ABSTRACT
Inflammation induced by Helicobacter pylori infection related to gastric carcinogenesis. In this study, we have investigated the anti-inflammatory effect and its mechanism of kaempferol in the inflammatory response caused by H. pylori infection in vitro. We found that kaempferol reduced the expression of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-8) and production of IL-8 in AGS cells. In addition, kaempferol suppressed translocation of cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A (VacA) of H. pylori to AGS cells. It was due to decreased transcription of type IV secretion system (T4SS) components involved in CagA injection and secretion system subunit protein A (SecA) of type V secretion system (T5SS) involved in VacA secretion by kaempferol. In conclusion, kaempferol shows the anti-inflammatory effect by suppressing the translocation of CagA and VacA proteins and leading to the down-regulation of pro-inflammatory cytokines.
Abbreviations: CagA: cytotoxin-associated gene A; VacA: vacuolating cytotoxin A; T4SS: type IV secretion systems; SecA: secretion system subunit protein A; T5SS: type V secretion system;
Graphical Abstract
Kaempferol has an anti-inflammatory effect on H. pylori infection by suppressing the translocation of CagA and VacA proteins.
Helicobacter pylori is a spiral-shaped Gram-negative bacterium, which penetrates the gastric mucus and persists in the stomach [Citation1,Citation2]. More than half of the world’s population are carriers that have H. pylori on their stomach [Citation3]. Continuous infection of H. pylori on stomach induces various kinds of gastrointestinal diseases such as chronic gastritis, gastric ulcer, atrophic gastritis, and leads to gastric cancer in severe cases [Citation4,Citation5]. For this reason, the World Health Organization (WHO) classified H. pylori as a class I carcinogen in 1994 [Citation6].
Development of gastric cancer is initiated by an inflammatory response that is continuously induced by bacterial infection [Citation7]. When H. pylori infection occurs, expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-8 increases in gastric epithelial cells and immune cells. These cytokines are induced by various virulence factors generated by H. pylori [Citation7,Citation8]. Typical virulence factors of H. pylori include cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A (VacA), urease, and high temperature requirement A (HtrA) [Citation9].
Among them, CagA, which is well known to play an important role in inflammation caused by H. pylori infection, is encoded by cag pathogenicity island (PAI) and translocated to host cells via the type IV secretion system [Citation7,Citation9]. Type IV secretion system (T4SS) is usually composed of 12 proteins consisting of VirB1 to VirB11 proteins and VirD4 protein [Citation10,Citation11]. CagA injected into the cells by T4SS activates nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in cells and induces production of IL-8 [Citation7,Citation9]. Furthermore, NF-κB affects gene expression of pro-inflammatory cytokines such as TNF-α, IL-1β as well as IL-8 [Citation12].
Another virulence factor VacA is a toxin secreted from H. pylori via type V secretion system (T5SS), which is a Sec-mediated process. Secretion system subunit protein A (SecA) among Sec proteins is an intracellular ATPase associated with secretion of bacterial protein through bacterial plasma membrane [Citation13,Citation14]. VacA usually acts on the host cell to induce vacuolation and induces apoptosis via mitochondria-dependent pathway [Citation8]. In addition to this effect, it is also involved in gastric mucosal inflammation by activating ERK1/2 signaling pathway and promoting expression of pro-inflammatory cytokines in gastric epithelial cells [Citation15,Citation16].
Kaempferol is one of the flavonoids that is a polyphenolic compound extracted from multiple kinds of edible plants (e.g. broccoli, strawberry, onion, and papaya) and medicinal plants (e.g. Aloe vera, Ginkgo biloba, and Acacia nilotica) [Citation17,Citation18]. It was reported that kaempferol has anti-bacterial effect against bacteria such as Staphylococcus aureus, Escherichia coli, Enterococcus faecalis, Mycobacterium tuberculosis as well as H. pylori [Citation17]. In addition, kaempferol shows an anti-inflammatory effect by inhibiting the activity of NF-κB, signal transducer and activator of transcription 1 (STAT-1), and activator protein-1 (AP-1) and reducing gene expression of TNF-α, IL-1β, and IL-8 in various kinds of cell lines [Citation19–Citation22].
Currently, as a study on H. pylori and kaempferol, the minimum inhibitory concentration (MIC) or minimum bactericidal concentration (MBC) of kaempferol against H. pylori was investigated in vitro and in vivo [Citation23–Citation26]. However, the effect of kaempferol on the inflammatory response induced by H. pylori infection has not been elucidated yet. In this study, therefore, the anti-inflammatory mechanism of kaempferol in the inflammatory response induced by H. pylori infection was investigated in vitro.
