ABSTRACT
A variety of extreme environments, characterized by extreme values of various physicochemical parameters (temperature, pressure, salinity, pH, and so on), are found on Earth. Organisms that favorably live in such extreme environments are called extremophiles. All living organisms, including extremophiles, must acquire energy to maintain cellular homeostasis, including extremophiles. For energy conversion in harsh environments, thermodynamically useful reactions and stable biomolecules are essential. In this review, I briefly summarize recent studies of extreme environments and extremophiles living in these environments and describe energy conversion processes in various extremophiles based on my previous research. Furthermore, I discuss the correlation between the biological system of electrotrophy, a third biological energy acquisition system, and the mechanism underlying microbiologically influenced corrosion. These insights into energy conversion in extremophiles may improve our understanding of the “limits of life”.
Abbreviations: PPi: pyrophosphate; PPase: pyrophosphatase; ITC: isothermal titration microcalorimetry; SVNTase: Shewanella violacea 5ʹ-nucleotidase; SANTase: Shewanella amazonensis 5ʹ-nucleotidase
Graphical Abstract
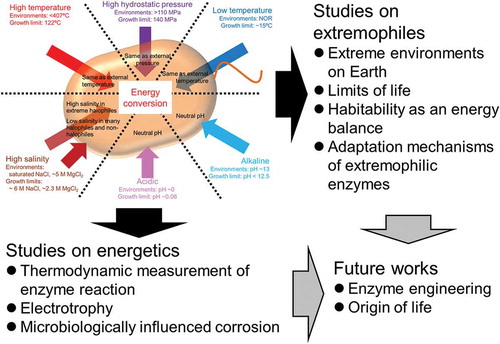
Energy conversions in extremophiles proceed under harsh condition. Understanding the systems may connect to understand “limits of life.”
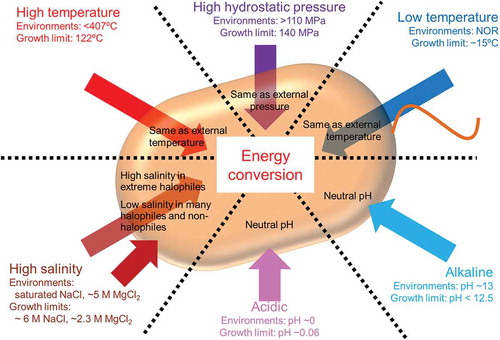
Organisms inhabit a wide range of environments on Earth. Liquid water is an absolute requirement for living organisms. Therefore, organisms can live in almost all environments where liquid water is available, including extreme environments (e.g. high and low temperature, high pressure, high salinity, and acidic or alkaline pH conditions). Such organisms are termed extremophiles. Since extreme environments are characterized by various physicochemical parameters (such as the temperature, hydrostatic pressure, pH, and salinity), extremophiles are exposed to a range of external environmental conditions. Although the stress of chemical parameters, such as pH and salinity, are blocked by cell membranes, the stress of physicochemical parameters, such as temperature and pressure, is transferred to the intracellular region, beyond the cell membrane ().
Figure 1. Influences of extreme physicochemical stress on biological energy conversion.
Each arrow represents each physicochemical parameter. Each value of “Environments” and “Growth limit” represents maximal value observed in each extreme environment where liquid water exists and upper or lower limit value which each extremophile can grow, respectively; NOR represents non official record.
All organisms must acquire energy to maintain cellular homeostasis by means of enzymes related to energy conversion, and enzymes in extremophiles must be adapted to harsh conditions. Such extremophilic enzymes has usefulness in the industrial application, many extremophilic enzymes containing thermophilic and alkaliphilic one have been used in the various fields as well as many readers has used DNA polymerase in PCR experiments. In addition, there are a lot of applications which whole cells (both resting and living cells) and cellular components are used. For a more details, the reader may refer to previous reviews on industrial application [Citation1–Citation4]. My research has focused on energy conversion in extremophilic microorganisms. In this review, I first describe the characteristics of extremophilic environments and microorganisms, with an emphasis on understanding the limitations of life. Next, I focus on energy conversion in extremophiles together with the mechanisms underlying the adaptation of several enzymes to extreme conditions.
