ABSTRACT
Auxin is thought to be an important factor in the induction of galls by galling insects. We have previously shown that both galling and nongalling insects synthesize indole-3-acetic acid (IAA) from tryptophan (Trp) via two intermediates, indole-3-acetaldoxime (IAOx) and indole-3-acetaldehyde (IAAld). In this study, we isolated an enzyme that catalyzes the last step “IAAld → IAA” from a silk-gland extract of Bombyx mori. The enzyme, designated “BmIAO1”, contains two 2Fe–2S iron–sulfur-cluster-binding domains, an FAD-binding domain, and a molybdopterin-binding domain, which are conserved in aldehyde oxidases. BmIAO1 causes the nonenzymatic conversion of Trp to IAAld and the enzymatic conversion of IAOx to IAA, suggesting that BmIAO1 alone is responsible for IAA production in B. mori. However, a detailed comparison of pure BmIAO1 and the crude silk-gland extract suggested the presence of other enzymes involved in IAA production from Trp.
Abbreviations: BA: benzoic acid; CE: collision energy; CXP: collision cell exit potential; DP: declustering potential; IAA: indole-3-acetic acid; IBI1: IAA biosynthetic inhibitor-1; IAAld: indole-3-acetaldehyde; ICA: indole-3-carboxylic acid; IAOx: indole-3-acetaldoxime; IEtOH: indole-3-ethanol; LC–MS/MS: liquid chromatography–tandem mass spectrometry; Trp: tryptophan
Graphical Abstract
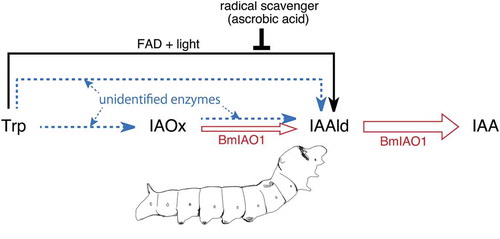
An enzyme, BmIAO1, that converts IAAld to IAA was identified in Bombyx mori, which could be responsible for IAA production in a broad range of insects
We have previously reported that galling insects contain indole-3-acetic acid (IAA), the active form of the phytohormone auxin, at higher levels than their host plant tissues. For example, a galling sawfly (Pontania sp.) larva contains about 1000 ng g−1 IAA, which is about 100 times higher than that in the leaf tissue of its host, the willow plant [Citation1]. We have also shown that galling insects can biosynthesize IAA from tryptophan (Trp) [Citation1–Citation3]. Based on the finding that not only galling insects but also nongalling insects can convert Trp to IAA [Citation4], we hypothesized that the biosynthesis of IAA is intrinsic to insects, and that galling insects evolutionarily acquired a more efficient system of IAA production than other insects. Based on this hypothesis, we used the nongalling silkworm, Bombyx mori, as a source of large amounts of experimental material at any time of the year, with which to study the IAA biosynthesis pathway. We proposed the pathway tryptophan (Trp) → indole-3-acetaldoxime (IAOx) → indole-3-acetadehyde (IAAld) → indole-3-acetic acid (IAA) is a commonly operating pathway in the galling sawfly and nongalling B. mori [Citation4]. Although IAOx was not detected as a metabolite of Trp and IAAld was not detected as a metabolite of Trp or IAOx in B. mori, this pathway was deduced from the finding that the conversion rates of each precursor to IAA were in the following order: “Trp → IAA” < “IAOx → IAA” < “IAAld → IAA”. We demonstrated the inclusion of the conversion “IAAld → IAA” in IAA production from both Trp and IAOx using a compound we isolated from a chemical library that inhibits the conversion “IAAld → IAA”. We designated it “IAA biosynthetic inhibitor-1” (IBI1). IBI1 inhibits IAA production not only from IAAld, but also from Trp and IAOx [Citation4]. A detailed analysis of the enzymatic activity of a B. mori silk-gland extract indicated that the apparent conversion rate for the conversion “IAOx → IAA” is about 60 times higher than that for “Trp → IAA”, and that the rate for “IAAld → IAA” is about 3000 times higher than that for “IAOx → IAA”. These results explain the failure of IAOx and IAAld to accumulate in the reaction mixture, and therefore our inability to detect them as metabolic intermediates in the conversion [Citation5]. We also concluded that IAOx is converted to IAA through IAAld because IAAld is converted to indole-3-ethanol (IEtOH) in the presence of IBI1, and the product of IAOx is altered from IAA to IEtOH by the inhibitor [Citation5].
