Maruthi MN, Bouvaine S, Tufan HA, et al Transcriptional response of virus-infected cassava and identification of putative sources of resistance for cassava brown streak disease. PloS one. 2014;9(5):e96642.
- Moorthy SN. Physicochemical and functional properties of tropical tuber starches: a review. Starch‐Stärke. 2002;54(12):559–592.
- Narayanan NN, Ihemere U, Ellery C, et al. Overexpression of hydroxynitrile lyase in cassava roots elevates protein and free amino acids while reducing residual cyanogen levels. PloS one. 2011;6(7):e21996. PubMed PMID: 21799761; PubMed Central PMCID: PMC3143114.
- Neuhaus HE, Wagner R. Solute pores, ion channels, and metabolite transporters in the outer and inner envelope membranes of higher plant plastids. Biochimica Et Biophysica Acta (Bba)-Biomembranes. 2000;1465:307–323.
- Maple J, Vojta L, Soll J, et al. ARC3 is a stromal Z-ring accessory protein essential for plastid division. EMBO Reports. 2007 Mar;8(3):293–299. PubMed PMID: 17304239; PubMed Central PMCID: PMC1808034.
- Yoshida Y, Miyagishima SY, Kuroiwa H, et al. The plastid-dividing machinery: formation, constriction and fission. Curr Opin Plant Biol. 2012 Dec;15(6):714–721. PubMed PMID: 22824141.
- Glynn JM, Miyagishima SY, Yoder DW, et al. Chloroplast division. Traffic. 2007 May;8(5):451–461. PubMed PMID: 17451550.
- KEELING PJ. Diversity and evolutionary history of plastids and their hosts. Am J Bot. 2004;91(10):1481–1493.
- Lutkenhaus J. Assembly dynamics of the bacterial MinCDE system and spatial regulation of the Z ring. Annu Rev Biochem. 2007;76:539–562. PubMed PMID: 17328675.
- Boer P. Advances in understanding E. coli cell fission. Curr Opin Microbiol. 2010 Dec;13(6):730–737. . PubMed PMID: 20943430; PubMed Central PMCID: PMC2994968.
- Natale P, Pazos M, Vicente M. The Escherichia coli divisome: born to divide. Environ Microbiol. 2013 Dec;15(12):3169–3182. . PubMed PMID: 23962168.
- Lutkenhaus J, Pichoff S, Du S. Bacterial cytokinesis: from Z ring to divisome. Cytoskeleton. 2012 Oct;69(10):778–790. . PubMed PMID: 22888013; PubMed Central PMCID: PMC3931253.
- Osteryoung KW, Nunnari J. The division of endosymbiotic organelles. Science. 2004 Dec 05;302(5651):1698–1704. . PubMed PMID: 14657485.
- Bi E, Lutkenhaus J. Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J Bacteriol. 1993;175(4):1118–1125.
- Pichoff S, Lutkenhaus J. Escherichia coli division inhibitor MinCD blocks septation by preventing Z-ring formation. J Bacteriol. 2001 Nov;183(22):6630–6635. . PubMed PMID: 11673433; PubMed Central PMCID: PMC95494.
- Pajd BOER, Re CROSSLEY, Li ROTHFIELD. Central role for the Escherichia coli minC gene product in two different cell division-inhibition systems. Proc Natl Acad Sci. 1990;87(3):1129–1133.
- Boer PAJD, Re CROSSLEY, Li ROTHFIELD. Roles of MinC and MinD in the site-specific septation block mediated by the MinCDE system of escherichia coli. J Bacteriol. 1992;174(1):63–70.
- PAJd B, Re C, Ar H, et al The MinD protein is a membrane ATPase required for the correct placement of the Escherichia coli division site. EMBO J. 1991;10(13):4371–4380.
- Hu Z, Lutkenhaus J. Topological regulation of cell division in escherichia coli involves rapid pole to pole oscillation of the division inhibitor MinC under the control of MinD and MinE. Mol Microbiol. 1999;34(1):82–90.
- C-R ZHAO, Pajd BOER, Li ROTHFIELD. Proper placement of the escherichia coli division site requires two functions that are associated with different domains of the MinE protein. Proc Natl Acad Sci. 1995;92(10):4313–4317.
- King GF, Y-L S, Maciejewski MW, et al. Structural basis for the topological specificity function of MinE. Nat Struct Biol. 2000 Nov;7(11):1013–1017. PubMed PMID: 11062554.
- Dennis R. Conformation studies of cell division regulator MinE by nuclear magnetic resonance and circular dichroism spectroscopy. Ottawa (Canada): University of Ottawa; 2006.
- Kim MJ, Baek K, C-M P. Optimization of conditions for transient Agrobacterium-mediated gene expression assays in Arabidopsis. Plant Cell Rep. 2009 Aug;28(8):1159–1167. . PubMed PMID: 19484242.
