ABSTRACT
A potato fraction library was constructed to investigate functional secondary metabolites from 8 cultivars: Kitahime, Pilka, Sakurafubuki, Atlantic, Toyoshiro, Snowden, Kitamurasaki, and Northern Ruby, which were divided into flower, leaf, stem, roots, tuber peel, and tuber. Each fraction was a semi-purified extract and about 800 fractions were prepared for the library. They were analyzed by DAD-LC/MS to obtain structural information and were evaluated for various biological activities. LC/MS data showed that each part had a specific characteristic for their constituents supported by principal component analysis (PCA). Approximately 40% of fractions showed significant biological activities at 30 μg/mL, especially the flower fractions showed strong cytotoxicity. PCAs based on the activity and LC/MS data suggested that the strong cytotoxicity of flowers was derived from a complex mixture of potato glycoalkaloids. In addition, tuber peel fractions showed strong antimalarial activity, which had not been reported before. Also, some fractions showed significant antibacterial activities.
Graphical Abstract
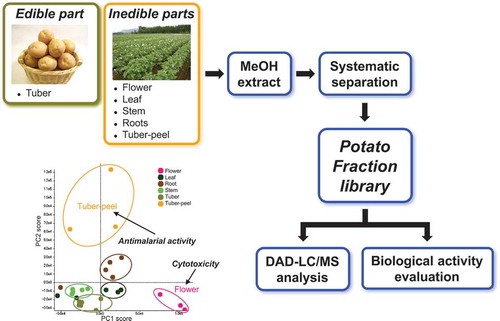
Potato fraction library was constructed to search for functional secondary metabolites. Flower and tuber-peel fractions showed potent cytotoxicity and antimalarial activity, respectively.
Potato (Solanum tuberosum L.) has been cultivated in most of the world as an edible plant or a staple food [Citation1,Citation2]. The edible part, tuber, contains various nutrients such as vitamins, folate, and several minerals: sodium, potassium, and magnesium, in addition to potato starch as the main component [Citation3,Citation4]. Also, polyphenols including chlorogenic acid and ferulic acid, and GABA (4-aminobutanoic acid) are contained as functional ingredients [Citation5–Citation7]. Meanwhile, the tuber peel contains functional compounds and has been investigated against some biological activities for food safety [Citation8]. A potato also contains potato glycoalkaloids (PGAs), such as α-solanine and α-chaconine, as major secondary metabolites, which are mainly observed around the sprout of the tuber [Citation1,Citation9,Citation10]. In general, potato ingredients have been investigated with focus on the tuber from the viewpoint of nutrition and safety, and its major metabolites, the PGAs. Recently, metabolome analysis based on MS and NMR has been reported to distinguish potato cultivars using tuber peels by searching biomarkers [Citation11]. So far, the secondary metabolites in potatoes have not been investigated systematically for isolation and identification of structurally interesting bioactive compounds using a large amount of sample, especially the inedible parts such as the flower, leaf, stem, and roots.
Previously, we constructed a fraction library to investigate the secondary metabolites of microbes [Citation12,Citation13]. Microbes, such as actinomycetes and fungi have a big potential to produce various secondary metabolites with interesting biological activities. The microbial metabolites have been used not only as drugs, but also as bioprobes, which are chemical tools for chemical biology studies [Citation14–Citation16]. A fraction is a semi-purified extract obtained by chromatographic techniques including HPLC and medium pressure liquid chromatography (MPLC). Minor metabolites, which are not detected in the simple extract because of low content, are concentrated in the fraction. Thus, the construction of a fraction library was done to discover and investigate minor metabolites efficiently. Fractions were subjected to LC/MS analysis and various biological activity tests to obtain structural and biological information of the metabolites. In the investigation of structurally interesting metabolites based on the LC/MS data, we developed an original database named Natural Products Plot (NPPlot) [Citation12,Citation13]. Each detected metabolite was plotted in two-dimensions: retention time and m/z value, for the X- and Y-axes, respectively. The NPPlot shows a distribution map of metabolites, and a specific metabolite for each strain can be easily compared and discovered. Based on this methodology, we discovered and isolated several new secondary metabolites from bacteria and fungi such as verticilactam [Citation17], spirotoamides [Citation18], pyrrolizilactone [Citation19], and recently, the wakodecalines [Citation20]. Some of these have novel skeleton and interesting biological activity. We applied this approach to investigate the secondary metabolites of potatoes with the goal to isolate each metabolite and evaluate individual biological activity or function.
Eight cultivars of potatoes: Kitahime, Pilka, Sakurafubuki, Atlantic, Toyoshiro, Snowden, Kitamura-saki, and Northern Ruby, were collected in Hokkaido, which has the highest potato production in Japan [Citation21]. Toyoshiro, which is the most widely farmed for processing into crisps and fries, was also collected from Ibaraki, Japan [Citation21]. The parts were divided into flower, leaf, stem, roots, tuber peel, and tuber; and were used to construct a potato fraction library. Herein, we report the construction of a potato fraction library and our findings based on LC/MS analysis and biological activity screening and subsequently, the identification and isolation of active substances supported by the principle component analysis (PCA).