In this study, we isolated an enzyme responsible for the conversion of IAAld to IAA from the B. mori silk gland and identified it. Although the results discussed above indicate that this conversion is not a rate-limiting step in the biosynthesis of IAA, we regard this enzyme as a key player in IAA production in a broad range of insects.
Materials and methods
Preparation of crude silk-gland extract
The crude silk-gland extract was prepared from about 30 g of mature silk gland of B. mori, as previously described [Citation5].
Conversion activity assay
To detect the enzyme activity after the chromatographic fractionation of the silk-gland extract, a portion of each fraction was incubated with 0.5 μg of IAAld for 10–30 min in a total volume of 50 μL. To determine the optimum pH of the reaction, it was performed at various pHs, ranging from pH 4.0 to 10.5, in the following buffers (all 25 mM): acetate buffer for pH 4.0–6.0, phosphate buffer for pH 6.0–7.0, Tris-HCl buffer for pH 7.0–9.5, and glycine buffer for pH 9.0–10.5. To determine the optimum temperature of the reaction, it was performed at various temperatures ranging from 0 to 90 °C. The enzyme and substrate were preheated separately at each test temperature for 3 min, and the reaction commenced when they were mixed. To analyze the thermal stability of the enzyme, the enzyme solution was incubated for 10 min at various temperatures ranging from 0 to 90 °C before the substrate IAAld was added. The Michaelis constant (KM) and maximal velocity (Vmax) were calculated from a Lineweaver–Burk plot, which was constructed by incubating 5.6, 7.0, 14, 28, and 560 μM IAAld in 25 mM Tris-HCl (pH 8.0) at 25 °C for 10 min. To measure the IAA produced during incubation, the reaction was stopped with the addition of 10 μL of 1 M HCl, and the mixture was spiked with 5 ng of [13C6]IAA, which was extracted with 100 μL of ethyl acetate. The ethyl acetate extract was concentrated in vacuo and subjected to liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis.
Each of the other candidate substrates (0.2 mM indole-3-carboxaldehyde, benzaldehyde, or xanthine) was incubated with the purified enzyme in 50 mM Tris-HCl (pH 9.0) for 5–30 min. The indole-3-carboxylic acid and benzoic acid produced from indole-3-carboxaldehyde and benzaldehyde, respectively, were extracted with ethyl acetate under acidic conditions and subjected to LC–MS/MS analysis, as for IAA. The uric acid produced from xanthine was analyzed with high-performance liquid chromatography (HPLC), as described below. One unit of enzyme activity was defined as the amount of protein that produced 1 μmol of product per minute, and the enzyme activities for different substrates were compared as the number of units in 1 mL of enzyme solution.
To examine the possible conversion of Trp or IAOx to IAA, 10 μg of Trp or 0.5 μg of IAOx was incubated with the purified enzyme or crude silk-gland extract for 16 h at 25 °C. For incubation under light, the reaction mixture was placed at about 25 cm from a 32 W fluorescent light (FHF32EX-N-H; Mitsubishi, Tokyo, Japan). To denature the enzyme, the reaction solution, including the enzyme(s), was treated at 100 °C for 30 min before the addition of the substrates. Ascorbic acid (0.1 mg mL−1) was used as a radical scavenger. IAAld (as a metabolite of Trp) and IAOx (as a metabolite of Trp) were extracted from the reaction mixture with ethyl acetate at neutral pH. The concentrated ethyl acetate extract was analyzed directly for its IAOx content with LC–MS/MS, or incubated in 0.25 M 2-aminoethanethiol to convert IAAld to its thiazolidine derivative, which was then analyzed with LC–MS/MS [Citation5]. The IAAld metabolite of IAOx was converted to IAA by incubation with purified BmIAO1 for 10 min and analyzed as IAA, because IAAld is easily formed nonenzymatically from a fraction of IAOx during the purification procedure. Note that neither IAAld nor IAA was detected after the treatment of IAOx with BmIAO1 for 10 min.
