ABSTRACT
We determined if Japanese Rice Wine (Sake) had inhibitory effects on stress-induced enhancement of masseter muscle (MM) nociception in the rats. Male rats were subjected to the repeated forced swim stress (FS) or sham conditionings from Day −3 to −1. Daily administration of Sake or saline was conducted after each stress conditioning. At Day 0 the number of Fos positive cells, a marker for neural activity, was quantified at the trigeminal subnucleus caudalis (Vc) region by MM injury with formalin. FS increased MM-evoked Fos expression in the Vc region, which was inhibited by Sake compared to saline administration. Sake did not alter the number of Fos positive cells under sham conditions, indicating that inhibitory roles of Sake on neural activity in the Vc region were seen under FS conditions. These findings indicated that Sake had inhibitory roles on stress-induced MM nociception at the Vc region in our experimental conditions.
Graphical Abstract
Sake has inhibitory roles on psychophysical stress responses such as pain.
Chronic stress is often causes psychological distress such as anxiety and depression, which lowered our quality of life. Thus, we need to have better ways to go out and cope with adverse stress responses on the daily basis, because stress is unavoidable factors in our life. So called, life style medicine approach is documented to promote healthier lives through salutary environments and healthier life choices such as nutritional interventions [Citation1], and could be reasonable ways to prevent stress-related negative body reactions in terms of medical care and economical issues [Citation2]. Alcohol intake as a recreational substance is known to reduce stress responses, when it is used with an appropriate dose and displayed varieties of health benefits including relaxation and pain relief. In human research, the mortality rate was lower in men who had moderate alcohol intake than in either abstainers or heavier drinkers in western countries [Citation3] and in Japan [Citation4]. Moderate, but not heavy alcohol intake was associated with lower risk of incident of depression [Citation5] and dementia [Citation6].
Ample studies have provided the evidence on critical roles of chronic stress in the etiology of depression that is found to increase susceptibility to develop pain response and exacerbate existing pain, referred to as stress-induced hyperalgesia [Citation7–Citation10]. These findings indicated a linkage of brain circuits to induce depression and pain. Several reports showed that increases in neural activities at the level of second order neurons including the spinal dorsal horn and trigeminal subnucleus caudalis (Vc) regions could be essential to induce stress-induced hyperalgesia under stress conditions [Citation10–Citation14]. Recently we reported that preventing psychological distress by antidepressant drug inhibited jaw muscle nociception indicated by Fos protein expression in the Vc and cervical spinal cord regions [Citation15]. These findings suggested that regulation of neural excitability in the second order neurons could be important to prevent facilitatory effects of stress on nociception. However, it is not fully understood if consumption of alcohol beverage has modulatory roles on neural dysfunction that could inhibit stress-induced hyperalgesia.
Among various alcohol beverages in the world, Japanese Rice Wine, Sake, is a traditional alcohol drink in Japan, and it is known that Sake could contribute to the health promotion including reduction of stress responses. Sake derives from fermented rice and generally contains an ethanol content of approximately 15 percent and non-ethanol contents, and both contents have potentials to modulate and/or improve physiological function under stress conditions. For example, Sake showed hepatoprotective [Citation16,Citation17], anti-depressive [Citation18], and anti-inflammatory [Citation19] effects, while Sake contains gamma-aminobutyric acid (GABA), which has inhibitory roles on neural activity [Citation20,Citation21]. These findings indicated that various contents in Sake could have potential to improve impaired neural functions under chronic stress conditions; however, it remains unclear the modulatory roles of Sake on deep craniofacial nociception.
The aim of this study was thus to determine (1) whether ethanol and Sake intake affected depression-like behaviors, and (2) whether ethanol and Sake displayed inhibitory effects on increases in masseter muscle nociception indicated by Fos expression [Citation22] at the Vc region after repeated psychophysical stress conditioning.
Materials and methods
Animals
Experiments were conducted in accordance with International Association for the Study of Pain [Citation23] and approved by the Institutional Animal Care and Use Committee. All efforts were made to minimize the number of animals used for experiments and their suffering. Adult male Sprague-Dawley rats (250–300 g, SLC Japan, Shizuoka, Japan) were used. Animals were housed in plastic cages (two rats per cage), with free access to food and water for at least 7 days before the start of stress conditioning. Cages remained in temperatures of 25 ± 2°C and were light-controlled protected units (12:12 h light: dark cycle with light at 7:00 a.m.).
Repeated forced swim stress (FS) treatment ()
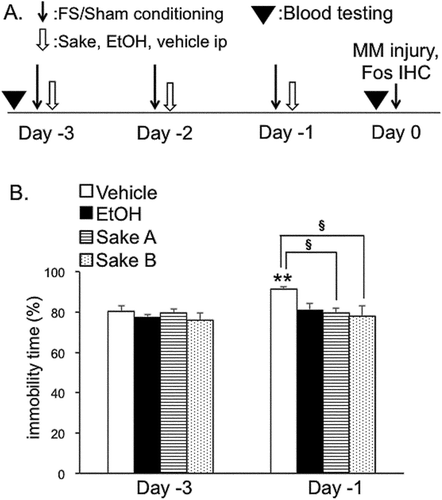
Repeated FS treatment protocol involved placing a rat in a plastic cylinder (diameter 30 cm, height 50 cm) containing 30 cm water (25–27°C) for 30 min/day between 09:00 and 11:00 am for 3 days (Days −3, −2, and −1) as described previously [Citation13,Citation24]. Fresh water was used in each session. Sham rats served as controls and were placed in an empty swim chamber using the same schedule. During the FS conditioning “immobility” times were recorded. Immobility was defined as the time spent in minimum body movement to keep the head above water, while struggling was defined as active diving, jumping or moving of the limbs to break the surface of the water and attempting to escape the cylinder. Rats were dried in a warm environment after each FS conditioning. The number of rats for measurements of immobility time was as follows: vehicle (n = 10), ethanol (n = 7), Sake A (n = 5), and Sake B (n = 5).