Materials and methods
General
Analytical grade solvents and reagents were purchased from commercial sources. α-Solanine and α-chaconine standards were purchased from Funakoshi Co., Ltd., Japan. Solamargine standard was purchased from Namiki Shoji Co., Ltd., Japan. Optical rotation was recorded on a HORIBA SEPA-300 high sensitive polarimeter. NMR data were obtained at 500 MHz for 1H NMR and 125 MHz for 13C NMR on a JEOL JNM-ECA-500 spectrometer. Methanol-d4 was used as the solvent and reference (δH 3.31 and δC 49.2). Teredyne ISCO CombiFlash Companion was used for MPLC. DAD-LC/MS analysis was performed on a Waters Alliance system with a 2996 PDA detector connected to an AB Sciex Qtrap with an ESI probe and Waters UPLC H-class connected to AB Sciex API3200 with an ESI probe. Waters Xterra C18 (2.1 x 150 mm, 5 μm) and BEH C18 UPLC (2.1 x 50 mm, 1.7 μm) columns were used for LC and UPLC analyses, respectively. HRESITOFMS was recorded on a Waters VION QTof system.
Plant material
Nine potato samples including 8 kinds of cultivar: Kitahime, Pilka, Sakurafubuki, Atlantic, Toyoshiro, Snowden, Kitamurasaki, and Northern Ruby were used for constructing a fraction library. Toyoshiro was collected at two prefectures, Ibaraki and Hokkaido, Japan, and the other samples were collected in Hokkaido (Table S1). Collected plants were divided into flower, leaf, stem, roots, tuber peel, and tuber to obtain a total of 27 samples (). Voucher specimens were stored at Calbee Inc.
Table 1. Plants materials collected for the construction of a fraction library.
Extraction and partition
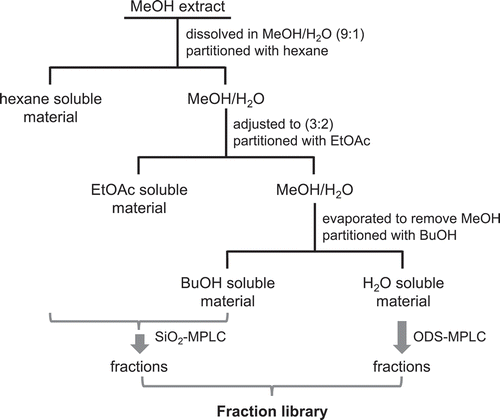
Each part of the plant was freeze-dried separately, except for the leaves of Kitahime, Toyoshiro, and Snowden, which were chopped and directly used for extraction with no drying. They were ground into powder and soaked in MeOH of ca. 5 times of the gross weight of a sample for a week (Table S1). Each sample was extracted for 3 times by the same procedure. The extracts were evaporated in vacuo to obtain crude extracts. The extract yield was approximately 50, 20, 15, 3, and 1% for the flower, stem, roots, tuber peel, and tuber, respectively. The yield from the leaf extract was approximately 30% and 4% from dry and wet weights, respectively (Table S1). Each extract was subjected to a liquid/liquid partition process to separate the constituents by polarity, except for the root extracts from Atlantic, which had insufficient amount (). A part of each extract (ca. 3 g and 1.6 g for Pilka flower) was dissolved in MeOH/H2O (9:1) and partitioned 3 times with hexane to obtain nonpolar compounds. The MeOH/H2O part was adjusted (3:2) by the addition of H2O and partitioned 3 times with EtOAc to afford moderately polar substances. The remaining MeOH was evaporated in vacuo to give the H2O portion, which was partitioned twice with BuOH to afford polar compounds. In total, 4 different extracts: hexane, EtOAc, BuOH, and H2O, were prepared from each sample to afford 104 partitioned extracts. A part of the 27 extracts and 104 partitioned extracts was used for biological activity tests and LC/MS analysis as a part of the fraction library. The remaining sample was used for the preparation of subsequent fractions.
Preparation of fractions
The hexane extract was subjected to SiO2-MPLC with a hexane/acetone stepwise gradient (acetone: 0, 1, 2, 5, 10, 20, 50, 100% in 8 steps) to afford 8 fractions, F001 ~ F008. The EtOAc and BuOH extracts were subjected to SiO2-MPLC with a CHCl3/MeOH stepwise gradient (MeOH: 0, 1, 2, 5, 10, 20, 50, 100% for EtOAc extract and 0, 5, 10, 20, 30, 40, 50, 100% for BuOH extract) to afford 6 or 8 fractions, F001 ~ F006 or F008. The water extract was separated by ODS-MPLC with a linear gradient of MeCN/H2O (MeCN: 20 to 100%) to obtain 5 or 6 fractions, F001 ~ F005 or F006. A part of each fraction was used for biological activity tests and LC/MS analysis. The remaining fraction was dried and stored as a core of the fraction library. A total of 754 fractions were prepared.