LC–MS/MS and HPLC–UV analyses
The products of the enzyme reaction (except uric acid) were analyzed with a 3200 QTrap LC–MS/MS instrument (AB Sciex, Foster City, CA, USA). The samples were separated with a Capcell Pak C18 MGIII column (2.0 mm i.d. × 250 mm; Shiseido, Tokyo, Japan). The elution conditions for IAA, IAAld, and IAOx were as previously reported by Yokoyama et al. [Citation5]. For the analysis of indole-3-carboxylic acid (ICA), the elution conditions were: isocratic elution for 2 min with 20% B, a linear gradient from 20% B to 100% B over a 5 min period, and isocratic elution for 3 min with 100% B. For the analysis of benzoic acid (BA), the elution conditions were: isocratic elution for 1 min with 20% B, a linear gradient from 20% B to 100% B over a 4 min period, and isocratic elution for 5 min with 100% B. These elution conditions gave retention times of 8.32 min for ICA and 7.34 min for BA. The mass spectrometer was operated in the multiple-reaction-monitoring mode. The analysis of IAAld was according to the method of Yokoyama et al. [Citation5]. The ion source (Turbo V Ion Source) was operated in the positive electrospray ionization (ESI) mode for IAA and IAOx and the negative ESI mode for ICA and BA. The source parameters were as follows: curtain gas, 10 psi for IAA and IAOx, 40 psi for ICA, 20 psi for BA; temperature, 600 °C for IAA, IAOx, and ICA, and 300 °C for BA; spray gas (GS1), 60 psi for IAA and IAOx, 50 psi for ICA, and 70 psi for BA; dry gas (GS2), 60 psi for IAA and BA, 80 psi for IAOx and ICA; and ion spray voltage, 5500 V for IAA, 4500 V for IAOx, and −4500 V for ICA and BA. The following transitions were monitored (collision energy, CE; collision cell exit potential, CXP; declustering potential, DP): IAA: m/z 176 → 130 (CE 19 V, CXP 2.0 V, DP 26 V); [13C6]IAA: m/z 182 → 136 (CE 19 V, CXP 2.0 V, DP 26 V); IAOx: m/z 175 → 158 (CE 15 V, CXP 12 V, DP 26 V); ICA: m/z 160 → 116 (CE −14 V, CXP −8.0 V, DP −30 V); BA: m/z 121 → 77 (CE −12 V, CXP −6.0 V, DP −25 V). Both quadrupoles were set at unit resolution. Uric acid (the product of xanthine) was analyzed with HPLC (Inertsil® ODS-EP column, 6.0 mm i.d. × 250 mm, 5 μm; GL Sciences, Tokyo, Japan), with UV absorption detection at 284 nm. The samples were eluted with 0.1% acetic acid in 2% aqueous methanol at 1.0 mL min−1. Under these conditions, uric acid was eluted at 7.9 min.
Purification of aldehyde oxidase (AO)
The crude silk-gland extract was dialyzed against 50 mM Tris-HCl (pH 8.0) and centrifuged at 2,000 × g for 10 min. The supernatant was applied to a Q-Sepharose Fast Flow column (16 mm i.d. × 170 mm; GE Healthcare Life Sciences, Buckinghamshire, England, UK) equilibrated with 50 mM Tris-HCl (pH 8.0). After the column was washed with 100 mL of Tris-HCl buffer, the proteins were eluted with a linear gradient of NaCl from 0 to 0.5 M in the same buffer (300 mL in total). The elution of the proteins was monitored at 280 nm, and the enzyme activity in each fraction collected was assayed as described above. The active fractions were pooled and dialyzed against 50 mM Tris-HCl (pH 7.0) containing 1 M ammonium sulfate. After centrifugation at 2,000 × g for 10 min, the supernatant was applied to a Butyl Cellulofine column (14 mm i.d. × 240 mm; Seikagaku Industries, Tokyo, Japan) equilibrated with the same buffer. After the column was washed with 250 mL of Tris-HCl/ammonium sulfate buffer, the proteins were eluted with a linear gradient of ammonium sulfate from 1 M to 0 M in 50 mM Tris-HCl (pH 7.0) (300 mL in total), and further eluted with 100 mL of 50 mM Tris-HCl (pH 7.0). The active fractions were pooled and dialyzed against 50 mM Tris-HCl (pH 7.0) containing 0.15 M NaCl. The protein solution was concentrated with ultrafiltration using an Amicon® Ultra-15 Centrifugal Filter Unit (Merck, Kenilworth, NJ, USA). After the concentrate was centrifuged at 18,000 × g for 5 min, the supernatant was applied to a Sephacryl S-300 column (16 mm i.d. × 500 mm; GE Healthcare Life Sciences) equilibrated with the same buffer. The proteins were eluted with 100 mL of the same buffer. The protein concentration in the pooled active fraction from each purification step was measured with a BCA Protein Assay Kit (Pierce, Rockford, IL).