Extreme environments and extremophilic microorganisms
Humans live in mild conditions and accordingly are limited to narrow regions on Earth, whereas microorganisms, especially extremophiles, can exist in almost all extreme environments where liquid water is available. Since Homo sapiens expanded their range by cultural development, they live on almost all land areas on the Earth and have even traveled to the moon. In contrast, extremophiles can “live” in the deep sea without getting into a submarine, in polar sites without wearing clothes for cold weather, and in hot springs without feeling dizzy. In this review, extreme environments are described with regard to physical aspects, i.e. temperature and pressure, and chemical aspects, i.e. salinity and pH, considering the maximal recorded conditions and probabilities of the presence of liquid water ().
Table 1. Extreme environments, extremophiles, and adaptation strategies.
High temperatures – There are various types of high-temperature environments, such as mantles, hydrothermal vents, and hot springs. Of these, the most extreme high-temperature environments where liquid water exists are deep sea hydrothermal vents, with a recorded temperature of 407°C (transient 464°C) [Citation5,Citation6]. Although these environments (over 100°C) are also characterized by a high pressure to maintain water in liquid form, I focus on temperature. Many thermophiles live in hydrothermal vents, hot springs, and similar artificial high-temperature environments. Thermophiles can be classified into four categories based on their optimal growth temperature; thermophile, moderate thermophile, extreme thermophile, and hyperthermophile can grow optimally at >55°C, >65°C, >75°C, and >85°C, respectively [Citation7]. The world record of hyperthermophile is the archaeon Methanopyrus kandleri, which can grow at 122°C [Citation8]. Since some viable but non-culturable microorganisms have been detected in hydrothermal vents at 365°C [Citation9], the development of appropriate culture methods are needed to update the maximal values. The limits of living organisms in high-temperature environments would depend on the heat stability and reproduction of biomolecules.
Low temperatures – Polar regions, the deep sea, and alpine regions are low-temperature environments. The lowest temperature recorded at atmospheric pressure is −89.2°C, where water is in the form of ice [Citation10], and the official record for the lowest temperature in liquid water is unknown. Many psychrophiles have been isolated from deep-sea and polar regions at circa- or below-zero degrees Celsius. Low temperatures do not have lethal effects on organisms; almost all organisms can survive for long periods in low temperature conditions. For instance, thermophilic microorganisms are detected in accretion ice in the subglacial Lake Vostok [Citation11]. However, many organisms cannot grow in such low-temperature environments due to reduced enzyme catalysis activity, enzyme stability, membrane fluidity, and availability of liquid water [Citation12]. Among psychrophiles, the bacterium Planococcus halocryophilus holds the record for growth at low temperatures; it can grow at −15°C [Citation13]. The limit of living microorganisms in low temperatures is restricted by the ability to acquire energy and maintain cellular homeostasis.
High pressure – The center of the Earth has an extremely high temperature and pressure. Water penetrates the Earth’s deep crust and this environment has a high hydrostatic pressure, although the precise pressure has not been recorded. Huge numbers of microorganisms are expected to live in the oceans and terrestrial subsurfaces [Citation14]. The deep sea has a high hydrostatic pressure, and the highest hydrostatic pressure recorded is 110 MPa in the Challenger Deep of the Mariana Trench [Citation15]. Kusube et al. isolated the bacterium Colwellia marinimaniae from a deep-sea sample and found that it can grow at 140 MPa, the highest value recorded to date [Citation16]. It is classified into hyperpiezophile, which can grow at >50 MPa, based on the optimal growth pressure. Others are classified as piezophilic or piezotolerant, which can grow at 10–50 MPa and <10 MPa, respectively [Citation17]. Similar to low temperatures, a high hydrostatic pressure acts as a bacteriostatic factor, rather than as a lethal factor. Thus, non-piezophilic microorganisms, such as Escherichia coli, can also survive after experiencing a high hydrostatic pressure of about 100 MPa, despite cell deviation and the formation of an abnormally elongated cell shape [Citation18]. Extremely high pressures of over 300 MPa are lethal [Citation19]. Recently, these effects have been the focus of research in the food industry because sterilization by high hydrostatic pressure does not result in the deterioration of texture, color, flavor, and chemical composition of food products.