LC/MS analysis
Each sample was dissolved in MeOH or H2O at a concentration of 5 mg/mL for partitioned extracts and 1 mg/mL for fractions, and centrifuged to remove insoluble materials. An aliquot of 10 μL was injected to a column and analyzed by Qtrap DAD-LC/MS with elution using an MeCN/0.05% aqueous formic acid linear gradient system (5–100% MeCN in 30 min and hold at 100% MeCN for 15 min at 0.2 mL/min). A single LC/MS condition was used to compare the retention time of the metabolites in each extract and fraction. A mass range of m/z 50 to 1500 Da was covered with 2.0 s of scan speed. Various ESI parameters were set as follows; curtain gas: 15 psi, source temperature: 400 °C, gas 1: 40 psi, gas 2: 50 psi, ion spray voltage: 5500 V. For the UPLC-API3200 DAD-LC/MS system, an aliquot of 2 μL was injected to a column and eluted by a linear gradient of MeCN/0.05% aqueous formic acid (5–100% MeCN in 4 min and hold at 100% MeCN for 2 min at 0.5 mL/min). The mass parameters were set as follows; mass range: m/z of 50 to 1500 Da with 0.4 s of scan speed, curtain gas: 15 psi, source temperature: 400 °C, gas 1: 40 psi, gas 2: 50 psi, ion spray voltage: 5500 V. Mass spectra were recorded in positive mode for both Qtrap and API3200 systems.
Biological activity tests
Each extract, partitioned extract, and fraction in the library was dissolved in DMSO at a concentration of 10 mg/mL for biological activity evaluation. The in vitro cytotoxicity assay against various cell lines: human cervical epidermoid carcinoma cell line (HeLa), human promyelocytic leukemia cell line (HL60), mouse temperature-sensitive cdc2 mutant cell line of the mammary carcinoma FM3A (tsFT210), rat kidney cells infected with ts25, a T-class mutant of Rous sarcoma virus Prague strain (srcts-NRK), and murine mesenchymal stem cell line (C3H10T1/2), were described in a previous report [Citation22]. The microdilution assay against Staphylococcus aureus 209, Escherichia coli HO141, Aspergillus fumigatus Af293, Pyricularia oryzae kita-1, and Candida albicans JCM1542 was also done as described [Citation22]. The antimalarial test against Plasmodium falciparum 3D7 was conducted as previously reported [Citation23]. The activity was evaluated at three points at final concentrations of 0.3, 3, and 30 μg/mL; and the strength was expressed by an index of 0, 1, 2, and 3 for 0 – 20%, 20 – 50%, 50 – 80%, and > 80% of growth inhibition, respectively.
Multivariate analysis
Principle component analysis (PCA) and PCA-discriminant analysis (DA) were performed by AB Sciex MarkerView 1.2.1 software. The signals by Qtrap-LC/MS analysis were extracted by the following parameters; extract range: 0.5 to 44.5 min, subtraction offset: 10 scans, subtraction mult factor: 1.3, noise threshold: 20,000, minimum spectral peak width: 0.50 Da, minimum RT peak width: 10 scans, retention time tolerance: 0.50 min, and mass tolerance: 0.50 Da, and performed by Pareto with no weighting. The t-test was performed by the same software based on the extracted data for PCA and PCA-DA. The volcano plot was visualized by p-value and log (fold change) based on the t-test. Likewise, the PCA of the biological activities was performed by Pareto with no weighting based on the results at a final concentration of 30 μg/mL. The strength of activities was expressed by an index as described in the Biological activity tests.
Isolation of compounds 1 and 2
The fraction F005 of Kitahime BuOH extract was prepared from a part of the MeOH extract (2.9 g of the total 9.3 g), which was obtained from 18.6 g of dried flower. It was subjected to Sephadex LH-20 column chromatography eluted with MeOH 100% to afford 4 fractions. The 2nd fraction was separated by SiO2-MPLC with a CHCl3/MeOH linear gradient (MeOH: 0–100%) to obtain 7 subfractions including 37.3 mg of compound 1 as a white powder in the 2nd subfraction. The 3rd subfraction was further purified by SiO2-MPLC with a CHCl3/MeOH linear gradient containing 0.1% TFA (MeOH: 0 to 100%) to afford 21.5 mg of compound 2 as a pale-yellow powder.