Polyacrylamide gel electrophoresis (PAGE) and activity staining
Sodium dodecyl sulfate (SDS)-PAGE was performed with a 10% or 15% polyacrylamide gel, and the proteins were stained with either Coomassie Brilliant Blue (CBB) or silver stain with conventional methods. Unless otherwise stated, the samples were treated at 95 °C for 5 min before loading onto the polyacrylamide gel. To determine the activities of the protein bands observed with silver staining, the protein sample was incubated at 37 °C for 1 h before loading. After electrophoresis, the gel was washed with 2.5% Triton X-100 for 1 h to remove the SDS, and cut into 5 mm-wide segments. Each segment was homogenized in 50 mM Tris-HCl (pH 7.0) and incubated overnight at 4 °C. The homogenate was centrifuged at 18,000 × g for 5 min, and the supernatant was recovered and subjected to an enzyme activity assay.
For activity staining, the proteins were separated on a 10% polyacrylamide gel without SDS at 4 °C. The gel was immersed in 100 mM Tris-HCl (pH 9.0) for 5 min, and then the activity was stained by incubating it in the same buffer containing 0.1 mM phenazine methosulfate, 0.4 mM 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and 1 mM IAAld at the ambient temperature, according to Koshiba et al. [Citation6].
Amino acid sequencing
The purified protein was transferred to polyvinylidene difluoride (PVDF) membrane after SDS-PAGE. The N-terminal amino acid sequence of the protein band was determined with a protein sequencer (Procise 494-HT, Applied Biosystems, Foster City, CA).
Results
The enzyme required to convert IAAld to IAA was purified from B. mori silk-gland extract by successive rounds of chromatographic fractionation. The elution profiles of the proteins and the conversion activities after each purification step are shown in Suppl. Figure 1 (A, anion exchange chromatography on a Q-Sepharose Fast Flow column; B, hydrophobic interaction chromatography on a Butyl Cellulofine column; C, gel filtration chromatography on a Sephacryl S-300 column). The purification results are summarized in . The major protein responsible for the enzyme activity was obtained after three rounds of chromatography as an almost single band after CBB staining (Suppl. Figure 2A). Based on the Lineweaver-Burk plot (Suppl. Figure 3), KM and Vmax were determined to be 1.4 × 10−4 M and 1.3 μM min−1, respectively. The presence of other enzymes with the same activity for IAAld in the silk gland was examined by staining the crude silk-gland extract for activity (Suppl. Figure 4). Only one band, corresponding to BmIAO1, was detected.
Table 1. Purification of IAAld oxidase from silkworm silk-gland.
The amino acid sequence of the most-purified fraction was determined without any protease treatment, and showed the single N-terminal sequence STTKELTLKDGVKWFTVTQI, which is found in a B. mori protein (XM_004925903), not as its N-terminal sequence but as the internal sequence starting from 201Ser. When the purified fraction was separated with nondenaturing SDS-PAGE after treatment at 37 °C, the enzyme activity was detected in a region of the gel containing a major protein band of about 260 kDa (Suppl. Figure 2B). The N-terminal sequence of this 260-kDa protein band was the same as that described above. When the fraction was heat treated at 95 °C before SDS-PAGE, the major band shifted to smaller band of about 130 kDa. This band was also faintly observed when the protein was not treated with high temperature (Suppl. Figure 2C), suggesting that the enzyme takes a homodimeric form. The 95 °C treatment also produced a band of about 15 kDa, which we inferred to be the N-terminal fragment that had dissociated from the enzyme complex. The identified clone from the databank was annotated as a xanthine dehydrogenase-like protein (accession no. D38159). Xanthine dehydrogenases (XDHs) are highly homologous to AOs, and have been considered to be the evolutionarily antecedents of AOs. We designated this clone “BmIAO1”, for “B. mori IAAld oxidase 1”. Both XDH and AO are known to form a homodimeric structure, consistent with the inference noted above. BmIAO1 contains two 2Fe–2S iron–sulfur-cluster-binding domains, an FAD binding domain, and a molybdopterin-binding domain, all of which are conserved in both eukaryotic XDHs and AOs (). The amino acid residues involved in the recognition of the xanthine substrate of XDHs are well conserved among XDHs from various organisms, whereas BmIAO1 does not share these residues, except for 1119Glu (, Suppl. Fig. 5), which is usually conserved in both XDHs and AOs. This suggests that BmIAO1 only has AO activity. This was confirmed experimentally. When 3.0 μg of xanthine was incubated with the purified enzyme, no uric acid (the product of XDH) was detected at all (Suppl. Fig. 6). Because detection limit of uric acid was less than 1 ng on HPLC, it was concluded that the conversion rate is practically zero. In contrast, benzaldehyde was a good substrate of BmIAO1, comparable to IAAld (6.7 × 10−4 units/mL for IAAld and 5.8 × 10−4 units/mL for benzaldehyde), whereas indole-3-carboxaldehyde was barely converted by BmIAO1 (7.4 × 10−7 units/mL).