High salinity – Next, I focus on chemical parameters. Seventy percentage of the surface of the Earth is covered by sea water, containing approximately 3.1% (w/v) salt. Hyper-saline environments, with saturated NaCl (over 20%), such as salt lakes and saltern soils, are observed on the Earth. Many types of halophiles live in these salty environments, and they sometimes overlay the environment, e.g. some salt lakes are colored red by the cells of halophilic microorganisms. On the basis of the optimal NaCl concentration for cell growth, halophiles are classified as slight (0.2−0.5 M), moderate (0.5−2.0 M), borderline extreme (2.0−3.0 M), and extreme (3.0−4.0) [Citation20]. Many extremely halophilic microorganisms growing under saturated NaCl conditions (approximately 5.5−6.5 M) have been reported [Citation21]. Apparently, an upper limit of high salt concentrations where halophiles can grow is lacking. However, such broad cultivable concentrations may be limited to NaCl.
In addition to NaCl-rich environments, MgCl2-rich environments, e.g. the Discovery basin in the Eastern Mediterranean Sea where the concentration of MgCl2 reaches approximately 5 M [Citation22], also exist. In such MgCl2-rich condition, genes (16S rRNA and dsrAB or mcrA) of sulfate-reducing bacteria (family Desulfobacteraceae and Desulfohalobiaceae) and methanogens (genus Methanohalophilus and phylum Euryarchaeota) were detected by direct PCR experiment. However, there are no reports of extreme halophiles growing in such MgCl2-rich conditions (~ 5 M). The present record for life in MgCl2-rich environments is the archaeon Halorubrum sodomense, which can grow at 2.3 M MgCl2 [Citation23].
Extreme pH – Strongly acidic and alkaline environments are observed in natural and artificial environments. Extremely acidic environments (around zero pH) are observed in acidic hot springs, solfataric fields, and acid mine drainages, which are formed by high concentrations of sulfuric acid [Citation24]. Since many of these extremely acidic environments are also high-temperature environments, thermophilic acidophiles in addition to mesophilic taxa are isolated [Citation4]. Among these, archaea Picrophilus oshimae and Picrophilus torridus grow optimally at pH 0.7, and both show significant growth at a pH around 0. Furthermore, P. torridus can grow at pH −0.06, which is the lower limit for all known organisms [Citation25].
Mildly alkaline waters (pH 8–9) are frequently observed in natural environments, such as alkaline lakes and groundwater discharged from ultramafic rocks. In contrast, strongly alkaline environments (pH >10) are formed by human activities, and the hyper-alkaline Górka pit Lake in Poland leaches at a pH of approximately 13 [Citation26]. Unfortunately, there are no descriptions of living organisms in this site. On the other hand, in similar hyper-alkaline condition (pH >12) the presence of some microorganisms is detected by PCR [Citation27]. Furthermore, in an ultra-deep gold mine with strongly alkaline conditions, the bacterium Alkaliphilus transvaalensis has been isolated and grow optimally at pH 10.0 [Citation28]. It can grow at pH 12.5, which is the higher limit for all known organisms. Alkaliphiles are classified into three categories based on the pH dependency of growth; obligate alkaliphilic (minimum: ≥7.5, optimum: ≥8.5, and maximum: ≥10); facultative alkaliphilic (minimum: <7.5 and optimum: >8.5); and alkalitolerant (minimum: ≥6.0, optimum: <8.5, and maximum: >9) [Citation21]. Although extreme alkaliphiles have been isolated from strongly alkaline environments, many alkaliphiles have also been isolated from neutral and acidic environments [Citation29].
Complex extreme environments – Combinations of extreme physicochemical parameters form severely extreme environments, and these conditions are often lethal, even for many extremophiles. However, the existence of polyextremophiles, which adapt to multiple extreme parameters, such as thermoalkaliphiles, haloalkaliphiles, and thermo-halo-alkaliphiles, has been recently reported [Citation30]. The most of extremophiles that can grow in the highest or lowest values for individual physicochemical parameters, except the alkaliphile A. transvaalensis, are polyextremophiles because such extreme environments are established by complex physicochemical parameters. For instance, a hyperthermophile M. kandleri can grow under high pressure condition (about 20–30 MPa) because high pressure is essential for maintain liquid water under high temperature [Citation8], and an acidophile P. torridus, which is isolated from heat, solfataric soil, has also thermophilic features [Citation25]. Such (poly)extremophiles may represent the edges of the “limits of life,” and the studies on adaptation mechanisms and energy conversion of these taxa would be excellent resources for understanding the limits of life.