Compound 1: white powder; [α]58926 −83° (c 0.1, EtOH); HRESITOFMS m/z: 868.5064 [M + H]+ (calcd. for C45H74NO15: 868.5053); 13C NMR data (methanol-d4) δC: 16.1, 17.5, 18.1, 18.2, 19.9, 20.0, 22.2, 27.8, 29.5, 31.0, 31.8, 32.9, 33.4, 33.7, 38.3, 38.8, 39.7, 41.3, 42.0, 43.7, 50.9, 51.9, 57.4, 62.2, 63.7, 70.0, 70.9, 73.4 (2C), 72.6, 72.7, 73.9, 74.1, 76.8, 78.3, 79.47, 79.50, 80.2, 80.5, 100.1, 100.6, 102.5, 103.2, 122.8, and 142.1.
Compound 2: pale-yellow powder; [α]58925 −46° (c 0.1, EtOH); HRESITOFMS m/z: 884.4990 [M + H]+ (calcd. for C45H74NO16: 884.5002); 13C NMR data (methanol-d4) δC: 14.8, 17.3, 18.2, 18.8, 20.0, 22.2, 26.9, 27.4, 29.9, 30.9, 32.9, 33.1, 33.3, 38.3, 38.7, 39.6, 41.0, 42.1, 42.4, 50.0, 51.7, 57.4, 62.6, 62.7, 63.2, 69.9, 70.4, 71.4, 72.3, 72.5, 74.2, 75.3, 75.9, 76.2, 78.1, 78.4, 79.1, 82.7, 85.8, 97.8, 101.0, 102.4, 105.9, 122.5, and 142.3.
Results
Potato fraction library
Nine potato samples, Kitahime (KH), Pilka (PL), Sakurafubuki (SF), Atlantic (AT), Toyoshiro (TS-H), Toyoshiro (TS-I), Snowden (SD), Kitamurasaki (KM), and Northern Ruby (NR) were divided into flower, leaf, stem, roots, tuber peel, and tuber, and extracted with MeOH to obtain 27 crude extracts (). They were dissolved in MeOH/H2O and partitioned with hexane, EtOAc, and BuOH sequentially to obtain related soluble materials and the remaining H2O soluble portion (). Each partitioned extract was separated by SiO2- or ODS-MPLC to afford fractions, which became the core of the fraction library. The fraction library consisted of total 885 samples including 754 fractions with 27 extracts and 104 partitioned extracts. The fraction library was subjected to DAD-LC/MS analysis on an AB Sciex Qtrap system to obtain physicochemical properties such as retention time, UV, and the mass spectral data of the metabolites. The same run conditions were implemented for each analysis.
Comparison of metabolites based on LC/MS analysis
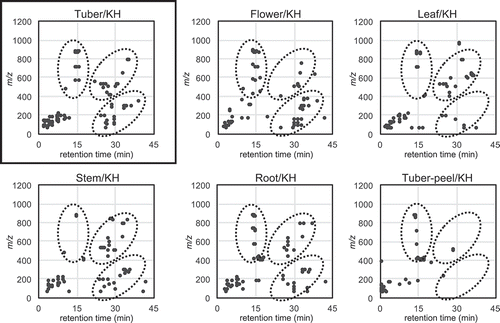
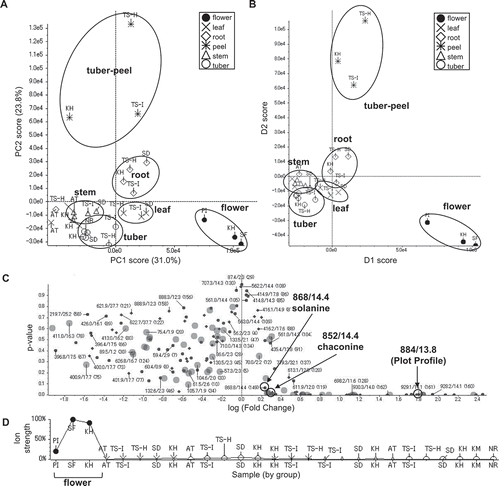
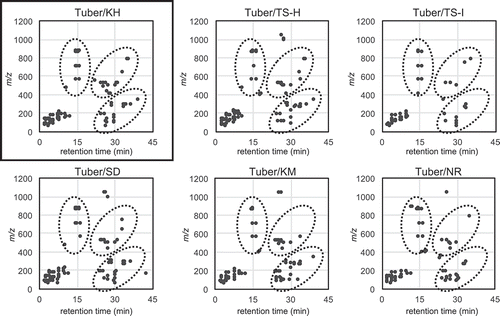
To take advantage of the fraction library for investigating structurally interesting metabolites and comparing metabolites between different cultivars and parts of potatoes, metabolites detected by LC/MS analysis were visualized in 2D on the basis of our NPPlot methodology. In the case of the potato fraction library, metabolites were surveyed by mass spectra. At first, metabolites were compared from the tubers of 6 cultivars: KH, TS-H, TS-I, SD, KM, and NR (). There was no significant difference in the metabolites of the tubers and similar tendencies were observed for the remaining parts of the cultivars. The distribution of metabolites from different parts, the tuber, flower, leaf, stem, roots, and tuber peel of KH showed slight differences with specific characteristics (). The flower showed complex metabolites around the retention time of 15 min and m/z value of 800. On the other hand, the tuber peel showed fewer metabolites than the others. These results implied that there might be specific or characteristic metabolites for each part of the potato. Principal component analysis (PCA) was applied on the basis of LC/MS data to visualize the differences in several parts of the potato. The result was expressed as a score plot