Figure 1. Schematic drawing of BmIAO1 and amino acid residues conserved in XDHs for substrate recognition. (A) BmAOX1 contains two [2Fe–2S]-cluster-binding domains ([2Fe–2S]-B), an FAD-binding domain (FAD-B), and a molybdopterin-binding domain (Molybdopterin-B), all of which are highly conserved in both XDHs and AOs. (B) Five amino acid residues involved in substrate recognition conserved in XDHs are boxed; four of these are not found in BmIAO1 and other AOs. The most strongly conserved identical amino acids are shown in bold. Accession numbers are: BmIAO1 (XM_004925903), Maize_AO-1 (D88451), Silkworm_XDH_1 (D38159), Fruit_fly_XDH (BT015293), Silkworm_XDH_2 (D43965), Human_XDH (D11456), Rat_XDH (J05579), Cow_XDH (X83508), and Chicken_XDH (D13221).
![Figure 1. Schematic drawing of BmIAO1 and amino acid residues conserved in XDHs for substrate recognition. (A) BmAOX1 contains two [2Fe–2S]-cluster-binding domains ([2Fe–2S]-B), an FAD-binding domain (FAD-B), and a molybdopterin-binding domain (Molybdopterin-B), all of which are highly conserved in both XDHs and AOs. (B) Five amino acid residues involved in substrate recognition conserved in XDHs are boxed; four of these are not found in BmIAO1 and other AOs. The most strongly conserved identical amino acids are shown in bold. Accession numbers are: BmIAO1 (XM_004925903), Maize_AO-1 (D88451), Silkworm_XDH_1 (D38159), Fruit_fly_XDH (BT015293), Silkworm_XDH_2 (D43965), Human_XDH (D11456), Rat_XDH (J05579), Cow_XDH (X83508), and Chicken_XDH (D13221).](/cms/asset/c3bfa6bb-86cf-4318-8a5b-e7eef261a24f/tbbb_a_1525275_f0001_b.gif)
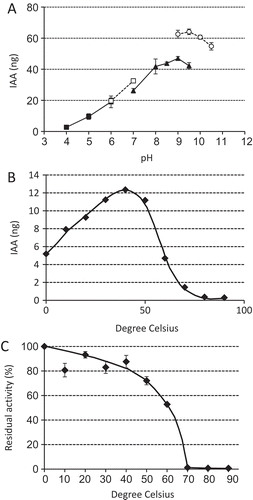
The enzyme was further characterized. The pH dependence of the activity is shown in . The optimum pH was determined to be about 9. The optimum temperature is about 40 °C (). The thermal stability is shown in . Incubation at > 50 °C reduced the enzyme activity, which was completely lost after incubation at 70 °C for 10 min.
Figure 2. Some properties of BmIAO1. (A) pH dependence (acetate buffer, filled squares; phosphate buffer, open squares; Tris-HCl buffer, filled triangles; glycine buffer, open circles); (B) temperature dependence; and (C) heat stability.