Materials and methods
Bacterial and mammalian cell culture
H. pylori reference strain ATCC 49503 strain was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). H. pylori was cultured on Brucella agar plates (Becton-Dickinson, Braintree, MA, USA) containing 10% bovine serum (BRL Life Technologies, Grand Island, NY, USA) under the microaerophilic condition with a humidified atmosphere at 37°C for 3 days. H. pylori were suspended in Mueller Hinton broth (Becton-Dickinson) supplemented with 10% bovine serum and incubated under the humidified atmosphere with 5% CO2 at 37°C for 3 days.
AGS cells, a gastric adenocarcinoma cell line (ATCC CRL-1739), were cultured in DMEM medium (BRL Life Technologies) containing 10% fetal bovine serum (BRL Life Technologies) and streptomycin-penicillin (100 μg/mL and 100 IU/mL) (BRL Life Technologies). AGS cells were incubated in a humidified atmosphere with 5% CO2 at 37°C.
Broth dilution test
H. pylori grown on Brucella agar plates were isolated and suspended in Mueller Hinton broth supplemented with 10% bovine serum. Kaempferol was treated and the bacteria were incubated for 3 days. An absorbance of bacterial broth was measured at 600 nm by spectrophotometry.
WST cell viability assay using EZ-Cytox
AGS cells were treated with kaempferol and then incubated for 24 h. Water soluble tetrazolium salt (WST) assay by using EZ-Cytox cell viability assay kit (Daeil Lab Service, Seoul, Korea) was conducted to determine the cytotoxicity of kaempferol on AGS cells according to manufacturer’s instruction. WST solution was treated to the culture media and incubated for 4h in the CO2 incubator. The optical density of media was measured optical at 450 nm by a spectrophotometer.
RT-PCR (reverse transcription-polymerase chain reaction)
H. pylori grown on Mueller Hinton broth for 3 days and cultured AGS cells were rinsed twice with phosphate-buffered saline (PBS). After washing, total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) as explained in the manufacturer’s instructions. cDNA was synthesized by reverse transcription with 2 μg total RNA, 0.25 μg of random hexamer (Invitrogen) and 200 U of MMLV-RT (Invitrogen) for 50 min at 37°C and 15 min at 70°C. Subsequent PCR amplification was conducted using 0.2 U of Taq polymerase in a thermocycler using specific primers. The PCR primer sequences used in this study are listed in .
Table 1. List of primer sequences and PCR conditions for RT-PCR.
ELISA (enzyme-linked immunosorbent assay)
AGS cells were infected with H. pylori and treated with kaempferol for 9 hours. To determine the production of IL-8, the cell culture supernatants were analyzed by IL-8 human uncoated ELISA kit (Invitrogen) according to manufacturer’s instruction.
Western blotting
H. pylori and AGS cells grown on the medium were rinsed with PBS and then lysed with radioimmunoprecipitation assay (RIPA) buffer containing a protease inhibitor cocktail (Sigma-Aldrich) for 10 min at 4°C. The cell lysates were centrifuged at 14,000 rpm for 10 min at 4°C and then the supernatants were collected. The proteins were quantified using Lowry Protein assay (Bio-rad, Hercules, CA, USA) were separated by SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The membrane was blocked with 5% (W/V) skim milk (BD Biosciences, Franklin Lakes, NJ, USA) for 1 hour. Then, it was incubated with the optimal concentrations of CagA, VacA, and β–actin primary antibody (Santa Cruz Biotechnology, Dallas, TX, USA), respectively overnight at 4°C. Polyclonal antibody against whole H. pylori (ATCC 49503) was produced as previously described [Citation27]. After incubation, the membrane was incubated with a suitable secondary antibody (anti-rabbit or anti-mouse IgG) (Santa Cruz Biotechnology) for 2 h at room temperature. The immune-labeled proteins were visualized using enhanced chemiluminescence (ECL) kit (Thermo Scientific, Waltham, MA, USA). β–actin and polyclonal anti-H.pylori antibody were used as an internal control.
Statistical analysis
The statistical analyses were conducted using GraphPad Prism 5.02 software (GraphPad Software, San Diego, CA, USA). Data in the bar graphs are expressed as a mean ± standard error of the mean (SEM). Data were evaluated by Student’s t-test and P < 0.05 was considered to be statistically significant (*P < 0.05, **P < 0.01 and ***P < 0.001).