consisting of synthetic variables, principal component (PC) 1 and PC2. The extracts prepared from each part of potatoes were independently clustered in the score plot with 31.0% and 23.8% contribution rates for PC1 and PC2, respectively; especially flower extracts were clearly clustered by PC1 ()). This result was consistent with the observation in the NPPlot and suggests that there might be characteristic constituents in each part of a potato. Therefore, supervised discriminant analysis PCA-DA was applied which showed that the flower samples were clearly clustered by D1 suggesting a common characteristic constituent in the flower samples ()). The responsible components were searched by a volcano plot of the p-value versus log (fold change) for the flower against all of other parts based on the t-test ()). The major secondary metabolites, chaconine and solanine with m/z 852 [M + H]+/tR 14.4 min and 868/14.4, were found on the center of the plot with small fold change (< 0.4) suggesting that these were not the specific metabolites for the flower samples. By searching components with low p-value and large log (fold change), a signal of m/z 884 [M + H]+/tR 13.8 min was found as a specific metabolite (p-value < 0.1 and log (fold change) > 1.5). It was mainly detected in the flower extracts confirmed by the plot profile, in which a selected signal was plotted by ion strength for each sample reflecting relative quantities ()).
Figure 1. Comparison of metabolites of tubers from different cultivar by modified NPPlot.
Highlighted zones by dotted circles showed characteristic distributions observed in KH metabolites.
Biological activities
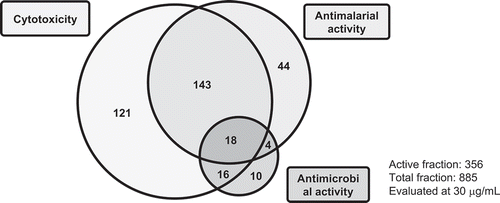
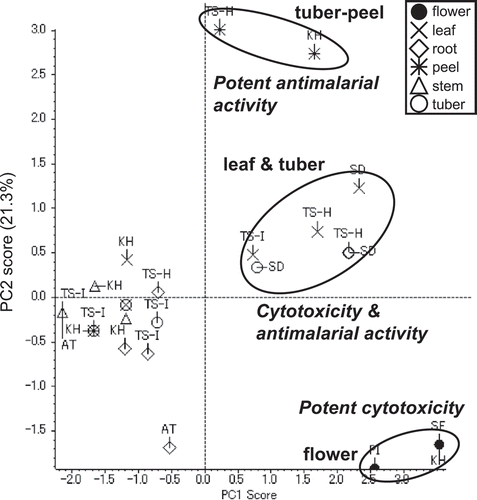
The fraction library including extracts and partitioned extracts were evaluated for cytotoxicity against HeLa, HL60, tsFT210, srcts-NRK, and C3H10T1/2 cells; and antimicrobial activities against S. aureus, E. coli, A. fumigatus, P. oryzae, and C. albicans, and antimalarial activity against P. falciparum. Almost 40% of the fractions had strong growth inhibition against mammalian cells, bacteria, and/or malaria at a final concentration of 30 μg/mL (). Furthermore, some active fractions showed specific activity against mammalian cells, bacteria or malaria. The detailed results of the activities of the extracts and partitioned extracts showed that the flower extracts had strong cytotoxicity against all of the mammalian cells and the tuber peel extracts had potent antimalarial activity and cytotoxicity (Table S2). These results suggested that similar to the observation in the LC/MS results, there might be specific or characteristic metabolites for each part of the potato. This was supported by the PCA score plot of the activity data, in which three flower extracts and 2 tuber peel extracts of KM and TS-H were clustered, respectively (). The flower extracts were supposed to be grouped by potent cytotoxicity against all of 5 mammalian cells (Table S2). Two tuber peels were grouped by potent antimalarial activity and cytotoxicity against HL60 cells (Table S2). Most of the extracts generated from the leaves and tubers were clustered and they showed cytotoxicity against HeLa and HL60 cells and/or weak antimalarial activity ( and Table S2).