Although BmIAO1 was identified as an enzyme responsible for the conversion of IAAld to IAA, the possibility that BmIAO1 functions enzymatically in other IAA biosynthetic steps was examined (). Because FAD, one of the prosthetic groups of AO, can nonenzymatically convert Trp to IAAld under light [Citation7], we also examined the effect of light. When 10 μg of Trp was incubated with BmIAO1, 5.5 ng of IAA was produced, whereas the yield of IAA increased 4-fold in the presence of light. The production of IAA was completely abolished by boiling the enzyme, suggesting that photosensitized FAD nonenzymatically converted Trp to IAAld, which was then converted to IAA enzymatically by BmIAO1 (). To confirm this interpretation, the amount of IAAld produced from Trp was examined under various conditions (). When intact BmIAO1 was used, IAAld was barely detectable because the enzyme activity of BmIAO1 in metabolizing IAAld to IAA was strong. When the enzyme was heat-deactivated, 5.9 ng of IAAld was detected, which was similar to the amount of IAA produced by the intact enzyme. The combination of heat-deactivation and light treatment extraordinarily enhanced the production of IAAld, which was abolished in the presence of ascorbic acid. This result suggests that the FAD molecules released from the denatured enzyme were photoactivated and converted Trp to IAAld as a radical reaction under light. However, no IAOx was detected as a product of Trp under any conditions (data not shown), suggesting that the nonenzymatic conversion of Trp to IAAld does not produce IAOx as an intermediate. These experiments were repeated with the crude silk-gland extract (). The activity that converted IAAld to IAA was adjusted so that it was equal in the pure enzyme and crude extract. The IAA (0.9 ng) detected even after treatment with the heat-denatured enzyme corresponded to the endogenous IAA in the crude silk-gland extract. The effects of both light (increasing IAA production) and ascorbic acid (reducing IAA production) were weaker in the crude extract than in the pure enzyme. Heat-denaturation of the crude extract only slightly increased the production of IAAld (from 0.2 to 1.1 ng) from Trp, which was much smaller than the reduction in IAA productivity (from 3.9 ng to 0.9 ng) caused by heat denaturation. These results suggest the presence of other enzymes responsible for the conversion “Trp → IAAld” in the crude extract. Although the combination of heat-denaturation and light treatment increased the level of IAAld, the effect was much smaller in the crude silk-gland extract than in pure BmIAO1.
Figure 3. Availability of Trp and IAOx as substrates of BmIAO1. Effects of light, heat denaturation, and ascorbic acid (VC) on the conversion by pure BmIAO1 (A, B) and crude silk-gland extract (C, D) of Trp to IAA (A, C) or to IAAld (B, D) were examined. The conversion activities of pure BmIAO1 (E) and crude silk-gland extract (F) from IAOx to IAAld or IAA were examined.
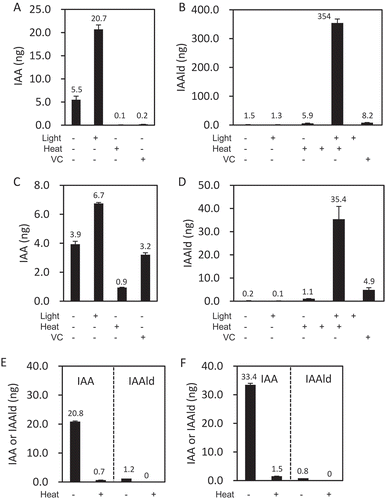
When 500 ng of IAOx was incubated with BmIAO1, 21 ng of IAA was detected, whereas no IAAld accumulated (). The production of IAA from IAOx was abolished by the heat-deactivation of the enzyme. Contrary to the conversion of Trp, IAAld did not accumulate after the deactivation of the enzyme. When the activity that converts IAAld to IAA in the pure BmIAO1 was adjusted to be equal to that in the crude silk-gland extract, the crude extract showed greater conversion of IAOx to IAA (), suggesting the presence of other enzyme(s) that convert IAOx to IAAld. This result is consistent with the conclusion drawn in our previous study that IAA is produced from IAOx through IAAld [Citation5]. Ascorbic acid had no effect on the production of IAA in either pure BmIAO1 or the crude extract (Suppl. Fig. 7).
Discussion
In this study, we isolated an enzyme responsible for the conversion of IAAld to IAA from a B. mori silk-gland extract, and identified the partial amino acid sequence. The sequence determined was not the N-terminal sequence but an internal sequence, starting from 201Ser. The detection of a ~ 15-kDa fragment on electrophoresis only when the protein was heat treated suggests that this fragment is the N-terminal portion of the enzyme, and was copurifed with the other portion. The scission of the molecule may have occurred during the purification procedure.
Although the clone (XM_004925903) identified, designated BmIAO1, had been annotated as “xanthine dehydrogenase-like”, it shows high homology to known AOs. Therefore, we conclude that this clone is the enzyme responsible for the conversion of IAAld to IAA. BmIAO1 showed no activity against xanthine, whereas benzaldehyde was a good substrate of BmIAO1. Several genes encode AOs in B. mori. Pelletier et al. [Citation8] examined AOX1 and AOX2 as candidate enzymes involved in pheromone degradation in the antenna, and localized the expression of their genes to the antenna. However, they did not confirm their enzymatic functions. The function of no B. mori AO has been reported until now. Activity staining of the silk-gland extract after native PAGE showed only one band, which corresponded to BmIAO1 (Suppl. Figure 4), suggesting that BmIAO1 is the only enzyme that converts IAAld to IAA in the silk gland. However, it is still possible that other AOs that localize differently from BmIAO1, outside the silk gland, have the same catalytic activity.