Results
Kaempferol inhibits the growth of H. pylori
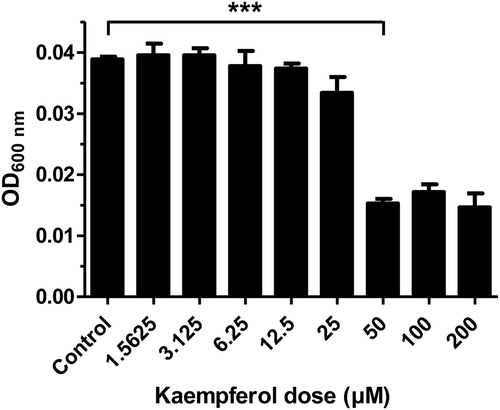
To investigate the minimum inhibitory concentration (MIC) of kaempferol against H. pylori, kaempferol was serially diluted 2-fold in Mueller Hinton broth and H. pylori was cultured in this medium for 3 days. It was determined that the MIC of kaempferol against H. pylori was 50 μM (). Based on this result, it was found that kaempferol suppresses the growth of H. pylori, and the MIC was determined.
Figure 1. Minimum inhibitory concentration (MIC) of kaempferol against Helicobacter pylori.
H. pylori was grown in Muller Hinton broth including 2-fold serially diluted concentrations of kaempferol (1.5625 to 200 μM). After 3 days of incubation, the absorbance of bacterial broth was measured at 600 nm in order to determine MIC of kaempferol. The experiments were conducted in triplicate and the results were evaluated by Student’s t-test (***P < 0.001).
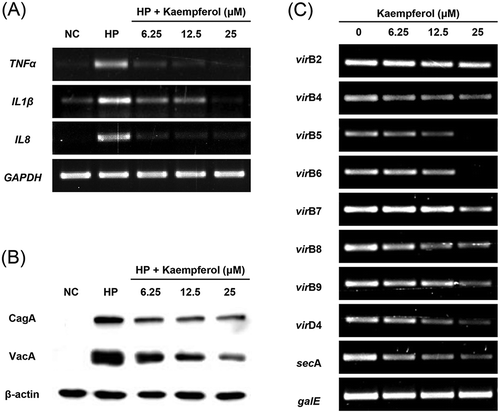
Kaempferol suppresses pro-inflammatory cytokines by H. pylori infection in AGS cells
It was determined whether kaempferol shows cytotoxicity in AGS cells. AGS cells were treated with varying doses of kaempferol and cell viability was measured using WST assay. Two hundred μM of kaempferol showed cytotoxicity to AGS cells while the cell viability of AGS cells was not decreased at 50 μM, the MIC of kaempferol against H. pylori (). However, 50 μM was excluded in the subsequent experiments as it leads to the inhibitory effect of growing on H. pylori. Instead, 6.25 μM, 12.5 μM, and 25 μM, which are the sub-MIC concentration of kaempferol, were used. To investigate the anti-inflammatory effects of kaempferol on AGS cells infected with H. pylori, mRNA levels of TNF-α, IL-1β, and IL-8 was measured by RT-PCR. The mRNA levels of TNF-α, IL-1β, and IL-8 in AGS cells increased upon infection with H. pylori and decreased when treated with kaempferol (). Among pro-inflammatory cytokines, IL-8 plays a key role in H. pylori-induced inflammation by activating neutrophils [Citation28,Citation29]. Therefore, it was examined whether kaempferol influences the production of IL-8 induced by H. pylori infection using ELISA. H. pylori infection increased the protein level of IL-8 in AGS cells as expected, and the increased IL-8 protein was inhibited by kaempferol treatment (). These results collectively suggest that kaempferol shows the anti-inflammatory effect by inhibiting the expressions of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-8) and the production of IL-8 induced by H. pylori infection in gastric epithelial cells.
Figure 2. Cytotoxicity of kaempferol on gastric adenocarcinoma cell line AGS.
AGS cells were treated with 2-fold serially diluted concentrations of kaempferol (1.5625 to 200 μM) for 24 hours. The cell viability of AGS cells was determined by WST assay. The experiments were conducted in triplicate. Error bars indicate a mean and standard error of the mean.
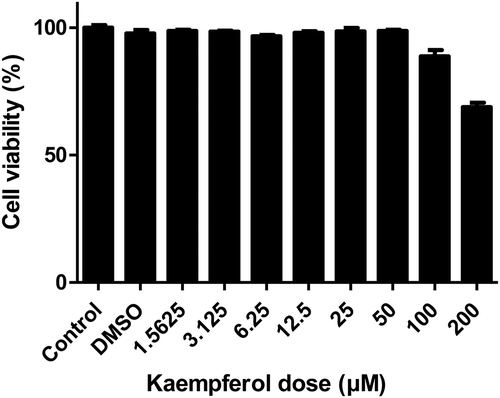
Figure 3. Inhibitory effects of kaempferol on the mRNA expression level of TNF-α, IL-1β, and IL-8 in AGS cells infected with H. pylori.
