ABSTRACT
To establish a reliable and practical ergothioneine (ERG) supply, we employed fermentative ERG production using Aspergillus oryzae, a fungus used for food production. We heterologously overexpressed the egt-1 and -2 genes of Neurospora crassa in A. oryzae and succeeded in producing ERG (231.0 mg/kg of media, which was 20 times higher than the wild type).
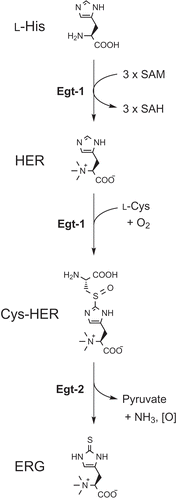
Abbreviations: ERG: ergothioneine; HER: hercynine; Cys-HER: hercynylcysteine-sulfoxide; SAM: S-adenosylmethionine; SAH: S-adenosylhomocysteine; l-His: l-histidine; l-Cys: l-cysteine; LC-ESI-MS: liquid chromatography-electrospray ionization-mass spectrometry
Ergothioneine (ERG), a histidine (His) betaine derivative with a thiol group at the C2 position of the imidazole ring (), is known as a very stable antioxidant. [Citation1] ERG is a natural compound and is found in the human body such as in red blood cells, the liver, the kidneys, and semen at high concentrations. However, humans cannot biosynthesize ERG and ingest it from diet via an organic cation transporter specific for ERG (OCTN1). [Citation2]
ERG was first isolated from an ergot fungus, Claviceps purpurea, more than a century ago, and mushrooms, fungi, fission yeast, actinobacteria, cyanobacteria, and a methylobacterium have recently been shown to synthesize ERG. [Citation3–Citation7] Mushrooms are major dietary sources but their slow growth, low contents, and time-consuming purification procedures lead to a high manufacturing cost. Therefore, alternative and sustainable sources of ERG are required.
A reliable and practical method for ERG production is a fermentative process using ERG-producing microorganisms, but the productivities are reported to be very low. We recently succeeded in heterologous production of ERG in Escherichia coli utilizing ERG biosynthetic genes identified in Mycobacteria smegmatis. [Citation8] In this study, we tried to produce ERG in a fungus with fungal biosynthetic genes. The biosynthetic pathway in Neurospora crassa was recently reported (). [Citation9,Citation10] Egt-1 is a bi-functional enzyme catalyzing successive reactions; the formation of hercynine (HER) with l-His and S-adenosylmethionine (SAM), followed by synthesis of hercynylcysteine-sulfoxide (Cys-HER) with HER, l-cysteine (l-Cys), and O2. Egt-2, a pyridoxal phosphate-dependent C–S lyase, catalyzes ERG formation from Cys-HER with concomitant release of pyruvate and ammonia as side-products. This pathway would be better for ERG production than that of M. smegmatis because it requires less genes and Cys as a sulfur source.
Aspergillus oryzae is a filamentous fungus used for traditional Japanese fermented beverages and seasonings, such as sake, soy sauce, and soybean paste, and is considered to be “generally recognized as safe” by the U.S. Food and Drug Administration. [Citation11] In addition, genetic engineering tools for A. oryzae have been established and utilized for heterologous expression of various foreign genes to produce many useful compounds. [Citation12] Therefore, we tried to heterologously produce Egt-1 and Egt-2 in A. oryzae.
There were no reports on ERG production by A. oryzae even though it has egt-1 and -2 orthologs, AO090012000265 and AO090026000291, which have 47% and 45% amino acid sequence identities, respectively (https://www.genome.jp/kegg-bin/show_organism?org=aor). Therefore, we first examined whether A. oryzae produces ERG in a solid medium containing 20 g of “Nanatsuboshi” rice (polishing ratio of 90%) steeped in deionized water for 6 h, 10 mg of adenine, and 4 mL of deionized water in a 50 mL glass petri dish. After sterilization, mycelia of A. oryzae NSAR1 [Citation13] () were inoculated into the solid medium and then cultured at 30°C for 5 days. After the whole culture was extracted with 100 mL methanol, an aliquot (1.2 mL) of the extract was centrifuged at 20,000 × g for 10 min to remove insoluble components. The supernatant (1 mL) was dried in vacuo and dissolved in 300 µL of water. Part of the solution (20 µL) was mixed with 0.05% (v/v) heptafluorobutyric acid (HFBA) solution (180 µL) and used for liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) analysis. Analytical conditions were as follows: UPLC, Waters ACQUITY system equipped with a photodiode array and a SQ Detector2 (Tokyo, Japan); column, XBridge BEH C18 XP (150 mm L × 2.0 mm ID; 2.5 µm; Waters); flow rate, 0.15 mL/min; temperature, 35°C; mobile phase, water containing 0.05% HFBA and 7% methanol; injection volume, 2 µL; detection, 258 nm for ERG. [Citation8] ERG productivity was estimated on the basis of the raw material (i.e. 20 g of steeped rice). As shown in , only a small amount of ERG production (11.5 ± 1.0 mg/kg of media) was detected. Therefore, we attempted to enhance the ERG productivity using a genetic engineering approach.