Figure 1. Experimental designs and repeated forced swim stress. (A). Experimental design. Rats were subjected to daily sessions of forced swim stress (FS) or sham conditioning from Day −3 to Day −1 (10 min/day). Vehicle (saline) or 15% ethanol or Sake A, B was administered intraperiotoneally 30 min after FS and sham conditionings. On Day 0, rats were euthanized 2 h after unilateral masseter muscle injection of 5% formalin to examine the Fos expression in the Vc region. In separate sets of rats for Fos experiments, blood samples were collected to measure the concentration of ethanol. (B). The effects of ethanol and Sake A, B on immobility time (IT) during FS for 10 min. IT was significantly increased in vehicle-treated rats at Day −1 compared to Day −3. IT in ethanol-, Sake A or B-treated group was significantly shorter than that in vehicle-treated rats. **P < 0.01 vs. Day −3. § P < 0.05 vs. vehicle-treated rats.
Effects of alcohol or Japanese Rice Wine (sake) on fos expression under repeated FS conditions
Systemic intraperiotoneal (i.p.) administration of alcohol (15% ethanol) and 2 different Sake (Sake A and B), was performed daily 30 min after each FS and sham conditionings on Day −3, −2 and −1. These beverages of Sake were produced at the different Company in Niigata Prefecture, Japan, and are categorized Junmai Dai-Ginjyo-Shu. The type of Junmai Dai-Ginjyo-Shu is made with rice that is polished extensively and is added no ingredients such as ethanol during a ferment process. Generally, it is well accepted that Junmai Dai-Ginjyo-Shu is well known to be premium Sake. Although it is documented that alcohol intake showed biphasic effects on nociception in human study [Citation25], a previous report revealed that drinking 9-34g/man (approximately 150–560 mg/kg) of ethanol showed beneficial effects to lower mortality [Citation3]. Accordingly, we set the dose of Sake at 300 mg/kg as ethanol concentration for the current preclinical experiments. For example, we made i.p. injection of Sake at the dose of 100 mg/rat (Body weight: ~300g) ethanol. This value (300 mg/kg) appeared to be equal to 18-21g/60–70 kg in a body weight of average men in Japan. For ethanol alone-treated group, we employed 15% ethanol, which is contained in Sake. Saline (i.p. 2.5 ml/kg) injections served as vehicle controls. Fos immunoreactivity was induced by the unilateral masseter muscle injection of 5% formalin (0.02 ml). The number of rats for Fos experiment was as follows: ethanol (n = 5), Sake A (n = 5), Sake B (n = 5) and vehicle (n = 10) + FST +masseter muscle injury, and ethanol (n = 5), Sake A (n = 5), Sake B (n = 5) and vehicle (n = 5) + sham + masseter muscle injury.
Masseter muscle injury
On Day 0 (), masseter muscle injury was induced by 5% formalin (0.02 ml) injection into the central portion of the left masseter muscle under general anesthesia with pentobarbital sodium (60 mg/kg ip). The plane of anesthesia left the rat areflex to noxious pinch stimulation to the hind paw until animals were euthanized. All rats were allowed to survive for 2 hours after the formalin injection.
Tissue preparation and Fos immunohistochemistry
Rats were deeply anesthetized with pentobarbital sodium (70mg/kg i.p) 2 hours after masseter muscle injury, and perfused through heart with 200 ml cold saline, followed by 400 ml cold fixative (4% paraformaldehyde in 0.01 M phosphate buffer saline (PBS), pH = 7.4, 4°C). The caudal brainstem and upper cervical spinal cord were removed and postfixed overnight, and placed in 30% sucrose in PBS for two days at 4°C. Transverse frozen sections (50 µm-thick) were cut using a freezing microtome and transferred serially to multiwell tissue culture plates that contained cold 0.01 M PBS. Every other section was used for Fos immunohistochemical study. Sections were incubated successively in normal goat serum (NGS, 5%) for 1.5 hours, affinity purified rabbit c-fos polyclonal antibody (Santa Cruz Biotechnology, 1:1000) for 40 hours in 0.01 M PBS containing Triton-X (0.3%) and NGS (5%) at 4°C, biotinylated goat anti-rabbit IgG antibody (Vector 1:200) for 1.5 hours at room temperature, and avidin-biotin-peroxidase complex (Vector) for 1 hour. Fos-positive nuclei were visualized by incubation in diaminobenzidine-nickel solution activated by 0.01% peroxidase. After washing in tris-buffered saline (TBS), sections were mounted on untreated glass slides and air-dried. Sections were dehydrated in a graded ethanol series, cleared in xylene and coverslipped. Under bright-field illumination, Fos-positive cells were distinguished as homogenous black-gray elements with well-defined borders. Specific staining was abolished by omission of primary antiserum.