(a) AGS cells infected with H. pylori (200 MOI) were incubated with increasing concentrations of kaempferol (6.25, 12.5, and 25 μM). After 6 hours, total RNA was extracted and TNF-α, IL-1β, and IL-8 mRNA levels were determined by RT-PCR. GAPDH was used as an internal control. (b-d) Representative histogram of the PCR bands was analyzed by ImageLab software. The experiments were conducted in triplicate and the results were evaluated by Student’s t-test (**P < 0.01 and ***P < 0.001). NC, normal control; HP, H. pylori infection; KF, kaempferol.
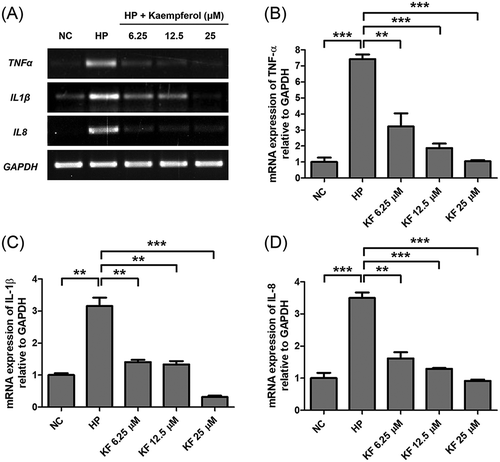
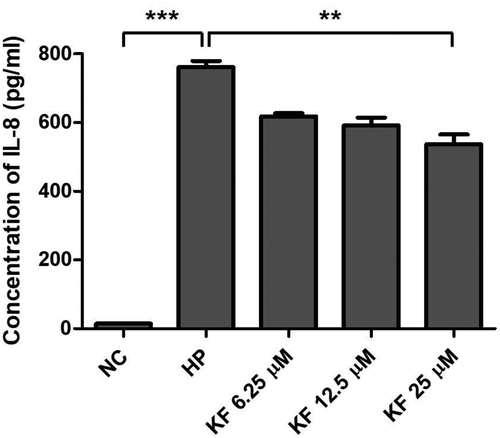
Figure 4. Inhibitory effects of kaempferol on the protein level of IL-8 in AGS cells infected with H. pylori.
AGS cells infected with H. pylori (200 MOI) were incubated with increasing concentrations of kaempferol (6.25, 12.5, and 25 μM). After 9 hours, the cell culture supernatants were harvested and IL-8 production was determined by ELISA. The experiments were conducted in triplicate and the results were evaluated by Student’s t-test (**P < 0.01 and ***P < 0.001).
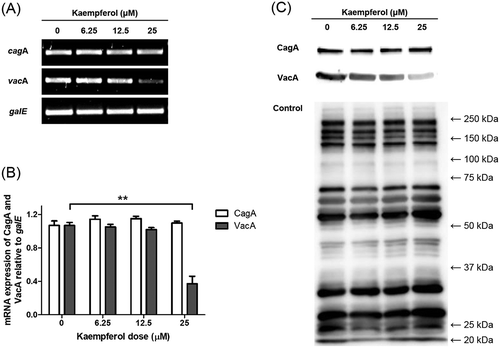
Kaempferol decreases the expression of VacA in H. pylori
Kaempferol was treated to H. pylori and it was examined whether kaempferol directly affects CagA and VacA expressions in H. pylori. The mRNA levels of CagA and VacA in kaempferol-treated H. pylori were observed using RT-PCR. As a result of RT-PCR, it was found that VacA expression was decreased by kaempferol treatment, while CagA expression was unchanged (). The protein levels of CagA and VacA in H. pylori treated with kaempferol were also examined using Western blotting. The result showed that VacA protein was decreased but CagA protein was not decreased by kaempferol treatment in concordance with the result from RT-PCR ()).
Figure 5. Effects of kaempferol on CagA and VacA in H. pylori.