Table 1. Strains and plasmids used in this study.
Figure 2. ERG production by A. oryzae NSAR1 and its recombinants. Data are presented as mean values with standard errors from three independent experiments.
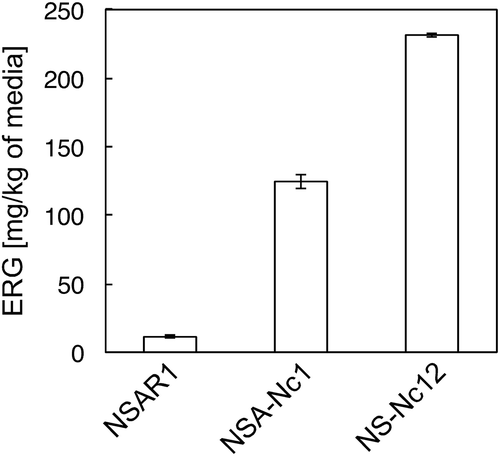
We first attempted to overexpress the N. crassa egt-1 gene in A. oryzae. DNA fragments encoding egt-1 were amplified by PCR with N. crassa JCM 19,069 genomic DNA as the template, the primers 5′-AACAAACTAGTATGCCGAGTGCCGAATCCATGACCCCAAG-3′/5′-TCACACTAGTCAACGACTCACAAATCCCTAACAACTCTCGC-3′ (SpeI restriction sites are underlined), and Tks Gflex DNA polymerase (Takara Bio Inc., Shiga, Japan). The PCR products (3,491 bp) were cloned into the same restriction site in the expression vector pUARA2 [Citation14] to express the egt-1 gene under the control of the amyB promoter. The plasmid thus constructed, pUARA2-Nc1 (11,546 bp, ), was introduced into A. oryzae according to a method described previously. [Citation15] After the transformants were subcultured twice on a selection medium, integration of the Ncegt-1 gene was confirmed by PCR with genomic DNA of the NSA-Nc1 strain and the same primers used for gene cloning (Figure S1A). Moreover, Southern blot analysis using a DNA fragment carrying Ncegt-1 as the probe showed only one strong band at approximately 11 kbp after digestion of the genomic DNA with XhoI, which has a unique restriction site in the Ncegt-1 gene (Figure S1B). In addition, only one band was detected by digestion with KasI or BplI, which have unique restriction sites in the vector. Taking these results together, the Ncegt-1 gene was suggested to be multiply integrated into the genomic DNA in a similar manner as previously reported. [Citation16] ERG productivity was then examined by the same methods as described above. As shown in , the ERG productivity of the transformant (strain NSA-Nc1) drastically increased to 124.5 ± 5.0 mg/kg of media. This result suggested that the egt-1 gene was efficiently expressed in A. oryzae.
Therefore, we next introduced the N. crassa egt-2 gene into the strain NSA-Nc1. DNA fragments were amplified by PCR with N. crassa genomic DNA and the primers 5′-AACAAACTAGTATGGTCGCCACCACCGTCGAGCTGCCTCTG-3′/5′-CAAATACTAGTTCAGGCGCCTCCTTGTACTCCCCCTTAGCCAC-3′ (SpeI restriction sites are underlined). The amplicon (1,498 bp) was digested with SpeI and inserted into the SpeI site of pAdeA2 [Citation17] to construct pAdeA2-Nc2 (8,086 bp, ). After the plasmid was introduced into the strain NSA-Nc1, integration of the Ncegt-2 gene into the genomic DNA was confirmed by PCR and Southern blot analysis (Figure S2). In this case, genomic Southern blot analysis with DNA fragment carrying the Ncegt-2 gene as the probe showed at least three major bands after digestion of the genomic DNA with NsiI, which exists at 159 bp from 5′ of the Ncegt-2 gene. Considering that random integrations are usually dominant rather than homologous recombination in fungi [Citation18], the detection of more than three hybridized bands suggested that the Ncegt-2 was integrated into at least two different loci of the genome. Accordingly, two major bands were detected by digestion with HindIII or SalI, which could cut the vector at unique sites. The ERG productivity of the transformant (strain NS-Nc12) was then investigated by the same methods as described above. As shown in , ERG productivity was further increased up to 231.0 ± 1.1 mg/kg of media, suggesting that the egt-2 gene was also efficiently expressed in A. oryzae.
However, significant amounts of HER (32.7 ± 1.1 mg/kg) were accumulated in the culture, suggesting Egt-1 reaction was a bottleneck. Egt-1, the bi-functional enzyme, uses l-His, SAM, and l-Cys as the substrates. Considering that ERG productivity was drastically enhanced by the integration of egt-1 gene, their substrates were suggested to be sufficiently supplied. Indeed, the ERG productivity with A. oryzae were higher than that with E. coli [Citation5]. Therefore, the HER accumulation might be caused by a low Cys-HER synthetic activity of Egt-1.