- Sheen J. Signal transduction in maize and arabidopsis mesophyll protoplasts. Plant Physiol. 2001;127(4):1466–1475.
- Tan B, Xu M, Chen Y, et al Transient expression for functional gene analysis using populus protoplasts. Plant Cell, Tissue and Organ Culture (PCTOC). 2013;114(1):11–18.
- E.C.Cocking. A. Method for the isolation of plant protoplasts and vacuoles. Nature. 1960;187(4741):962.
- Anthony P, Davey MR, Power JB, et al An improved protocol for the culture of cassava leaf protoplasts. Plant Cell, Tissue and Organ Culture (PCTOC). 1995;42(3):299–302.
- Wu J-Z, Liu Q, Geng X-S, et al Highly efficient mesophyll protoplast isolation and PEG-mediated transient gene expression for rapid and large-scale gene characterization in cassava (Manihot esculenta crantz). BMC Biotechnology. 2017;17(1):29.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001 Dec;25(4):402–408. . PubMed PMID: 11846609.
- Guilini C, Baehr C, Schaeffer E, et al. New fluorescein precursors for live bacteria detection. Anal Chem. 2015 Sep 1;87(17):8858–8866. PubMed PMID: 26260548.
- Larkin PJ. Purification and viability determinations of plant protoplasts. Planta. 1976;128(3):213–216.
- Fishman JM, Lowdell MW, Urbani L, et al Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model. Proc Natl Acad Sci. 2013;110(35):14360–14365.
- Wang L, Wu Z, Li L, et al A chlamydomonas reinhardtii nuclear-encoded MinE homologue recognizes the escherichia coli division site, and the evolutionary implications of MinE gene transfer from chloroplast to nucleus. J Plant Biol. 2017;60(2):154–162.
- Guo J, Morrell-Falvey JL, Labbe´ JL, et al. Highly efficient isolation of Populus mesophyll protoplasts and its application in transient expression assays. PloS one. 2012;7(9):e44908. PubMed PMID: 23028673; PubMed Central PMCID: PMC3441479.
- S-D Y, Y-H C, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. PubMed PMID: 17585298 Nat Protoc. 2007;27:1565–1572.
- Davey MR, Anthony P, Jb P, et al. Plant protoplasts: status and biotechnological perspectives. Biotechnol Adv. 2005 Mar;23(2):131–171. PubMed PMID: 15694124.
- Maple J, Chua N-H, Møller SG. The topological specificity factor AtMinE1 is essential for correct plastid division site placement in Arabidopsis. Plant J. 2002;31(3):269–277.
- Fu X, Shih YL, Zhang Y, et al. The MinE ring required for proper placement of the division site is a mobile structure that changes its cellular location during the Escherichia coli division cycle. Proc Natl Acad Sci U S A. 2001 Jan 30;98(3):980–985. PubMed PMID: 11158581; PubMed Central PMCID: PMC14695.
- Reddy MS, Dinkins R, Collins GB. Overexpression of the Arabidopsis thaliana MinE1 bacterial division inhibitor homologue gene alters chloroplast size and morphology in transgenic arabidopsis and tobacco plants. Planta. 2002 Jun;215(2):167–176. . PubMed PMID: 12029464.
- Leech AK. RM. Chloroplast Division and Expansion Is Radically Altered by Nuclear Mutations in Arabidopsis Thaliana1. Plant Physiol. 1992;99(3):1005–1008.
- Maple J, Chua N-H, Møller SG. The topological specificity factor AtMinE1 is essential for correct plastid division site placement in Arabidopsis. Plant J. 2002;31(3):269–277.
- Itoh R, Yoshida S. Reduced expression of the arabidopsis minE gene affects size and number of chloroplasts. CYTOLOGIA. 2001;66(4):427–430.
- Robertson EJ, Rutherford SM, Leech RM. Characterization of chloroplast division using the arabidopsis mutant arc5. Plant Physiol. 1996;112(1):149–159.
- Raskin DM, Boer P. The MinE ring: an FtsZ-independent cell structure required for selection of the correct division site in E. coli. Cell.. 1997;91(5):685–694.
- Aldridge C, Maple J, Moller SG. The molecular biology of plastid division in higher plants. J Exp Bot. 2005 Apr;56(414):1061–1077. . PubMed PMID: 15753112.
- Colletti KS, Tattersall EA, Pyke KA, et al A homologue of the bacterial cell division site-determining factor MinD mediates placement of the chloroplast division apparatus. Curr Biol. 2000;10(9):507–516.
- Wakasugi T, Nagai T, Kapoor M, et al Complete nucleotide sequence of the chloroplast genome from the green alga Chlorella vulgaris: the existence of genes possibly involved in chloroplast division. Proc Natl Acad Sci. 1997;94(11):5967–5972.
- Moehs CP, Tian L, Osteryoung KW, et al Analysis of carotenoid biosynthetic gene expression during marigold petal development. Plant Mol Biol. 2001;45(3):281–293.