(a) H. pylori was incubated with increasing concentrations of kaempferol (6.25, 12.5, and 25 μM). After 24 hours, total RNA was extracted and mRNA levels of CagA and VacA were determined by RT-PCR. galE was used as an internal control. (b) Representative histogram of the PCR bands were analyzed by ImageLab software. The experiments were conducted in triplicate and the results were evaluated by Student’s t-test (**P < 0.01). (c) H. pylori was treated as in (a). After 24 hours, the cell lysates were analyzed by Western blotting to determine protein levels of CagA (120 kDa) and VacA (58 kDa) in H. pylori. Polyclonal anti-H. pylori antibody was used as an internal control. The experiments were conducted in triplicate.
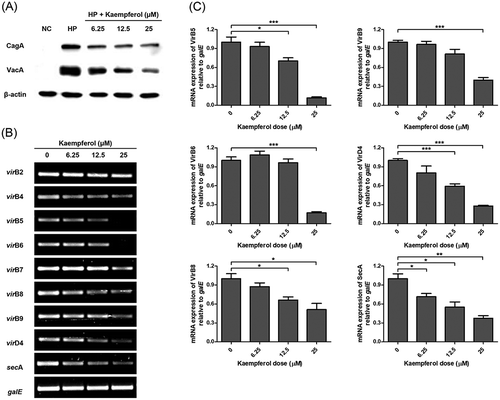
Kaempferol suppresses CagA and VacA translocation to AGS cells
In order to investigate whether kaempferol affects translocation of CagA and VacA, the protein levels of CagA and VacA in the AGS cells was measured using Western blotting. CagA and VacA proteins translocated into the AGS cell infected with H. pylori, and kaempferol treatment decreased the translocated CagA and VacA protein levels ()). The mRNA levels of T4SS components and a regulatory protein SecA of T5SS were investigated using RT-PCR in order to confirm whether kaempferol affects the secretion system of H. pylori. According to the results of RT-PCR, the expression of virB5, virB6, virB8, virB9, virD4, and secA decreased in H. pylori, when kaempferol was treated (). Therefore, it can be suggested that kaempferol decreases mRNA level of T4SS component and suppresses translocation of CagA. In addition, the reduced translocation of VacA was at least in part associated with down-regulation of secA and subsequent inhibition of T5SS.
Figure 6. Inhibitory effects of kaempferol on the translocation of CagA and VacA to AGS cells.
(a) AGS cells infected with H. pylori (200 MOI) were incubated with increasing concentrations of kaempferol (6.25, 12.5, and 25 μM). After 6 hours, the cell lysates were analyzed by Western blotting for expression of CagA (120 kDa) and VacA (58 kDa) proteins. β-actin (45 kDa) was used as an internal control. The experiments were conducted in triplicate. (b) H. pylori was incubated with increasing concentrations of kaempferol (6.25, 12.5, and 25 μM). After 24 hours, total RNA was extracted and mRNA levels of Type IV secretion system components and secA were determined by RT-PCR. galE was used as an internal control. (c) Representative histogram of the PCR bands were analyzed by ImageLab software. The experiments were conducted in triplicate and the results were evaluated by Student’s t-test (*P < 0.05, **P < 0.01 and ***P < 0.001).
Discussion
Inflammation caused by prolonged infection of H. pylori is the first stage of gastrointestinal disease induction. According to some reports, kaempferol has been shown to inhibit the expression of the pro-inflammatory cytokines (TNF-α, IL-1β, and IL-8) in various tissues and cells [Citation19,Citation30–Citation32]. Based on these reports, the anti-inflammatory effect and its mechanism of kaempferol against the inflammation caused by H. pylori infection were confirmed in this study. In the current study, kaempferol treatment decreased the mRNA level of pro-inflammatory cytokines including TNF-α, IL-1β, and IL-8 as well as the production of IL-8 that were increased in AGS cell by H. pylori infection. These results indicated that kaempferol has an inhibitory effect on up-regulation of pro-inflammatory cytokine induced by H. pylori infection. However, the protein levels of TNF-α and IL-1β were hardly measured in H. pylori-infected AGS cells using ELISA. Jung et al reported that the expression time and amount of pro-inflammatory cytokines were different depending on gastric epithelial cell type infected with H. pylori [Citation33]. According to these findings, probably the results of this study can be caused by differences in cell type and experiment conditions.
Chronic inflammation caused by H. pylori infection is induced by increased expression of pro-inflammatory cytokine by CagA protein [Citation34]. It was reported that CagA was essential for H. pylori-induced NF-κB activation. CagA translocated to gastric epithelial cells through T4SS and promoted IL-8 secretion by activating a Ras-Raf-Mek-Erk-NF-κB signaling pathway [Citation34–Citation36]. VacA also promotes expression of pro-inflammatory cytokines by affecting ERK1/2 signaling pathway [Citation15,Citation16]. In this study, kaempferol suppressed translocation of CagA and VacA protein of H. pylori to AGS cell. Kaempferol treatment did not decrease mRNA level and protein level of CagA in H. pylori, but mRNA level and protein level of VacA decreased.