Purified BmIAO1 had a KM of 140 μM. The Zea mays AO, zmAO-1, was isolated as an enzyme activity that converts IAAld to IAA [Citation6]. ZmAO-1 shows higher substrate affinity (KM = 3.2 μM) than BmIAO1. The pathway “Trp → indole-3-pyruvic acid → IAA” is the main IAA biosynthetic pathway in plants [Citation9,Citation10]. Although zmAO-1 was isolated as a candidate enzyme responsible for IAA production in maize, it is unlikely that the pathway via IAAld is involved in IAA production in plants. An Arabidopsis AO, AOα, also catalyzed the conversion of IAAld to IAA when prepared as a recombinant protein, with KM of 39 μM [Citation11], but its biological function is also unknown. Because benzaldehyde is a good substrate for zmAO-1, AOα, and BmIAO1, and because BmIAO1 has even lower affinity for IAAld than zmAO-1 or AOα, it is possible that BmIAO1 has not been optimized for IAA production and has biological functions other than its role in IAA production.
Contrary to our expectation, BmIAO1 converted Trp to IAA. This conversion seems to involve the nonenzymatic step “Trp → IAAld” and the enzymatic step “IAAld → IAA” (). This assumption is supported by the findings that IAA production was increased by light irradiation; the amount of IAAld (5.9 ng) produced by the heat-deactivated enzyme was nearly equal to the amount of IAA (5.5 ng) produced by the intact enzyme; and the heat-denatured protein showed strong activity in converting Trp to IAAld under light (). Heat treatment seems to have released FAD from BmIAO1, which was then photoactivated and induced a radical reaction that converted Trp to IAAld [Citation7]. The involvement of a radical reaction is supported by the suppression of this reaction by ascorbic acid ().
Figure 4. Summary of the IAA production system in B. mori. Open arrows, enzymatic conversion by BmIAO1; solid arrow, nonenzymatic conversion by BmIAO1; dotted arrows, conversion by unidentified enzymes present in the crude silk-gland extract.
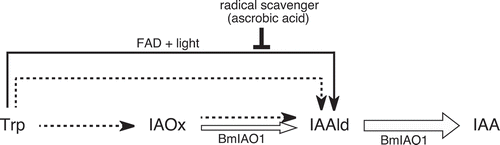
The crude silk-gland extract produced less IAA from Trp than did BmIAO1, and this conversion was also less enhanced by light irradiation (3.9 → 6.7 ng vs 5.5 → 20.7 ng, respectively) (). The heat-denatured extract also produced less IAAld (1.1 ng) from Trp than did pure BmIAO1 (5.9 ng) (). Although IAAld production was enhanced by light irradiation, the level of IAAld (35.4 ng) was only one tenth of that produced by BmIAO1 (354 ng). These results suggest that the nonenzymatic activity is weaker in the crude extract than in pure BmIAO1. The “IAAld → IAA” activity in the crude extract was adjusted to equal that in the pure BmIAO1. Because the amount of FAD released from BmIAO1 should be the same in both the pure and crude reaction mixtures, the weaker nonenzymatic activity in the crude extract suggests the presence of endogenous radical scavengers in the crude extract. IAA production (5.5 ng) with intact BmIAO1 without light irradiation seems to be attributable to the partial release of FAD from BmIAO1 and the weak light irradiation that occurred during experimental handling, because the IAA yield was reduced to nearly zero (0.2 ng) by ascorbic acid. However, the amount of IAA produced by the crude extract was calculated to be 3.0 ng (by subtracting 0.9 ng, the endogenous IAA in the crude extract, from 3.9 ng), which is even lower than the amount produced by the pure enzyme (5.5 ng) (). This result can be attributed to radical scavengers present in the crude extract. The crude extract produced 2.3 ng of IAA (when 0.9 ng is subtracted from 3.2 ng), even in the presence of ascorbic acid, suggesting the presence of other conversion systems, including enzymes that produce IAA from Trp. Moreover, the amount of IAAld produced by the heat-denatured BmIAO1 (5.9 ng) was nearly equal to that of the IAA produced by intact BmIAO1 (5.5 ng) (), whereas the heat-denatured crude extract produced only 1.1 ng of IAAld, which is much less than the level of IAA produced by the intact crude extract (3.0 ng) (). The different degrees of the effect that heat denaturation exerted on pure BmIAO1 and the crude extract also suggest that Trp is enzymatically converted to IAAld in the crude extract. This could be either the one-step conversion “Trp → IAAld” or the two-step conversion “Trp → IAOx → IAAld”.