Search for active components by PCA based on activities and LC/MS analyses
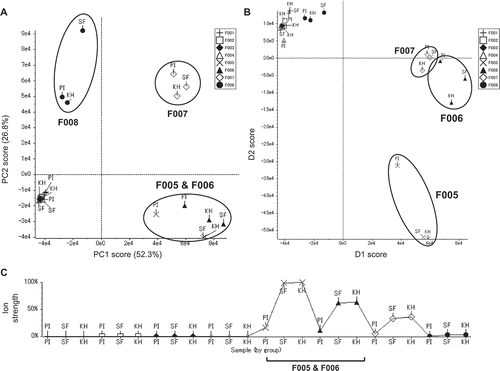
The NPPlot and PCA results of LC/MS analyses and activity tests suggested that there might be a characteristic metabolite or metabolite group for each part of the potato, especially in the flower extracts that showed strong cytotoxicity. To mine a potential cytotoxic component in the flower extracts, we compared the activities of partitioned extracts, i.e. hexane, EtOAc, BuOH, and H2O by the same PCA. The results showed that the active principles for the cytotoxicity were found in the EtOAc and BuOH extracts ( and Figure S1A). The cytotoxicity was further concentrated in 2 fractions, F005 and F006, which were obtained by SiO2-MPLC separations of the active BuOH extracts ( and Figure S1B). F007 also showed the same potent cytotoxicity as F005 and F006, but was clustered apart from them due to its strong antimalarial activity ( and Figure S1B). These results implied that an active component might be contained in F005, F006, and F007. The PCA score plot based on the LC/MS data was consistent with the result of the activity data showing clusters of F005 and F006, and with F007 being clustered apart from them ()). The result of PCA-DA confirmed that there was a common characteristic for each of the F005, F006, and F007 fractions by clustering separately ()). The responsible component was searched by volcano plots based on the t-test for each of the inactive fractions F001–F004 against the active fraction F005, and for F005 against the active fractions F006 and F007 (Figures S2A to S2F). Major secondary metabolites, solanine and chaconine, were mainly observed in the cytotoxic fractions F005, F006, and F007. This was supported by the large log (fold change) in the absolute values (> 1.5) of the inactive fractions F001–F004 against F005, and the small log (fold change) values (< 0.5) of F005 against the active fractions, F006 and F007. Also, a signal of m/z 884 [M + H]+ with tR 14.0 min, which was identical with the responsible component in the flower extracts cluster observed at tR 13.8 min, was clearly identified as a probable component by the large log (fold change) values in absolute values (> 3.0) against the inactive fractions and by the small log (fold change) values (< 0.5) against active ones. These observations showed that all of active fractions contained a compound with 884/14.0 and the major PGAs, chaconine and solanine. The relative quantity of the compound with a signal of 884/14.0 for F001 to F008 was confirmed by the plot profile, and the compound was contained in the cytotoxic fractions F005, F006, and F007 (. F008 contained small amounts of the compound (884/14.0) and the 2 PGAs which was consistent with the relatively small log (fold change) for F005 ( and S2G). These results suggested that this compound (884/14.0) and the 2 PGAs were possibly the responsible metabolites for the cytotoxicity, especially the former was found in the flower extracts.
Table 2. Biological activities of flower partitioned extracts and BuOH fractions of KH, PI, and SF.
Identification of the potential cytotoxic component in the flowers
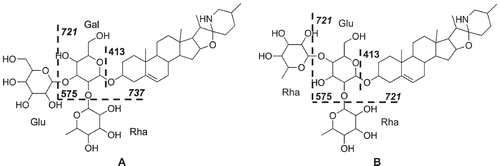
A structure of the potential cytotoxic component observed as a signal with m/z 884 [M + H]+/tR 14.0 min in the flower fractions was predicted to belong to PGAs such as solanine and chaconine for its close molecular weight and retention time. The mass spectra of standard α-solanine and α-chaconine were measured in positive mode and compared with an extracted mass spectrum from the potential retention time to speculate the structure. They showed characteristic fragment ions for a triglucoside with fragment ions assigned as diglucoside, monoglucoside, and aglycone (Figure S3A and S3B). Specifically, α-solanine contained fragment ions at m/z 722 and 706 for two types of diglucosides, aglycone/Gal/Glu and aglycone/Gal/Rha, respectively. The mass spectrum from the potential signal was similar to those of the two PGAs and implied that it belonged to a triglucoside showing a characteristic fragmentation pattern (Figure S3C). However, the signal consisted of two metabolites with nominal masses of 883 and 867, which were observed at exactly the same retention time in the LC/MS analysis similar to chaconine and solanine, which were observed as a single overlapped peak. On the basis of the interpretation of the fragmentation patterns of solanine and chaconine, the compounds had the same aglycone unit with a nominal mass of 413. The metabolite with m/z 884 was predicted to have an identical sugar moiety with that of solanine because of the similar fragmentation pattern derived from the 2 types of diglucosides. The other was predicted to have the chaconine-type sugar moiety. These results suggested that the structures of two metabolites with molecular weight (MW) of 883 and 867 were possibly the spiro derivatives of solanine and chaconine, solasonine and solamargine, respectively () [Citation24]. Then, we isolated the metabolites as compounds 1 and 2 with MW 867 and 883, respectively, from cytotoxic fraction F005 of KH-flower BuOH extract. The direct comparison of 1, which had been predicted to be solamargine, to the standard solamargine by UPLC/MS showed the identical mass spectra with m/z values of 868 [M + H]+ with characteristic fragmentation patterns. However, their retention times were slightly different; 1 had 2.04 min and the standard solamargine had 2.10 min recorded as m/z: 868 [M + H]+ in 6 min analyses (Figure S4). Therefore, the structure of 1 was investigated by the HRMS and by comparison of the 1H and 13C NMR spectra to those of the standard solamargine, the planar structure of 1 was identified as solamargine. The planar structure of 2 (MW 883) was also identified as solasonine by the comparison of 1D NMR data with those of 1 and HRMS analysis. However, the 13C NMR chemical shifts around the spiro moiety of the 3 PGAs, 1, 2, and standard solamargine, which shared exactly the same aglycone in the planar structure, were slightly different from each other (Figure S5). These results suggest that 1 and 2 might have slightly different stereochemistry or conformation around the spiro carbon to the reported solamargine and solasonine, respectively. In summary, 2 compounds, 1 and 2, which had the same planar structure with those of solamargine and solasonine, and 2 major PGAs, chaconine and solanine were identified as the major components of the cytotoxic fraction, F005 of the flower extracts.