Data analysis-Fos immunohistochemistry
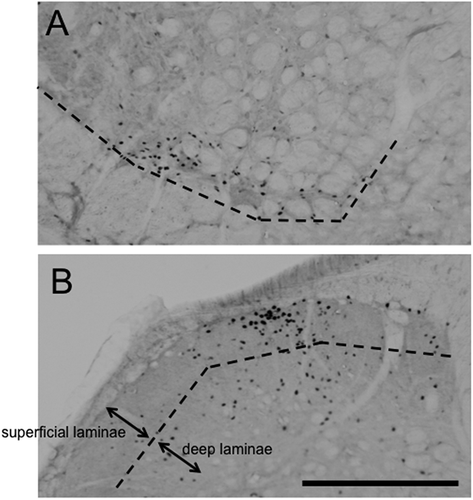
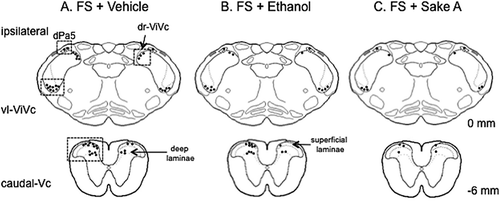
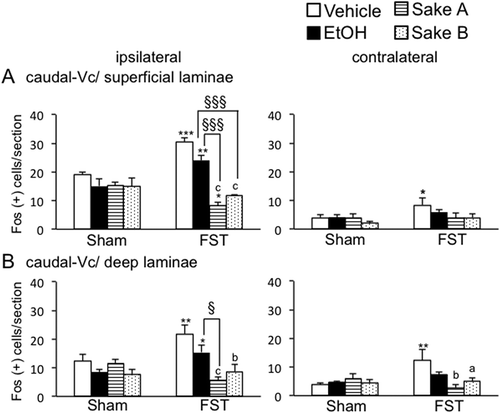
Sections were categorized in accordance with the rostral-caudal level at 1-mm intervals from 1 mm rostral to 6 mm caudal to the obex. The obex is a surface landmark defined by the caudal end of the 4th ventricle approximately 0.5 mm rostral to the most caudal tip of the trigeminal subnucleus interporalis (Vi) [Citation26]. Typically, 70–80 sections were obtained from a rat and there were 8–10 sections in 1-mm intervals. Sections were identified by the landmark structures at each level using an atlas [Citation27] and several reports [Citation28–Citation30]. The average number of Fos-positive cells was calculated in each area from 4 to 5 sections. Sections of individual rats were observed at a magnification of x 100 and the examiners were blinded to the treatment groups. To facilitate statistical analysis across multiple treatments, the average number of Fos-positive cells/section was compared for the following the brainstem portions. (): (1) the ventrolateral pole (vl) or the dorsal area (dr) of the trigeminal subnucleus interpolaris (Vi) and caudalis (Vc) transition (vl-Vi/Vc, dr-Vi/Vc, + 1 to −1 mm); dorsal paratrigeminal nucleus (dPa5, +1 to −1 mm), located along the dorsolateral edge of the main body of the trigeminal spinal nucleus and within the trigeminal spinal tract; the Vc and upper cervical spinal cord junction region (caudal-Vc, −4 to −6 mm, ). Separate cell counts were performed at the caudal-Vc regions for superficial (laminae I-II) and deep (laminae III-V) laminae [Citation28].
Figure 2. An example of the expression pattern of Fos-positive cells in vehicle (A). 15% ethanol (B). and Sake A (C). Treated FS rats drawn onto a series of camera lucida outlines. One dot = 5 Fos-positive cells. The boxed areas indicate the region with a high density of Fos expression being analyzed. Numbers to the right of the outline refer to the distance from the obex in mm.
Measurement of ethanol concentration in blood serum
We assessed the concentration of ethanol in blood serum to determine (1) the time course effect of ethanol concentration after Sake administration and (2) if daily administration of Sake for 3 days increases in ethanol level at Day 0, which might affect Fos expression by masseter muscle injury in sham and FS rats (n = 4 each). Blood samples were collected to assess the time course effects on the ethanol concentration before, 30 min and 60 min after Sake B administration (n = 4). Further, in separate experiments, blood samples were collected from repeated FS and sham rats 24 hours after the last Sake administration at Day 0 and just before rats were sacrificed (). The samples were centrifuged at 1,200 x g for 15 min at 4°C after coagulation, and then serums were collected. The concentration of ethanol in serum was measured by using enzymatic ethanol assay (Roche, Basel, Switzerland).
Statistical analysis
Variables are expressed as the mean ± standard error of the mean (SEM) and statistical analysis was performed using SPSS statistic (version 21.0; IBM, Armonk, NY, USA). The repeated forced swim stress test data for the immobility time (%) and ethanol concentration of blood serum were assessed using analysis of variance (ANOVA) and post hoc analysis comprised individual comparisons made using Bonferroni tests. For Fos immunohistochemistry, the number of Fos-positive cells was compared across treatment groups were made using ANOVA followed by Bonferroni tests. A probability level less than 0.05 was considered significant.
Results
Sake and ethanol effects on depression-like behavior
Rats were subjected to either FS (n = 27) or sham (n = 20) conditionings, and FS rats were employed to quantify FS-induced behavioral activity. The effects of ethanol and Sake A, B on immobility time expressed as a percentage of FS conditioning for 10 min were compared between Day −3 and Day −1. Two-way ANOVA revealed that ethanol and Sake had modulatory effects on the immobility time (F (3, 36) = 3.9, P < 0.05). In accordance with previous reports [Citation15,Citation31], post hoc analysis revealed that repeated FS significantly increased immobility time on Day −3 compared to Day −1 in vehicle-treated rats (P < 0.05, ), indicating that psychophysical stress increases depression-like behaviors. Next, the effects of Sake and ethanol (given daily, at 30 min after each FS) on immobility time were compared between Day −3 and Day −1 (). Ethanol, Sake A and Sake B (P < 0.05, ) prevented increases in immobility time during FS conditioning compared to vehicle treatment (vehicle ip: n = 10, ethanol; n = 7, Sake A: n = 5, Sake B: n = 5). These results indicated that FS-induced depression-like behavioral activity was reduced by daily administration of Sake in our experimental conditions.