In addition, the expression of H. pylori T4SS components including VirB5, VirB6, VirB8, VirB9, and VirD4 was decreased by kaempferol treatment. NTPases such as VirD4 provide important energy to assemble the secretion system structure and secrete CagA [Citation10,Citation11]. VirB6, VirB8, and VirB9 allow the bacterial protein to pass through the periplasmic space, and VirB5 promotes pilus biogenesis and attachment to epithelial cells [Citation11,Citation37,Citation38]. Based on the results, it can be supposed that decreased expression of T4SS components by kaempferol led to reduced translocation of CagA. Moreover, the expression of SecA, a regulator protein of T5SS, was decreased in H. pylori by kaempferol treatment. Thus, both reduced SecA expression of T5SS and down-regulation of VacA by kaempferol contributed to decreased VacA translocation.
In conclusion, it was found that kaempferol shows the anti-inflammatory effect by inhibiting the translocation of CagA and VacA protein of H. pylori to AGS cells thereby down-regulating the expression of pro-inflammatory cytokines. We suggest that kaempferol is a candidate of a supportive agent to treat inflammation induced by H. pylori infection.
Author Contributions
Min Ji Yeon and Jong-Bae Kim conceived and designed the study. Min Ji Yeon, Min Ho Lee, and Do Hyun Kim performed the experiments and analysed data. Ji Yeong Yang, Hyun Jun Woo, Hye Jin Kwon, Cheol Moon, and Sa-Hyun Kim contributed to the technology supporting. Min Ji Yeon wrote the manuscript and Jong-Bae Kim reviewed the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- O’Rourke J, Bode G. Morphology and Ultrastructure. In: Mobley HLT, Mendz GL, Hazell SL, editors Helicobacter pylori: physiology and genetics. Washington (DC): ASM Press; 2001. PubMed PMID: 21290748.
- Krishnamurthy P, Phadnis SH, DeLoney CR. Biosynthetic pathways related to cell structure and function. In: Mobley HLT, Mendz GL, Hazell SL, et al. editors. Helicobacter pylori: physiology and Genetics. Washington (DC): ASM Press; 2001. PubMed PMID: 21290717.
- Bisignano C, Filocamo A, La Camera E, et al. Antibacterial activities of almond skins on cagA-positive and-negative clinical isolates of Helicobacter pylori. BMC Microbiol. 2013 May;9(13):103. PubMed PMID: 23659287; PubMed Central PMCID: PMCPMC3654990.
- Zabala Torrres B, Lucero Y, Lagomarcino AJ, et al. Review: prevalence and dynamics of Helicobacter pylori infection during childhood. Helicobacter. 2017 Oct;22(5). PubMed PMID: 28643393. DOI:10.1111/hel.12399
- Hitkova I, Yuan G, Anderl F, et al. Caveolin-1 protects B6129 mice against Helicobacter pylori gastritis. PLoS Pathog. 2013;9(4):e1003251. PubMed PMID: 23592983; PubMed Central PMCID: PMCPMC3623771.
- Roesler BM, Costa SC, Zeitune JM. Eradication treatment of Helicobacter pylori infection: its importance and possible relationship in preventing the development of gastric cancer. ISRN Gastroenterol. 2012;2012:935410. PubMed PMID: 22778979; PubMed Central PMCID: PMCPMC3384894.
- Lamb A, Chen LF. Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer. J Cell Biochem. 2013 Mar;114(3):491–497. PubMed PMID: 22961880; PubMed Central PMCID: PMCPMC3909030.
- Wang F, Meng W, Wang B, et al. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014 Apr 10;345(2):196–202. PubMed PMID: 23981572.
- Alzahrani S, Lina TT, Gonzalez J, et al. Effect of Helicobacter pylori on gastric epithelial cells. World J Gastroenterol. 2014 Sep 28;20(36):12767–12780. PubMed PMID: 25278677; PubMed Central PMCID: PMCPMC4177462.
- Terradot L, Waksman G. Architecture of the Helicobacter pylori Cag-type IV secretion system. FEBS J. 2011 Apr;278(8):1213–1222. PubMed PMID: 21352491.
- Christie PJ, Cascales E. Structural and dynamic properties of bacterial type IV secretion systems (review). Mol Membr Biol. 2005 Jan-Apr;22(1–2):51–61. PubMed PMID: 16092524; PubMed Central PMCID: PMCPMC3921681.
- Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001 Jan;107(1):7–11. PubMed PMID: 11134171; PubMed Central PMCID: PMCPMC198552.
- Kim SH, Woo H, Park M, et al. Cyanidin 3-O-glucoside reduces Helicobacter pylori VacA-induced cell death of gastric KATO III cells through inhibition of the SecA pathway. Int J Med Sci. 2014;11(7):742–747. PubMed PMID: 24904230; PubMed Central PMCID: PMCPMC4045794.
- Leyton DL, Rossiter AE, Henderson IR. From self sufficiency to dependence: mechanisms and factors important for autotransporter biogenesis. Nat Rev Microbiol. 2012 Feb 16;10(3):213–225. PubMed PMID: 22337167.
- Fahimi F, Tohidkia MR, Fouladi M, et al. Pleiotropic cytotoxicity of VacA toxin in host cells and its impact on immunotherapy. Bioimpacts. 2017;7(1):59–71. PubMed PMID: 28546954; PubMed Central PMCID: PMCPMC5439391.
- Palframan SL, Kwok T, Gabriel K. Vacuolating cytotoxin A (VacA), a key toxin for Helicobacter pylori pathogenesis. Front Cell Infect Microbiol. 2012;2:92. PubMed PMID: 22919683; PubMed Central PMCID: PMCPMC3417644.
- Calderon-Montano JM, Burgos-Moron E, Perez-Guerrero C, et al. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011 Apr;11(4):298–344. PubMed PMID: 21428901.
- Devi KP, Malar DS, Nabavi SF, et al. Kaempferol and inflammation: from chemistry to medicine. Pharmacol Res. 2015 Sep;99:1–10. PubMed PMID: 25982933.
- Kowalski J, Samojedny A, Paul M, et al. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1beta and tumor necrosis factor-alpha genes in J774.2 macrophages. Pharmacol Rep. 2005 May-Jun;57(3):390–394. PubMed PMID: 15985724.
- Lee S, Kim YJ, Kwon S, et al. Inhibitory effects of flavonoids on TNF-alpha-induced IL-8 gene expression in HEK 293 cells. BMB Rep. 2009 May 31;42(5):265–270. PubMed PMID: 19470239.
- Hamalainen M, Nieminen R, Vuorela P, et al. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007;2007:45673. PubMed PMID: 18274639; PubMed Central PMCID: PMCPMC2220047.
- Chen CC, Chow MP, Huang WC, et al. Flavonoids inhibit tumor necrosis factor-alpha-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-kappaB: structure-activity relationships. Mol Pharmacol. 2004 Sep;66(3):683–693. PubMed PMID: 15322261.
- Kataoka M, Hirata K, Kunikata T, et al. Antibacterial action of tryptanthrin and kaempferol, isolated from the indigo plant (Polygonum tinctorium Lour.), against Helicobacter pylori-infected Mongolian gerbils. J Gastroenterol. 2001 Jan;36(1):5–9. PubMed PMID: 11211212.
- Konstantinopoulou M, Karioti A, Skaltsas S, et al. Sesquiterpene lactones from Anthemis altissima and their anti-Helicobacter pylori activity. J Nat Prod. 2003 May;66(5):699–702. PubMed PMID: 12762812.
- Martini S, D’Addario C, Colacevich A, et al. Antimicrobial activity against Helicobacter pylori strains and antioxidant properties of blackberry leaves (Rubus ulmifolius) and isolated compounds. Int J Antimicrob Agents. 2009 Jul;34(1):50–59. PubMed PMID: 19386474.
- Mafioleti L, Da Silva Junior IF, Colodel EM, et al. Evaluation of the toxicity and antimicrobial activity of hydroethanolic extract of Arrabidaea chica (Humb. & Bonpl.) B. Verl. J Ethnopharmacol. 2013 Nov 25;150(2):576–582. PubMed PMID: 24070833.
- Kim SH, Park M, Woo H, et al. Inhibitory effects of anthocyanins on secretion of Helicobacter pylori CagA and VacA toxins. Int J Med Sci. 2012;9(10):838–842. PubMed PMID: 23155357; PubMed Central PMCID: PMCPMC3498748.
- Crabtree JE, Lindley IJ. Mucosal interleukin-8 and Helicobacter pylori-associated gastroduodenal disease. Eur J Gastroenterol Hepatol. 1994 Dec;6 Suppl 1:S33–S38. PubMed PMID: 7735932.