Activities of PGAs
The biological activities of isolated 1 and 2 and standards α-chaconine and α-solanine were evaluated to confirm the cytotoxicity of potato extracts (). They showed cytotoxicity against 5 mammalian cell lines. In particular, 1 and α-chaconine showed significant cytotoxicity with IC50 values under 5 μM. They have identical sugar moieties containing Glu and two Rha, which may be a factor in the strong cytotoxicity. These results suggest that the potent cytotoxicity observed in flower extracts may be caused by the complex mixture of the 4 PGAs consisting of chaconine, solanine, 1, and 2.
Table 3. Biological activities of compounds 1 and 2 and two major PGAs, α-chaconine and α-solanine.
Discussion
The relative quantities of the 4 PGAs, compounds 1 and 2 and chaconine and solanine, and the total cytotoxicity were compared for BuOH fractions of 3 flowers, PI, SF, and KH (Figure S6). The relative quantity was calculated from the ion strength observed in the mass chromatogram recorded by the related m/z values for the 4 PGAs and expressed by a relative value against the KH flower BuOH-F005. The total cytotoxicity was the sum of the activity index at 30 μg/mL for the 5 mammalian cell lines. The relative quantity of the 4 PGAs was closely correlated to the total cytotoxicity. The active fractions, F005, F006, and F007 contained the 2 specific compounds 1 and 2 in addition to the large amounts of the 2 major PGAs, chaconine and solanine. Compounds 1 and 2 were not observed in other extracts, except for the root extracts of KH and SD, which only contained 2 as a very minor one ()). These observations suggested that 1 and 2 might be specifically produced or accumulated in the flowers, and also confirmed that the strong cytotoxicity of the flower extracts was derived from the mixture of the 4 PGAs, especially the additional quantities of 1 and 2 were important contributors.
The fractions from the tuber peel showed significant antimalarial activity in addition to mild cytotoxicity, and some fractions showed strong antibacterial activities ( and Table S2). However, the major PGAs, chaconine and solanine, did not show any effect against bacteria and malaria (). This result suggests that potatoes may produce some unknown secondary metabolites with antibacterial or antimalarial activities apart from PGAs, which are particularly accumulated in the tuber peel. Moreover, an increase in activity was observed after MPLC separation. For example, MPLC fractions F007 and F008 prepared from the BuOH extract of the flower showed antimalarial activity, which was not clearly observed for the extract ( and Figure S1). This is one advantage of using a fraction library to check various kinds of biological activities. The application of this method will lead to the isolation and identification of biologically and/or structurally interesting secondary metabolites.
Conclusion
The potato fraction library was constructed by the application of our methodology on microbial metabolite fraction library to investigate the functional secondary metabolites in the potato plant. Several parts of the potato plant including flower, leaf, stem, roots, tuber peel, and tuber from 8 cultivars including Kitahime, Pilka, Sakurafubuki, Atlantic, Toyoshiro, Snowden, Kitamurasaki, and Northern Ruby were used for the study. The fraction library was subjected to DAD-LC/MS analysis and several biological activity tests including cytotoxicity, antibacterial and antimalarial activity. The extracts of flowers were the most cytotoxic and the potent activity was found to be derived from the complex mixture of PGAs. In particular, the flower extracts contained large amounts of 2 specific compounds, 1 and 2, which had identical planar structures with solamargine and solasonine, respectively, in addition to the major PGAs, chaconine and solanine. The relative quantities of the 4 PGAs in flower fractions were related to their cytotoxicity.
In conclusion, with simple modifications, our methodology to investigate interesting secondary metabolites from microorganisms by using a fraction library was successfully applied to plant materials. The results demonstrated that potatoes might produce some novel secondary metabolites with unknown biological activities and functions.