Fos immunohistochemistry
FS effects on fos expression in the Vc region
shows an example of the Fos expression pattern evoked by masseter muscle injury with 5% formalin injection between treatment groups for FS rats. Fos expression appeared to be varied in number between treatments, but high density of Fos expression was seen mainly in the five spatially discrete regions within the Vc region including the vl-ViVc, dr-Vi/Vc, dPa5, superficial and deep laminae at the caudal-Vc regions as reported previously [Citation15,Citation32,Citation33]. Thus, we focused on these areas in which a substantial level of Fos positive cells (> 5 cells) was seen on the side ipsilateral to masseter muscle injury. demonstrated the micrographic examples for Fos expression evoked in the vl-Vi/Vc and caudal-Vc regions ipsilateral to formalin injection in FS rats. First, we determined the effect of FS on the number of Fos positive cells at the rostral-Vc region including the vl-Vi/Vc, dr-Vi/Vc, and dPa5 regions in vehicle-treated rats shown in . Fos expression evoked by formalin injection was significantly increased in all areas at the rostral-Vc region bilaterally in vehicle-treated FS rats compared to vehicle-treated sham rats (). Next, we analyzed FS effects on Fos expression in the caudal-Vc region in vehicle-treated rats. In both superficial and deep laminae at the caudal-Vc region, the number of Fos positive cells was significantly increased bilaterally in vehicle-treated FS rats compared to vehicle-treated sham rats ().
Figure 4. Effects of repeated FS on Fos expression in the rostral Vc regions including vl-Vi/Vc (A). dr-Vi/Vc (B). and dPa5 (C). regions after masseter muscle injection of 5% formalin in comparison to sham rats. The average number of Fos-positive neurons in the three areas of the Vc regions is shown. *P < 0.05, **P < 0.01, ***P < 0.001 vs. sham rats in each treatment groups. a P < 0.05, b P < 0.01, c P < 0.001 vs. vehicle-treated in FS rats. § P < 0.05, §§§ P < 0.001 vs. ethanol-treated in FS rats.
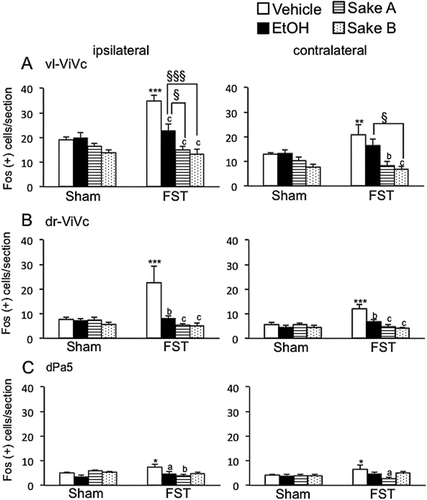
Figure 5. Effects of repeated FS on Fos expression in superficial and deep laminae at the caudal-Vc region after masseter muscle injection of 5% formalin in comparison to sham rats. The average number of Fos-positive neurons in each laminae of the Vc regions is shown. *P < 0.05, **P < 0.01, ***P < 0.001 vs. sham rats in each treatment groups. a P < 0.05, b P < 0.01, c P < 0.001 vs. vehicle-treated in FS rats. § P < 0.05, §§§ P < 0.001 vs. ethanol-treated in FS rats.
Ethanol and sake effects on Fos expression
We analyzed ethanol and Sake effects on Fos expression in each area in sham and FS rats. Two way-ANOVA revealed that ethanol and Sake had modulatory effects on the number of Fos positive cells significantly in the rostral Vc regions such as vl-Vi/Vc (F (3, 32) = 8.3, P < 0.001, ), dr-Vi/Vc (F (3, 32) = 5.3, P < 0.01, ) and dPa5 (F (3, 32) = 3.8, P < 0.05, ), superficial (F(3,32) = 11.4, P < 0.001, ) and deep (F (3, 32) = 7.4, P < 0.01, ) laminae at the caudal-Vc ipsilateral to formalin injection in FS rats. Post hoc analysis revealed that Fos expression under FS condition was significantly decreased after ethanol administration in the vl-Vi/Vc (P < 0.001, ), dr-Vi/Vc (P < 0.01, ) and dPa5 (P < 0.05, ) in comparison to vehicle treatment. However, ethanol did not affect Fos expression in the caudal-Vc region in FS rats (). These findings indicated that rostral-Vc region could be more sensitive to ethanol than caudal-Vc region in FS rats. In sham rats, ethanol had no effects on Fos expression evoked by masseter muscle injury in all areas (, ). Next, we determined the effect of Sake on the number of Fos-positive cells in the caudal-Vc region between sham and FS rats. Post hoc analysis revealed that the number of Fos positive cells was significantly decreased after Sake administration in the vl-Vi/Vc (P < 0.001, ), dr-Vi/Vc (P < 0.001, ) and dPa5 (Sake A; P < 0.01, ), superficial (P < 0.001, ) and deep (Sake A; P < 0.001, Sake B; P < 0.01, ) laminae at the caudal-Vc region ipsilateral to formalin injection compared to vehicle-treated rats.
On the contralateral side of formalin injection, two-way ANOVA revealed that sham and FS rats treated with ethanol and Sake exhibited significant effects of Fos expression in the vl-Vi/Vc (F (3, 32) = 9.8, P < 0.001, ), dr-Vi/Vc (F (3, 32) = 6.1, P < 0.01, ), deep laminae at the caudal-Vc regions (F (3, 32) = 4.0, P < 0.05, ). Post hoc analysis revealed that Fos expression was significantly decreased after Sake A, B administration in each area. In the rostral-Vc areas contralateral to masseter muscle injury, Sake significantly decreased Fos expression in the vl-Vi/Vc (Sake A: P < 0.01, Sake B: P < 0.001, ) and dr-Vi/Vc (Sake A, B: P < 0.001, ) regions compared to vehicle-treated FS rats. In the caudal-Vc areas contralateral to masseter muscle injury, Sake significantly decreased Fos expression in deep (Sake A: P < 0.01, Sake B: P < 0.05, ), but not in superficial laminae, indicating that that modulatory roles of Sake on neural activity in deep laminae seemed to be different from those in superficial laminae at the caudal-Vc region. In sham rats, Sake did not affect the number of Fos-positive cells in each area bilaterally (, ). These findings indicated that effect of daily administration of Sake on Fos expression seemed not to be carried over at Day 0.