The concept of habitability and extremophiles living on the edge
It is likely that the maximal values observed for known extremophiles do not represent the “limits of life” because life has been observed in increasingly severe environments, e.g. the hydrothermal vents, as described above. However, (poly)extremophiles are likely to be near the edge of these limits. In any extreme environment, extremophiles must acquire energy to maintain activity. The minimal energy potential reported for living organisms is −20 kJ/mol per reaction [Citation31]. For increased energy potential, can organisms maintain their activity? The answer is unquestionably no. This can be explained by the concept of habitability.
According to the concept of “habitability as an energy balance”, two factors are important for energy, i.e. energy potential (voltage) and energy load (power), and the biological demand for energy can be plotted in a 2-dimensional graph with power and voltage as the axes [Citation32]. In the plot, habitability can be calculated based on the region between the respective upper and lower limits. The lower limit of voltage is the minimum free energy level for sustaining the “complexity of the system” and is the driving force for biological processes. The upper limit of voltage is the voltage uptake limit for sustaining “complexity,” above which any additional energy potential results in a loss of control. The lower limit of power is the minimal maintenance energy required to sustain “complexity.” The upper limit of power is the maximal energy needed to control “complexity.”
Based on this concept, the lower limits of voltage and power correspond to extreme oligotrophic conditions and ultralow temperatures, in which it difficult to acquire an energy potential of −20 kJ/mol per reaction. In contrast, the upper limits of voltage and power correspond to a hyper-temperature environment. Therefore, the energy metabolism of hyperthermophiles would place them near the edges of the upper limits. Many researchers have reported free energy changes for various reactions, but almost all of these studies have considered the standard state. To understand the limits of energy conversion, it may be necessary to understand free energy changes for various biological reactions under abnormal conditions. Therefore, I focus on energy conversion and the underlying machinery in extremophilic microorganisms.
Energy conversion of halophiles
Generally, most non-halophilic, halotolerant, and halophilic microorganisms prefer a low salt concentration in cells and maintain this condition by blocking and exporting ions. In contrast, many extremely halophilic archaea and a few extremely halophilic bacteria accumulate salt within cells in order to eliminate osmotic pressure via the cell membrane [Citation33]. The intracellular salt concentrations reach saturation levels of KCl or NaCl depending on extracellular salt concentrations [Citation34]. This salt adaptation strategy is referred to as a salt-in strategy. Although it enables escape from osmotic pressure, other issues, such as the influence of ionic strength on reaction progression and the influence of dehydration on enzymes, substrates, and cofactors, are generated.
All living organisms use ATP as the biological energy currency, which is highly hydrated. Hydration energy is one of the origins of the Gibbs free energy change for the hydrolysis of ATP, together with resonance stabilization and electrostatic repulsion [Citation35–Citation38]. For example, the fee energy change for ATP hydrolysis in the gas phase is very small. Similarly, low water activity established by non-physiological concentrations of dimethyl sulfoxide also reduce the free energy change [Citation39]. Therefore, in this section, I introduce biochemical features of enzymes from extreme halophiles and the thermodynamic properties of enzymes involved in the hydrolysis of high-energy phosphate compounds in halophilic microorganisms.
Hydrolysis of high-energy phosphate compounds by extreme halophiles
Intracellular salt concentrations of extremely halophilic microorganisms, especially archaea and a few bacteria, reach saturation levels for KCl or NaCl, as described above. Therefore, almost all enzymes are exposed to such hyper-saline conditions. Almost all non-halophilic and many moderately halophilic enzymes are inactivated in such hyper-saline conditions. However, extremely halophilic enzymes must maintain the correct folding state and catalyze specific reactions. Fortunately, numerous studies have described the effects of salt on enzyme biochemical features and folding states [Citation40–Citation43].
Biochemical features of extremely halophilic enzymes
I studied the extremely halophilic archaeon Haloarcula japonica isolated from saltern soil in Japan [Citation44]; it grows preferentially at 20% NaCl (3.4 M NaCl). Accordingly, many studies have examined its intracellular, halophilic enzymes (e.g. cell division protein FtsZ1 [Citation45]; α-amylase [Citation46]; dihydrofolate reductase [Citation43]; 2-Deoxy-d-ribose-5-phosphate aldolase [Citation47]; and inorganic pyrophosphatase [Citation48]).