Author contributions
T.N. and H.O. conceived and designed the research. T.N., Y.F., A.O., M.S, J.N. and K.I performed experiments. T.N., Y.F. and J.N. wrote the manuscript with supports from all other authors. All authors reviewed and approved the manuscript.
BBB-180204_Supplementary.pdf
Download PDF (2.4 MB)Acknowledgments
We thank Ms H Aono, Ms M Tanaka, Dr J Otaka, and Mr K Yamamoto at RIKEN for performing the activity tests and Dr JAV Lopez at RIKEN for reading this paper. We also thank Dr H Koga, Mr M Ito, Dr R Sakuma, and Ms H Hasebe at Calbee Inc. for technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Maga JA, Fithzpatrick TJ. Potato glycoalkaloids. CRC Crit Rev Food Sci Nutr. 1980;12:371–405.
- Birch PRJ, Bryan G, Fenton B, et al. Crops that feed the world 8: potato: are the trends of increased global production sustainable? Food Sec. 2012;4:477–508.
- Han JS, Kozukue N, Young KS, et al. Distribution of ascorbic acid in potato tubers and in home-processed and commercial potato foods. J Agric Food Chem. 2004;52:6515–6521.
- Camire ME, Kubow S, Donnelly DJ. Potatoes and human health. Crit Rev Food Sci Nutr. 2009;49:823–840.
- Miyoshi T, Ishihara K, Nakamura K, et al. Antioxidative compounds in potato chips. J Cook Sci Jpn. 2006;39:282–727.
- Hamouz K, Lachman J, Pazderu K, et al. Effect of cultivar, location and method of cultivation on the content of chlorogenic acid in potatoes with different flesh colour. Plant Soil Environ. 2013;59:465–471.
- Ezekiel R, Singh N, Sharma S, et al. Beneficial phytochemicals in potato – a review. Food Res Int. 2013;50:487–496.
- Rodriguez de Sotillo D, Hadley M, Wolf-Hall C. Potato peel extraction a nonmutagenic antioxidant with potential antimicrobial activity. J Food Sci. 1998;63:907–910.
- Shindo T, Ushiyama H, Kan K, et al. Contents and its change during storage of α-solanine and α-chaconine in potato. Food Hyg Saf Sci. 2004;45:277–282.
- Korpan YI, Nazarenko EA, Skryshevskaya IV, et al. Potato glycoalkaloids: true safety or false sense of security? Trends Biotechnol. 2004;22:147–151.
- Huang W, Serra O, Dastmalchi K, et al. Comprehensive MS and solid-state NMR metabolomics profiling reveals molecular variations in native periderms from four Solanum tuberosum Potato cultivars. J Agric Food Chem. 2017;65:2258–2274.
- Osada H, Nogawa T. Systematic isolation of microbial metabolites for natural products depository (NPDepo). Pure Appl Chem. 2012;84:1407–1420.
- Kato N, Takahashi S, Nogawa T, et al. Construction of a microbial natural product library for chemical biology studies. Curr Opin Chem. 2012;16:101–108.
- Larsson J, Gottfries J, Muresan S, et al. ChemGPS-NP: tunes for navigation in biologically relevant chemical space. J Nat Prod. 2007;70:789–794.
- Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–661.
- Osada H. Bioprobes. Berlin: Springer;2017. 1–14. ed. Osada H.
- Nogawa T, Okano A, Takahashi S, et al. Verticilactam, a new macrolactam isolated from a microbial metabolite fraction library. Org Lett. 2010;12:4564–4567.
- Nogawa T, Takahashi S, Okano A, et al. Spirotoamides A and B, novel 6,6-spiroacetal polyketides isolated from a microbial metabolite fraction library. J Antibiot. 2012;65:123–128.
- Nogawa T, Kawatani M, Uramoto M, et al. Pyrrolizilactone, a new pyrrolizidinone metabolites produced by a fungus. J Antibiot. 2013;66:621–623.
- Nogawa T, Kato N, Shimizu T, et al. Wakodecalines A and B, new decaline metabolites isolated from a fungus Pyrenochaetopsis sp RK10-F058. J Antibiot. 2018;71:123–128.
- Deguchi T, Iwama K, Haverkort AJ. Actual and potential yield levels of potato in different production systems of Japan. Potato Res. 2016;59:207–225.
- Lim CL, Nogawa T, Uramoto M, et al. RK-1355A and B, novel quinomycin derivatives isolated from a microbial metabolites fraction library based on NPPlot screening. J Antibiot. 2014;67:323–329.
- Nogawa T, Okano A, Lim CL, et al. Opantimycin A, a new metabolite isolated from Streptomyces sp. RK88-1355. J Antibiot. 2017;70:222–225.
- Alzerreca A, Hart G. Molluscicidal steroid glycoalkaloids possessing stereoisomeric spirosolane structures. Txicol Lett. 1982;12:151–155.