Blood ethanol levels
First, we determined the time course effects of ethanol concentration for 60 min after Sake B administration in naive rats (). One way-ANOVA revealed that Sake B had significant modulatory roles on ethanol concentration in blood serum after Sake B administration (F (2, 10) = 4.7, P < 0.05, )). Post hoc analysis revealed that Sake B significantly increased the concentration of ethanol in blood serum at 30 min (95 ± 41 mg/L, P < 0.05) but not 60 min (39 ± 27 mg/L) compared to 0 min (0.1 ± 0.02 mg/L) in naive condition (). A greater concentration of ethanol at 30 min compared to 60 min indicated that ethanol level could reach at the maximum around 30 min.
Figure 6. Ethanol concentration. (A). Time course effects of ethanol concentration after systemic administration of Japanese Rice Wine (Sake B) in naive rats. *P < 0.05 versus 0 min. (B). Comparisons of ethanol concentration in blood serum at Day 0 between naive rats without Sake B, sham and FS rats with daily administration of Sake B from Day −3 to −1 immediately after sham or FS conditioning. *P < 0.05 versus naive rats without Sake B.
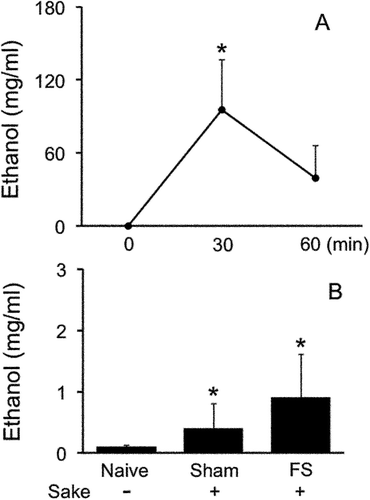
Next, we determined the ethanol concentration in blood serum after repeated Sake B administration for 3 days in sham and FS rats (, ). One-way ANOVA revealed that repeated Sham and FS rats with Sake B administration had significant influences on ethanol concentration (F (2, 10) = 5.1, P < 0.05, ). Post hoc analysis revealed that the ethanol concentration measured 24 hours after the last Sake administration at Day 0 in FS rats (0.9 ± 0.7 mg/L, P < 0.05) and Sham rats (0.4 ± 0.4 mg/L, P < 0.05) were significantly greater than those in naive rats treated with saline (0.1 ± 0.02 mg/L). Collectively, repeated Sake-treated rats regardless of FS conditionings showed small but significant increases in ethanol concentration compared to saline-treated naive rats.
Discussion
For centuries, alcohol beverages have been consumed around the world. Researchers have attempted to assess the effects of alcohol intake on stress reduction as well as its adverse effect. Accordingly, clinical studies revealed that stress conditionings can induce biological responses including psychophysical reactions, changes in skin conductance, muscle tension, cardiovascular responses and brain functions, while alcohol consumption could decrease magnitudes of such organ responses to various stress conditionings [Citation34,Citation35]. Preclinical studies revealed that intakes of alcohol beverages reduced conflict-induced stress reactions in cats [Citation36] and anxiolytic effects in rats [Citation37]. Our current results, in general, supported the hypothesis that daily administration of Japanese Rice Wine, Sake, had inhibitory roles on depression-like behaviors and enhanced masseter muscle nociception under psychophysical stress conditions.
Enhancement of masseter muscle nociception under FS condition
It is well documented that chronic stress as critical factors in the vulnerability to psychological distress could result in enhanced pain sensitivity due to dysfunction of the central nervous system [Citation7,Citation38,Citation39]. Evidence supported the idea that dysfunction of the central nervous system contributes to enhance pain responses under chronic stress conditions, so called stress-induced hyperalgesia [Citation7,Citation39]. Among various areas in the central nervous system, increases in neural activities at the spinal cord have been documented to play critical roles in stress-induced hyperalgesia in the hind paw using chronic restrain and repeated forced swim stress models [Citation10,Citation40,Citation41], while our previous and current studies revealed that repeated psychophysical stress were sufficient to amplify nociceptive responses in the trigeminal subnucleus caudalis (Vc) region, which is widely known to play critical roles in deep craniofacial nociception by masseter muscle [Citation15] and TMJ stimulation [Citation12–Citation14]. These findings indicated that neural signals induced by psychophysical stress and nociceptive processing to the deep craniofacial tissues were integrated by the central nervous system mechanisms that included the Vc region, and preexisting psychological distress had facilitatory roles on deep craniofacial nociception at the Vc region.
Recently, we determined if antidepressant drug, the selective serotonin-reuptake inhibitor (SSRI), which was administered daily just after each repeated FS for 3 days [Citation15], could prevent enhanced masseter muscle nociception indicated by Fos expression evoked by masseter muscle injury in the Vc region. Results revealed that SSRI decreased FS-enhanced Fos expression in the Vc region, indicating that regulation of psychophysical stress responses by antidepressant drug could be linked to the reduction of FS-enhanced masseter muscle nociception. These results clearly motivated us to investigate the beneficial effects of alcohol beverage consumption on stress-related enhanced MM nociception.