In conclusion, fermentative ERG production by a fungus was carried out to establish a reliable and practical method for ERG production. By heterologous overexpression of the egt-1 and -2 genes of N. crassa in A. oryzae, we achieved 20-fold higher ERG production (231.0 ± 1.1 mg/kg of media) than the wild type, which is comparable to mushrooms (16 to 417 mg/kg of wet weight) [Citation3]. To date, screening of ERG producers from fungi [Citation19] and production of ERG by engineered fungi [Citation20] have been carried out. Besides these approaches, our system will also be an alternative method for ERG supply.
Author contributions
T.D. conceived and designed the experiments. S.T., Y.S., and I.O. performed the experiments and analyzed the data. Y.S. and T.D. wrote the paper.
Acknowledgments
We are grateful to Professor Jun-ichi Maruyama (The University of Tokyo) and Professor Hideaki Oikawa (Hokkaido University) for providing A. oryzae NSAR1 and vectors (pUARA2, pAdeA2), respectively. We thank Robbie Lewis, MSc, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
BBB_SI_180918.docx
Download MS Word (15.7 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Hartman PE. Ergothioneine as antioxidant. Methods Enzymol. 1990;186:310–318.
- Gründemann D, Harlfinger S, Golz S, et al. Discovery of the ergothioneine transporter. Proc Natl Acad Sci USA. 2005;102:5256–5261.
- Kalaras MD, Richie JP, Calcagnotto A, et al. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017;233:429–433.
- Genghof DS. Biosynthesis of ergothioneine and hercynine by fungi and Actinomycetales. J Bacteriol. 1970;103:475–478.
- Pluskal T, Ueno M, Yanagida M. Genetic and metabolomic dissection of the ergothioneine and selenoneine biosynthetic pathway in the fission yeast, S. pombe, and construction of an overproduction system. PLoS One. 2014;9:e97774.
- Pfeiffer C, Bauer T, Surek B, et al. Cyanobacteria produce high levels of ergothioneine. Food Chem. 2011;129:1766–1769.
- Alamgir KM, Masuda S, Fujitani Y, et al. Production of ergothioneine by Methylobacterium species. Front Microbiol. 2015;6:1185.
- Osawa R, Kamide T, Satoh Y, et al. Heterologous and high production of ergothioneine in Escherichia coli. J Agric Food Chem. 2018;66:1191–1196.
- Bello MH, Barrera-Perez V, Morin D, et al. The Neurospora crassa mutant NcΔEgt-1 identifies an ergothioneine biosynthetic gene and demonstrates that ergothioneine enhances conidial survival and protects against peroxide toxicity during conidial germination. Fungal Genet Biol. 2012;49:160–172.
- Hu W, Song H, Sae Her A, et al. Bioinformatic and biochemical characterizations of C–S bond formation and cleavage enzymes in the fungus Neurospora crassa ergothioneine biosynthetic pathway. Org Lett. 2014;16:5382–5385.
- Barbesgaard P, Heldt-Hansen HP, Diderichsen B. On the safety of Aspergillus oryzae: a review. Appl Microbiol Biotechnol. 1992;36:569–572.
- Wakai S, Arazoe T, Ogino C, et al. Future insights in fungal metabolic engineering. Bioresour Technol. 2017;245:1314–1326.
- Jin FJ, Maruyama J, Juvvadi PR, et al. Development of a novel quadruple auxotrophic host transformation system by argB gene disruption using adeA gene and exploiting adenine auxotrophy in Aspergillus oryzae. FEMS Microbiol Lett. 2004;239:79–85.
- Tagami K, Minami A, Fujii R, et al. Rapid reconstitution of biosynthetic machinery for fungal metabolites in Aspergillus oryzae: total biosynthesis of aflatrem. ChemBioChem. 2014;15:2076–2080.
- Liu C, Tagami K, Minami A, et al. Reconstitution of biosynthetic machinery for the synthesis of the highly elaborated indole diterpene penitrem. Angew Chem Int Ed. 2015;54:5748–5752.
- Gomi K, Iimura Y, Hara S. Integrative transformation of Aspergillus oryzae with a plasmid containing the Aspergillus nidulans argB gene. Agric Biol Chem. 1987;51:2549–2555.
- Ugai T, Minami A, Fujii R, et al. Heterologous expression of highly reducing polyketide synthase involved in betaenone biosynthesis. Chem Commun. 2015;51:1878–1881.
- Lubertozzi D, Keasling JD. Developing Aspergillus as a host for heterologous expression. Biotechnol Adv. 2009;27:53–75.
- Fujitani Y, Alamgir KM, Tani A. Ergothioneine production using Methylobacterium species, yeast, and fungi. J Biosci Bioeng. 2018. DOI:10.1016/j.jbiosc.2018.05.021
- Hara S, Hirokawa K, Ichikawa K. Transformed fungus having enhanced ergothioneine productivity and method for producing ergothioneine. Patent, WO2016121285.