To understand the biochemical features of enzymes involved in energy conversion, I investigated the halophilicity of hydrolysis activities for ATP and PPi as high-energy phosphate compounds using cell-free extracts. The H. japonica cell-free extract preferentially hydrolyzed ATP at 2.5 M Na2SO4 (unpublished). Such salt-dependent hydrolysis activity was observed using NaCl and KCl, but maximal specific activities in NaCl and KCl were lower compared with that in Na2SO4. Similar activities were observed in the membrane fraction, most likely due to AOA1 ATP synthase. In contrast, PPi hydrolysis activities in NaCl, KCl, and Na2SO4 were observed in the cell-free extract and soluble fraction [Citation48]. Furthermore, the PPi hydrolysis activity was observed in 0 M NaCl plus 50–200 mM MgCl2 or MgSO4, and this activity is Mg-dependent. Both activities for high concentrations (molar order) of Na+ and relatively low concentrations (sub-molar order) of Mg2+ are catalyzed by a single pyrophosphatase. Extremely halophilic archaea require high concentrations of Mg2+ in addition to NaCl for cell growth, and the intracellular Mg2+ concentration is also higher than those of non-halophilic archaea [Citation34,Citation49]. Such robust activity over a broad range of ionic strengths may have biological significance against intracellular low salinity stress.
Adaptation mechanisms of extremely halophilic enzymes
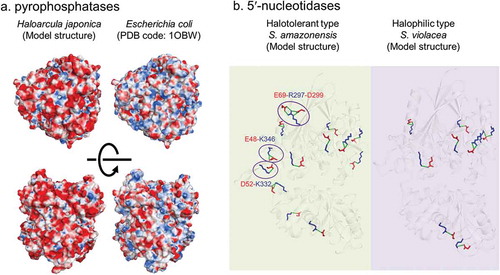
The solubilization of extremely halophilic enzymes depends on the electrostatic neutralization of acidic residues on the protein surface by cations, especially Na+ and K+ [Citation50,Citation51]. The most well-known feature of extremely halophilic enzymes is the increase in acidic residues on the protein surface. This feature is supported by crystal structures and bioinformatics analyses [Citation52]. As described above, high salt-dependent activities in H. japonica cell-free extracts can be attributed to this increase ()). Indeed, pyrophosphatase, which catalyzes PPi hydrolysis, shows the accumulation of acidic residues on the accessible surface area, whereas this accumulation is not observed at the interfaces among subunits in pyrophosphatase with a homohexameric structure [Citation53]. In addition, anions occasionally contribute to the stabilization of oligomeric structures. For instance, the quaternary structure of malate dehydrogenase from the extremely halophilic archaeon Haloarcula marismortui has anion binding sites at the interface between subunits and is stabilized by chloride ions [Citation54]. According to this stabilization with anions, sulfate ions may contribute to Na2SO4-depedent ATPase activity in H. japonica membrane fraction, because AOA1-ATP synthase is a multi-complex enzyme consisting of 10 kinds of subunits.
Figure 2. Examples of adaptation for high salt concentration in halophilic enzymes.
Pyrophosphatase (a): surface charges of pyrophosphatases from extreme halophile H. japonica and non-halophile E. coli. Red and blue color represent negative and positive charges, respectively. 5ʹ-nucleotidase (b): positions of salt bridges in SANTase and SVNTase are shown stick models. Purple circles represent halotolerant specific salt bridges with each residue.
Additionally, halophilic enzymes exhibit a decrease in the numbers of serine residues, which prefer interactions with water, based on a bioinformatics analysis [Citation55]. These enzymes exhibit decreased numbers of hydrophobic residues, which form a relatively small hydrophobic core [Citation42]. A small hydrophobic core prevents excessive rigidity, and as a result, the protein acquires flexibility in high salt concentrations. Furthermore, in some cases, an acidic peptide insertion provides halophilicity to proteins, without generally increasing acidic residues and reducing the hydrophobic core. For example, such insertions have been identified in cysteinyl-tRNA synthase, serinyl-tRNA, and ferredoxin from extremely halophilic archaea [Citation56–Citation58].
Halophilic enzymes are active in the presence of salts at a molar ratio. In such high salt concentrations, salt strengthens hydrophobic interactions and interferes with the electrostatic interactions between charged amino acids [Citation50,Citation51]. Therefore, to avoid these effects of the solvent, halophilic enzymes have many adaptation strategies, which are observed on the accessible surface area of each subunit and the subunit–subunit interfaces.