Roles of Japanese Rice Wine on enhanced masseter muscle nociception in the Vc region
In this study, we assessed the beneficial roles of Japanese Rice Wine, Sake, on decreases in negative reactions in the body like stress reactions. In Japan, Sake has traditionally played important roles in the life and culture of Japanese people. The unique and complex brewing processes that employed two different types of microbes, Aspergillus oryzae and yeast can produce ethanol and variety of non-ethanol constituents such as glucose, varieties of amino acid, and peptides [Citation42]. Although biochemical studies demonstrated that Sake also contained various constituents including ethanol and non-ethanol substances such as peptides, ion and glucose, which could regulate several physiological activities [Citation34], it remains unclear that Sake could reduce stress-evoked physiological responses including enhanced nociception.
We assessed if systemic administration of ethanol and Sake for 3 days just after each FS conditioning altered depression-like behaviors and masseter muscle nociception indicated by Fos expression. Ethanol and Sake A, B had inhibitory effects on depression-like behaviors. On the other hand, consistent with previous reports [Citation13,Citation15], our current results revealed that FS conditionings enhanced Fos expression in the Vc region, while Sake administered for 3 days decreased Fos expression by formalin injection at the Vc region. In sham rats, both Sake and ethanol had no modulatory effects on Fos expression in the Vc region. These findings, collectively, indicated that daily administration of Sake and ethanol had less modulatory effects of masseter muscle nociception in the absence of psychophysical stress. Despite a small, but significant inhibitory effect of ethanol alone on Fos expression in the vl-Vi/Vc, dr-Vi/Vc and dPa5 regions, a noteworthy finding indicated that inhibitory roles of Sake on Fos expression appeared to be greater than those of ethanol alone especially in the caudal-Vc region. These findings clearly indicated that non-ethanol constituents in Sake had significant roles to prevent enhanced masseter muscle nociceptive responses in the Vc region under FS conditions.
It has been demonstrated that Sake contained several non-ethanol constituents that could influence physical reactions. For example, pyroglutamyl peptides were identified in commercially available Sake, while wheat-derived pyroglutamyl peptides in wheat gluten hydrolysates exert biological activities such as hepatoprotective [Citation17], anti-inflammatory [Citation19] and anti-depressant effects after FS [Citation18]. At this point, it remains unclear if Sake-, but not wheat-derived pyroglutamyl peptides could exert modulatory roles on stress-related reactions; however, these finding suggested that inhibitory effects of pyroglutamyl peptides in Sake on depression-like responses could prevent increases in masseter muscle nociception after FS. At this point, it remains unclear how much doses of pyroglutamyl peptides appeared to be included in the Sake. Further, besides pyroglutamyl peptides, evidence demonstrated that gamma-aminobutyric acid (GABA), which plays critical roles in human health promotion, has been identified in Sake [Citation20,Citation43]. GABA is particularly known as a major inhibitory transmitter in mammalian brain tissues [Citation44]. Animal studies revealed that FS conditionings decreased GABA release in the spinal dorsal horn with increases in nociceptive behaviors [Citation10], while peripheral administration of GABA showed inhibitory effects on deep craniofacial nociception [Citation45]. The precise mechanism underlying GABAergic inhibitory effects on Fos expression in the Vc region remains unclear, but one might consider that peripheral rather than central effects of GABA derived form Sake reduced masseter muscle nociceptive responses that were enhanced under stress conditions [Citation46,Citation47], because GABA, in general, had less permeability of blood brain barrier. Although those findings shown above do not provide precise mechanisms for decreases in masseter muscle nociception, inhibitory effects of Sake on stress responses in the brain would be crucial factors to inhibit stress-induced increases in nociception.
One might consider that inhibitory roles of ethanol on masseter muscle nociception under FS conditions could be less, since inhibitory effects of ethanol alone were seen only in rostral-Vc (). Previous reports revealed that caudal- rather than rostral-Vc region play critical roles on nociception [Citation13–Citation15]. However, ample evidence has indicated antinociceptive roles of ethanol. For example, acute and chronic administration of ethanol produced anti-nociceptive effects in the hindpaw [Citation48], and those in the temporomandibular joint region [Citation49], and these findings, especially the latter one, indicated the inhibitory roles of ethanol on trigeminal nociception. Although precise mechanisms underlying the inhibitory effects of ethanol on nociception remains unclear, ethanol had modulatory roles on brain activity via several molecular functions. For example, several receptors like purinergic [Citation50], potassium [Citation51], GABA [Citation52], and NMDA [Citation53] receptors have been known to play critical roles of ethanol on stress and nociceptive responses. However, these reports shown above investigated the effect of ethanol on nociception in acute pain models, but not in repeated-FS-induced pain models. Further, a low concentration of ethanol in blood serum at Day 0 (~ 1.5 mg/L, ) after repeated administration of Sake indicated that direct effects of ethanol on Fos expression could be excluded, since ethanol has physiological effects on the brain system at blood concentration as low as 90 mg/L [Citation54].
Clinical relevance
It is documented that excessive consumptions of alcohol beverage could be linked to various health problems [Citation6,Citation55], while the appropriate quantity of ethanol consumption could be crucial to induce either positive or negative effects on health promotion [Citation3,Citation56]. In a large prospective study in Japan, current drinkers who consumed moderate dose of alcohol beverage displayed a significant reduction of all-cause mortality rate, whereas heavy drinker was associated with increases in mortality risk in men [Citation4]. Similar results can be found in Western countries that moderate alcohol drinker showed the lowest mortality [Citation3]. In terms of Sake consumption, Miyazaki and Une [Citation56] reported that Sake appeared to be associated with a low risk of all cause mortality in comparison to beer drinker, while the relative risks increased with amount of alcohol intake regardless of type of alcohol beverage [Citation57]. In our current study, daily administration of ethanol (300 mg/kg/day) had small but significant inhibitory effects on depression-like behaviors and masseter muscle nociception, and this dose of ethanol appeared to have a lower mortality rate than heavy drinkers or abstainers in human study [Citation3,Citation4,Citation57]. These results indicated that inhibitory roles of Sake on adverse stress responses in the body could be due to the effect of ethanol and non-ethanol constituents in Sake in our experimental conditions. It is, therefore, reasonable to mention that an appropriate consumption of Sake could contribute to reduce pain responses under psychophysical stress conditions. However, emphasis should be directed on the beneficial roles of Sake on the health promotion being restrictive, since drinking alcohol beverage independent of the quantity could induce health disturbance under various psychophysical conditions.