- Hammond ME, Lapointe GR, Feucht PH, et al. IL-8 induces neutrophil chemotaxis predominantly via type I IL-8 receptors. J Immunol. 1995 Aug 1;155(3):1428–1433. PubMed PMID: 7636208.
- Tang XL, Liu JX, Dong W, et al. Protective effect of kaempferol on LPS plus ATP-induced inflammatory response in cardiac fibroblasts. Inflammation. 2015 Feb;38(1):94–101. PubMed PMID: 25189464.
- Wall C, Lim R, Poljak M, et al. Dietary flavonoids as therapeutics for preterm birth: luteolin and kaempferol suppress inflammation in human gestational tissues in vitro. Oxid Med Cell Longev. 2013;2013:485201. PubMed PMID: 23840918; PubMed Central PMCID: PMCPMC3687483.
- Kim HK, Park HR, Lee JS, et al. Down-regulation of iNOS and TNF-alpha expression by kaempferol via NF-kappaB inactivation in aged rat gingival tissues. Biogerontology. 2007 Aug;8(4):399–408. PubMed PMID: 17278014.
- Jung HC, Kim JM, Song IS, et al. Helicobacter pylori induces an array of pro-inflammatory cytokines in human gastric epithelial cells: quantification of mRNA for interleukin-8, −1 alpha/beta, granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein-1 and tumour necrosis factor-alpha. J Gastroenterol Hepatol. 1997 Jul;12(7):473–480. PubMed PMID: 9257236.
- Lamb A, Yang XD, Tsang YH, et al. Helicobacter pylori CagA activates NF-kappaB by targeting TAK1 for TRAF6-mediated Lys 63 ubiquitination. EMBO Rep. 2009 Nov;10(11):1242–1249. PubMed PMID: 19820695; PubMed Central PMCID: PMCPMC2775174.
- Brandt S, Kwok T, Hartig R, et al. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci U S A. 2005 Jun 28;102(26):9300–9305. PubMed PMID: 15972330; PubMed Central PMCID: PMCPMC1166591.
- Papadakos KS, Sougleri IS, Mentis AF, et al. Presence of terminal EPIYA phosphorylation motifs in Helicobacter pylori CagA contributes to IL-8 secretion, irrespective of the number of repeats. PLoS One. 2013;8(2):e56291. PubMed PMID: 23409168; PubMed Central PMCID: PMCPMC3567036.
- Yeo HJ, Waksman G. Unveiling molecular scaffolds of the type IV secretion system. J Bacteriol. 2004 Apr;186(7):1919–1926. PubMed PMID: 15028675; PubMed Central PMCID: PMCPMC374423.
- Fischer W. Assembly and molecular mode of action of the Helicobacter pylori Cag type IV secretion apparatus. FEBS J. 2011 Apr;278(8):1203–1212. PubMed PMID: 21352490.
- Kwon DH, Osato MS, Graham DY, et al. Quantitative RT-PCR analysis of multiple genes encoding putative metronidazole nitroreductases from Helicobacter pylori. Int J Antimicrob Agents. 2000 Jun;15(1):31–36. PubMed PMID: 10856674.
- Niehues M, Stark T, Keller D, et al. Antiadhesion as a functional concept for prevention of pathogens: N-Phenylpropenoyl-L-amino acid amides as inhibitors of the Helicobacter pylori BabA outer membrane protein. Mol Nutr Food Res. 2011 Jul;55(7):1104–1117. PubMed PMID: 21520488.
- Kim SH, Lee MH, Park M, et al. Regulatory effects of black rice extract on Helicobacter pylori infection-induced apoptosis. Mol Nutr Food Res. 2018 Feb;62(3). PubMed PMID: 29035012. DOI:10.1002/mnfr.201700586
- Amornrit W, Santiyanont R. Effect of amaranthus on advanced glycation end-products induced cytotoxicity and proinflammatory cytokine gene expression in SH-SY5Y cells. Molecules. 2015 Sep 18;20(9):17288–17308. PubMed PMID: 26393562.
- Papadopoulos NG, Papi A, Meyer J, et al. Rhinovirus infection up-regulates eotaxin and eotaxin-2 expression in bronchial epithelial cells. Clin Exp Allergy. 2001 Jul;31(7):1060–1066. PubMed PMID: 11467997.
- Tharmalingam N, Park M, Lee MH, et al. Piperine treatment suppresses Helicobacter pylori toxin entry in to gastric epithelium and minimizes beta-catenin mediated oncogenesis and IL-8 secretion in vitro. Am J Transl Res. 2016;8(2):885–898. PubMed PMID: 27158376; PubMed Central PMCID: PMCPMC4846933.