Thermodynamic measurement of enzymatic reactions by halophilic enzymes
As described above, the minimal energy potential for living organisms and a reduction in the free energy change for the hydrolysis of high-energy phosphate compounds in conditions of low water activity have been reported. However, free energy changes have not been measured under biological high salinity conditions in the cells of extreme halophiles. Therefore, I and coworkers attempted the direct measurement of reaction heat for the hydrolysis of high-energy phosphate compounds under biological salt conditions.
Since the hydration of ATP and PPi is enthalpically driven [Citation59], we estimated the values of enthalpy change for enzymatic hydration under a broad range of salt concentrations by isothermal titration microcalorimetry (ITC) [Citation60]. Measurements of net enthalpy change are extremely complicated; accordingly, I first describe these methods. Many thermal events, such as reaction, dilution, and ionization, are involved in enzymatic reactions that are initiated by the injection of a substrate into an enzyme-containing buffer. Therefore, all thermal events must be measured or estimated [Citation61–Citation63].
The dilution heat (ΔHDilute,Sub) of a substrate can be measured by injecting the substrate into a reaction buffer lacking an enzyme ()). Similarly, the apparent enthalpy change (ΔHWhole,React) for an enzymatic reaction can be measured by injecting the substrate into a reaction buffer with the enzyme ()). Experimentally determined enthalpy changes (ΔHEXP) are calculated by subtracting ΔHWhole,React from ΔHDilute,Sub. Since almost all in vitro enzyme assays use a buffer system to avoid alterations in the pH, the (de)protonation of the buffer base, i.e. ionization, must be considered. Ionization enthalpy changes (ΔHION) are calculated from the apparent enthalpy change (ΔHWhole,Protonation) for buffer protonation and the dilution heat (ΔHDilute,HCl) of acid (). ΔHWhole,Protonation and ΔHDilute,HCl can be measured by injecting HCl solution into a buffer solution and pure water, respectively. Similar to the calculation of ΔHEXP, ΔHION is calculated by subtracting ΔHDilute,HCl from ΔHWhole,Protonation. Furthermore, all measurements described above must be conducted using at least three kinds of buffer salt with the same pH value. Finally, whole catalytic enthalpy changes (ΔHWhole) are estimated from ΔHEXP and the ΔHION ()). Plots of ΔHEXP and ΔHION in each buffer show a linear function described by the following equation:
Figure 3. ITC measurement to estimate the whole catalytic enthalpy change.
Panels a to d represent raw plots in ITC measurements. The heat of each event is calculated as the integrated area of each injection. To estimate the experimentally determined enthalpy change (ΔHEXP), the ΔHDilute,Sub (a) and ΔHWhole,React (b) are measured by injecting PPi solution into reaction solution without and with PPase, respectively. To estimate the ionization enthalpy change (ΔHION), the ΔHDilution,HCl (c) and ΔHWhole,Protonation (d) are measured by injecting HCl solution into pure water (or salt solution) and buffer solution, respectively. (e) Estimation of the ΔHWhole. The correlation between the resulting ΔHEXP and ΔHION values in different buffer system are plotted.
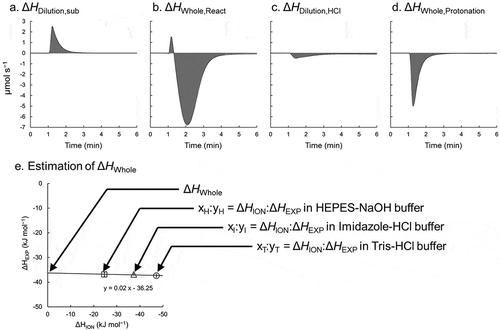
ΔHEXP = n ΔHION + ΔHWhole
where n represents the slope of the regression line for the experimentally determined number of protons released during the enzymatic reaction. Linear regression gives the whole catalytic enthalpy change (ΔHWhole) by the extrapolation of the line to zero heat of the buffer. For comparisons among salt concentrations, all measurement must be obtained under each concentration.