Conclusions
Repeated exposures to FS induced depression-like behaviors and enhanced masseter muscle nociception, which could be inhibited by daily administration of Sake just after each stress condition. These findings supported the idea that a certain dose of Sake consumption could have preventive roles on enhanced pain responses related to psychological distress.
Author contribution
K.O., Y.N., and R.T. conceived and designed the experiments. Y.N., S.S., M.K., M.S., and K.Y. performed the experiments. K.O. and Y.N. wrote the paper. Y.N., Y.K., S.S., T. S., M.K., M.S., R.T., and K.Y. discussed the results, commented on the manuscript, and approved the manuscript submission.
Acknowledgments
This work was supported by the JSPS KAKENHI under Grant number 16K11679.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Sagner M, Katz D, Egger G, et al Lifestyle medicine potential for reversing a world of chronic disease epidemics: from cell to community. Int J Clin Pract. 2014;68:1289–1292.
- Sarris J, O’neil A, Coulson CE, et al Lifestyle medicine for depression. BMC Psychiatry. 2014;14:107.
- Marmot MG, Rose G, Shipley MJ, et al. Alcohol and mortality: a U-shaped curve. Lancet. 1981;1:580–583.
- Lin Y, Kikuchi S, Tamakoshi A, et al Alcohol consumption and mortality among middle-aged and elderly Japanese men and women. Ann Epidemiol. 2005;15:590–597.
- Gea A, Beunza JJ, Estruch R, et al Alcohol intake, wine consumption and the development of depression: the PREDIMED study. BMC Medicine. 2013;11:192.
- Neafsey EJ, Collins MA. Moderate alcohol consumption and cognitive risk. Neuropsychiatr Dis Treat. 2011;7:465–484.
- Imbe H, Iwai-Liao Y, Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Front Biosci. 2006;11:2179–2192.
- Gameiro GH, Andrade Ada S, De Castro M, et al The effects of restraint stress on nociceptive responses induced by formalin injected in rat’s TMJ. Pharmacol Biochem Behav. 2005;82:338–344.
- Suarez-Roca H, Leal L, Silva JA, et al Reduced GABA neurotransmission underlies hyperalgesia induced by repeated forced swimming stress. Behav Brain Res. 2008;189:159–169.
- Quintero L, Cardenas R, Suarez-Roca H. Stress-induced hyperalgesia is associated with a reduced and delayed GABA inhibitory control that enhances post-synaptic NMDA receptor activation in the spinal cord. Pain. 2011;152:1909–1922.
- Ma X, Bao W, Wandg X, et al Role of spinal GABAA receptor reduction induced by stress in rat thermal hyperalgesia. Exp Brain Res. 2014;232:3413–3420.
- Duenes SL, Thompson R, Chang Z, et al Psychophysical stress increases the expression of phospho-CREB, Fos protein and neurokinin-1 receptors in superficial laminae of trigeminal subnucleus caudalis in female rats. Neurosci Lett. 2010;486:207–210.
- Okamoto K, Tashiro A, Chang Z, et al Temporomandibular joint-evoked responses by spinomedullary neurons and masseter muscle are enhanced after repeated psychophysical stress. Eur J Neurosci. 2012;36:2025–2034.
- Okamoto K, Thompson R, Katagiri A, et al Estrogen status and psychophysical stress modify temporomandibular joint input to medullary dorsal horn neurons in a lamina-specific manner in female rats. Pain. 2013;154:1057–1064.
- Nakatani Y, Kurose M, Shimizu S, et al Inhibitory effects of fluoxetine on increases in Fos responses in the trigeminal subnucleus caudalis evoked by masseter muscle injury after repeated forced swim stress conditionings in rats. Exp Brain Res. 2018;236:2209–2221.
- Izu H, Hizume K, Goto K, et al Hepatoprotective effects of a concentrate and components of sake against galactosamine (GalN)-induced liver injury in mice. Biosci Biotechnol Biochem. 2007;71:951–957.
- Kiyono T, Hirooka K, Yamamoto Y, et al Identification of pyroglutamyl peptides in Japanese rice wine (sake): presence of hepatoprotective pyroGlu-Leu. J Agric Food Chem. 2013;61:11660–11667.
- Yamamoto Y, Mizushige T, Mori Y, et al Antidepressant-like effect of food-derived pyroglutamyl peptides in mice. Neuropeptides. 2015;51:25–29.
- Wada S, Sato K, Ohta R, et al Ingestion of low dose pyroglutamyl leucine improves dextran sulfate sodium-induced colitis and intestinal microbiota in mice. J Agric Food Chem. 2013;61:8807–8813.
- Liu T, Zhou Y, Zhu Y, et al Study of the rapid detection of gamma-aminobutyric acid in rice wine based on chemometrics using near infrared spectroscopy. J Food Sci Technol. 2015;52:5347–5351.
- Briones-Aranda A, Rocha L, Picazo O. Alterations in GABAergic function following forced swimming stress. Pharmacol Biochem Behav. 2005;80:463–470.
- Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45:1–8.
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110.
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245.
- Holdstock L, De Wit H. Individual differences in the biphasic effects of ethanol. Alcohol Clin Exp Res. 1998;22:1903–1911.