Although BmIAO1 converted IAOx to IAA, IAAld did not accumulate after the heat-deactivation of BmIAO1, which differs from the conversion of Trp, during which IAAld accumulated after the deactivation of the enzyme. This supports the argument made above that the nonenzymatic conversion of Trp to IAAld does not include IAOx as an intermediate. This result also indicates that BmIAO1 enzymatically converts IAOx to IAA (). However, the result itself does not clarify whether this conversion involves the one-step process “IAOx → IAA” or the two-step process “IAOx → IAAld → IAA”. The crude extract showed higher activity than pure BmIAO1 in converting IAOx to IAA (33.4 ng vs 20.8 ng, respectively), suggesting the presence of enzymes other than BmIAO1 in the extract. Because we have demonstrated previously that the major route of IAA production from IAOx involves IAAld as an intermediate [Citation5], it is probable that BmIAO1 enzymatically converts IAOx to IAA via IAAld, and that additional unidentified enzymes in the crude extract convert IAOx to IAAld.
In this study, we found that BmIAO1, an AO, may cause a nonenzymatic reaction, although this reaction is not prominent in the crude extract. This difference may be attributable to the presence of radical scavengers in the crude extract. It is possible that this kind of regulation, which represses undesirable oxidative radical reactions, operates more effectively in living B. mori. However, it will be necessary to examine the possibility that a nonenzymatic reaction is also involved in the production of IAA in insects. A detailed comparison of pure BmIAO1 and the crude extract suggested the presence in the extract of other enzymes that generate IAA from Trp. Identification of these enzymes will extend our understanding of the mechanisms underlying the efficient IAA biosynthetic system in galling insects.
Author contributions
Y.S. and Y.K. conceived and designed the study; M.T., S.K. and C.Y. performed the experiment; T.M. and Y.S. wrote the manuscript. All authors have read and approved the final manuscript.
Suppl._Fig._7.pdf
Download PDF (32.5 KB)Suppl._Fig._6.pdf
Download PDF (39.4 KB)Suppl._Fig._5.pdf
Download PDF (127.7 KB)Suppl._Fig._4.pdf
Download PDF (983.3 KB)Suppl._Fig._3.pdf
Download PDF (44.9 KB)Suppl._Fig._2.pdf
Download PDF (3.7 MB)Suppl._Fig._1C.pdf
Download PDF (48 KB)Suppl._Fig._1B.pdf
Download PDF (47.2 KB)Suppl._Fig._1A.pdf
Download PDF (39.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Yamaguchi H, Tanaka H, Hasegawa M, et al Phytohormones and willow gall induction by a gall-inducing sawfly. New Phytol. 2012;196:586–595.
- Tanaka Y, Okada K, Asami T, et al Phytohormones in Japanese mugwort gall induction by a gall-inducing gall midge. Biosci Biotechnol Biochem. 2013;77:1942–1948.
- Takei M, Yoshida S, Kawai T, et al Adaptive significance of gall formation for a gall-inducing aphids on Japanese elm trees. J Insect Physiol. 2015;72:43–51.
- Suzuki H, Yokokura J, Ito T, et al Biosynthetic pathway of the phytohormone auxin in insects and screening of its inhibitors. Insect Biochem Mol Biol. 2014;53:66–72.
- Yokoyama C, Takei M, Kouzuma Y, et al Novel tryptophan metabolic pathways in auxin biosynthesis in silkworm. J Insect Physiol. 2017;101:91–96.
- Koshiba T, Saito E, Ono N, et al Purification and properties of flavin- and molybdenum-containing aldehyde oxidase from coleoptiles of maize. Plant Physiol. 1996;110:781–789.
- Koshiba T, Yamauchi K, Matsuyama H, et al Flavin-photosensitized production of indole-3-acetaldehyde from tryptophan. Tetrahedron Lett. 1993;34:7603–7604.
- Pelletier J, Bozzolan F, Solvar M, et al Identification of candidate aldehyde oxidases from the silkworm Bombyx mori potentially involved in antennal pheromone degradation. Gene. 2007;404:31–40.
- Mashiguchi K, Tanaka K, Sakai T, et al The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci USA. 2011;108:18512–18517.
- Stepanova AN, Yun J, Robles LM, et al The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell. 2011;23:3961–3973.
- Koiwai H, Akaba S, Seo M, et al Functional expression of two Arabidopsis aldehyde oxidases in the yeast Pichia pastoris. J Biochem. 2000;127:659–664.