We attempted to estimate the enthalpy change for ATP and PPi hydrolysis using H. japonica cell-free extracts. Unfortunately, enthalpy changes for ATP under high salt conditions with Na2SO4 could not be measured because salt deposits were generated in the injection syringe of the ITC apparatus. However, we successfully measured the reaction heat for PPi hydrolysis under 1.5 to 4.0 M NaCl. Furthermore, we measured the reaction heat for PPi hydrolysis under 0.1 to 2.0 M NaCl using non-halophilic PPase from Escherichia coli and Saccharomyces cerevisiae. As a result, the changes in enthalpy for PPi hydrolysis were constant in the range of 0.1 to 4.0 M NaCl. This result is unexpected because water activity values at 4.0 M NaCl are reduced by 0.84. This finding indicates that high salinity conditions in the cells of extreme halophiles do not influence the values for enthalpy change for PPi, a high-energy phosphate compound.
In addition, we attempted to estimate enthalpy changes under high magnesium conditions because the H. japonica PPase can catalyze PPi hydrolysis at 50–200 mM Mg2+ concentrations without NaCl. In contrast to a high salt concentration with NaCl, the enthalpy changes for 200 mM MgCl2 are clearly lower than those for a high concentration of NaCl with 10 mM MgCl2 (unpublished data). This result suggests that Mg2+ strongly influences the enthalpy change for PPi hydrolysis. This finding may provide insight into the apparent limits of life in Mg2+-rich environments.
ATP hydrolysis by halophilic and halotolerant microorganisms
Although areas with extremely high salt conditions are limited on Earth, sea water with a salinity of approximately 3.1% occupy approximately 70% of the surface area of the Earth. Slight and moderate halophiles and halotolerant organisms live in these sea water environments. Extracellular enzymes from these organisms also adapt to salt environments and exhibit halophilicity and halotolerance. Shewanella spp. live in various salt environments, such as sea water, brackish water, fresh water, and soil [Citation64], and their enzymes also show versatile halophilicity, including halotolerant, moderately halophilic, and strictly halophilic enzymes.
I and co-workers found the difference in halotolerance for ATP hydrolysis activities of deep-sea halophilic Shewanella violacea [Citation65] and brackish water halotolerant Shewanella amazonensis [Citation66] as part of biochemical studies using various Shewanella spp [Citation67]. The ATP hydrolysis activities were due to 5′-nucleotidase activity, and both nucleotidases (SVNTase and SANTase) were purified from membrane fractions of S. violacea and S. amazonensis, respectively. ATP hydrolysis activity of SVNTase showed over 90% relative activity at 2.5 M NaCl compared to the activity at 0 M NaCl, and it is more highly tolerant than SANTase, which exhibits only ~ 30% relative activity at 2.5 M NaCl. The predicted structure modeled from each estimated amino acid sequence is highly conserved at the level of the main carbon chain, but the acidic/basic amino acid ratio and the numbers of salt bridges vary. Therefore, we focus on the differences in NaCl tolerance and amino acid composition, especially in the number of salt bridges.
Ten model structures of 5′-nucleotidase from Shewanella spp. for which genome sequences are available can be separated into two groups as follows: a group with a relatively high acidic/basic ratio and few salt brides and a group with a relatively low acidic/basic ratio and many salt brides. Furthermore, the Shewanella group with the former type of 5′-nucleotidase is halophilic, and cannot grow on 0 M NaCl, and the Shewanella group with the latter 5′-nucleotidase is halotolerant, and can grow on 0 M NaCl and in sea water conditions. As strong ionic strength breaks interactions, such as salt bridges, the lower number of salt brides in the 5′-nucleotidase of the halophilic Shewanella group is reasonable. In addition, the positions of three salt bridges in the N-terminal domain are conserved in only halotolerant group ()). Since N-terminal domain in 5′-nucleotidase can catalyze without C-terminal domain, conformational stability in the N-terminus is significantly important to catalytic reaction. These conserved salt bridges are formed between far residues from each other on primary structure. Therefore, breaking down of these salt bridges may result in inactivation under the high salt condition.
Since Shewanella spp. do not use the salt-in strategy, the intracellular energy conversion system is not influenced by extracellular salt. However, some Shewanella spp. possess an extracellular electron transfer system as an energy conversion system [Citation68]. Proteins contributing to such an extracellular system would adapt to external physicochemical stress as well as halophilic 5′-nucleotidase.