- Yoshida A, Dostrovsky JO, Sessle BJ, et al Trigeminal projections to the nucleus submedius of the thalamus in the rat. J Comp Neurol. 1991;307:609–625.
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. New York (NY): Academic Press; 1997.
- Molander C, Xu Q, Rivero-Melian C, et al Cytoarchitectonic organization of the spinal cord in the rat: II. The cervical and upper thoracic cord. J Comp Neurol. 1989;289:375–385.
- Strassman AM, Vos BP. Somatotopic and laminar organization of Fos-like immunoreactivity in the medullary and cervical dorsal horn induced by noxious facial stimulation in the rat. J Comp Neurol. 1993;331:495–516.
- Bereiter DA, Okamoto K, Bereiter DF. Effect of persistent monoarthritis of the temporomandibular joint region on acute mustard oil-induced excitation of trigeminal subnucleus caudalis neurons in male and female rats. Pain. 2005;117:58–67.
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732.
- Imbe H, Dubner R, Ren K. Masseteric inflammation-induced Fos protein expression in the trigeminal interpolaris/caudalis transition zone: contribution of somatosensory-vagal-adrenal integration. Brain Res. 1999;845:165–175.
- Okamoto K, Kimura A, Donishi T, et al Contribution of peripheral 5-HT2A or 5-HT3 receptors to Fos expression in the trigeminal spinal nucleus produced by acute injury to the masseter muscle during persistent temporomandibular joint inflammation in rats. Neuroscience. 2006;143:597–606.
- Levenson RW, Sher KJ, Grossman LM, et al Alcohol and stress response dampening: pharmacological effects, expectancy, and tension reduction. J Abnorm Psychol. 1980;89:528–538.
- Sayette MA. Does drinking reduce stress? Alcohol Res Health. 1999;23:250–255.
- Masserman JH, Yum KS. An analysis of the influence of alcohol on experimental neuroses in cats. Psychosom Med. 1946;8:36–52.
- Gallate JE, Morley KC, Ambermoon P, et al The consequences of beer consumption in rats: acute anxiolytic and ataxic effects and withdrawal-induced anxiety. Psychopharmacology. 2003;166:51–60.
- Wood PB. Stress and dopamine: implications for the pathophysiology of chronic widespread pain. Med Hypotheses. 2004;62:420–424.
- Jennings EM, Okine BN, Roche M, et al Stress-induced hyperalgesia. Prog Neurobiol. 2014;121:1–18.
- Imbe H, Murakami S, Okamoto K, et al The effects of acute and chronic restraint stress on activation of ERK in the rostral ventromedial medulla and locus coeruleus. Pain. 2004;112:361–371.
- Imbe H, Okamoto K, Donishi T, et al Involvement of descending facilitation from the rostral ventromedial medulla in the enhancement of formalin-evoked nocifensive behavior following repeated forced swim stress. Brain Res. 2010;1329:103–112.
- Nakahara M, Mishima T, Hayakawa T. Effect of a sake concentrate on the epidermis of aged mice and confirmation of ethyl alpha-D-glucoside as its active component. Biosci Biotechnol Biochem. 2007;71:427–434.
- Kato Y, Kato Y, Furukawa K, et al Cloning and nucleotide sequence of the glutamate decarboxylase-encoding gene gadA from Aspergillus oryzae. Biosci Biotechnol Biochem. 2002;66:2600–2605.
- Roberts E, Frankel S. Gamma-Aminobutyric acid in brain: its formation from glutamic acid. J Biol Chem. 1950;187:55–63.
- Cairns BE, Sessle BJ, Hu JW. Evidence that excitatory amino acid receptors within the temporomandibular joint region are involved in the reflex activation of the jaw muscles. J Neurosci. 1998;18:8056–8064.
- Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. Pain. 2005;116:79–86.
- Khasar SG, Dina OA, Green PG, et al Sound stress-induced long-term enhancement of mechanical hyperalgesia in rats is maintained by sympathoadrenal catecholamines. J Pain. 2009;10:1073–1077.
- Gatch MB, Lal H. Effects of ethanol and ethanol withdrawal on nociception in rats. Alcohol Clin Exp Res. 1999;23:328–333.
- Gameiro GH, Arthuri MT, Tambeli CH, et al Effects of ethanol on deep pain evoked by formalin injected in TMJ of rat. Life Sci. 2003;73:3351–3361.
- Weight FF, Li C, Peoples RW. Alcohol action on membrane ion channels gated by extracellular ATP (P2X receptors). Neurochem Int. 1999;35:143–152.
- Ikeda K, Kobayashi T, Kumanishi T, et al Molecular mechanisms of analgesia induced by opioids and ethanol: is the GIRK channel one of the keys? Neurosci Res. 2002;44:121–131.
- Nestoros JN. Ethanol specifically potentiates GABA-mediated neurotransmission in feline cerebral cortex. Science. 1980;209:708–710.
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724.
- Davidson D, Camara P, Swift R. Behavioral effects and pharmacokinetics of low-dose intravenous alcohol in humans. Alcohol Clin Exp Res. 1997;21:1294–1299.
- Dina OA, Khasar SG, Alessandri-Haber N, et al Alcohol-induced stress in painful alcoholic neuropathy. Eur J Neurosci. 2008;27:83–92.
- Miyazaki M, Une H. Japanese alcoholic beverage and all cause mortality in Japanese adult men. J Epidemiol. 2001;11:219–223.
- Tsugane S, Fahey MT, Sasaki S, et al Alcohol consumption and all-cause and cancer mortality among middle-aged Japanese men: seven-year follow-up of the JPHC study Cohort I. Japan Public Health Center. Am J Epidemiol. 1999;150:1201–1207.