Energy conversion-related proteins in thermophiles
Energy conversion in biological processes is based on chemical reactions, and almost all chemical reactions in cells are catalyzed by enzymes. According to the Arrhenius equation, chemical reaction rates increase with elevating temperatures until substrates and products become unstable. Enzymatic catalysis in biological reactions is unstable at relatively low temperatures compared with the stability of chemical reactions without enzymes; this can be explained by the thermal instability of enzymes. Additionally, the Gibbs free energy change for catalytic reactions is larger under high temperatures than under low temperatures [Citation69] because the activation energy barrier is reduced by the entropy gain of the – TΔS term associated with a temperature elevation. Therefore, reactions by thermophilic enzymes are faster than those of mesophilic or psychrophilic enzymes under optimal conditions because they are thermally stable.
Energy conversion in thermophile has been well studied in extremophiles. For instance, free energy changes for various combinations of electron donors and acceptors have been calculated exhaustively [Citation70]. However, metabolic reactions related to energy conversion in thermophiles are highly versatile, as evidenced by the ability of various thermophiles to use both organic and inorganic materials as substrates for growth. Therefore, in this section I introduce the ATP hydrolysis of a thermophile and mechanisms for adaptation to heat based on our previous studies.
ATP hydrolysis in a thermophile
To study energy conversion in thermophiles, we previously focused on ATP synthase and the thermophilic, facultatively chemolithoautotrophic, hydrogen-oxidizing bacterium Hydrogenophilus thermoluteolus, which can grow heterotrophically on organic acids and autotrophically on hydrogen and carbon dioxide [Citation71]. Partially purified F-type ATP synthase from the membrane fraction of H. thermoluteolus showed thermally stable ATP hydrolysis activity with an optimal temperature at 65°C, similar to those of other thermophiles [Citation72]. Heterologous expression of H. thermoluteolus ATP synthase in mesophilic E. coli resulted in thermophilic ATP hydrolysis activity in the membrane fraction of the transformant. This result indicates that thermophilic ATP synthase is functionally assembled at 37°C in the cells of mesophiles. However, H. thermoluteolus ATP synthase could not complement the growth of authentic ATP synthase-defective E. coli under mesophilic conditions. The uncoupling of ATP synthesis suggests that thermophilic enzymes exhibit insufficient catalytic ability under mesophilic temperatures. In particular, ATP synthase comprises 8 kinds of subunits, the proton-translocating Fo and catalytic F1 parts, and the coupling of both parts is essential for ATP synthesis.
ATP synthase is a key enzyme in the energy conversion of H. thermoluteolus because this bacterium can grow heterotrophically and autotrophically via oxidative phosphorylation with various substrates. The inverted membrane vesicles derived from H. thermoluteolus cells autotrophically cultivated with H2 are able to generate a proton gradient with lactate and succinate as substrates for heterotrophic growth [Citation72]. The heterotrophic energy conversion pathway is maintained even when cells are growing with an autotrophic energy conversion pathway, and both energy conversion systems are consolidated by ATP synthase. Such a flexible energy conversion system may allow the microorganism to live in various environments. The environmental origin of molecular hydrogen as an autotrophic substrate is geochemically limited, whereas organic acids as heterotrophic substrates exist globally. Furthermore, a flexible biological system for energy acquisition would explain the wide distribution of thermophilic, facultative chemoautotrophs (i.e. Hydrogenophilus spp.) in versatile geochemical niches [Citation11].
Mechanisms underlying the adaptation of extremophilic enzymes against temperature and pressure
Thermophilic enzymes must have thermal stability to avoid the denaturation (unfolding) of proteins under high temperatures. Some hyper thermophiles are not only thermophiles, but also piezophiles because high pressure requires the maintenance of the liquid state of water over 100°C. Therefore, in this section, I summarize mechanisms by which thermophilic proteins adapt to temperature and pressure together, in addition to psychrophilic and piezophilic proteins.
Thermophilic proteins adapt to high temperatures by oligomerization and alterations of amino acid residues. Acetyl-CoA synthetase from the hyperthermophilic archaeon Ignicoccus hospitalis forms octamers, whereas such enzymes from mesophiles are monomers or dimers [Citation73–Citation75]. Such oligomerization increases the rigidity of the individual subunits, promotes tighter packing of the hydrophobic core, and reduces the exposure of hydrophobic residues to a solvent [Citation76]. As a similar oligomerization strategy is observed in piezophilic proteins, it is not clear whether it is an adaptation against heat or pressure. However, the formation of hydrophobic interactions at subunit–subunit interfaces is a significant strategy for thermophilic proteins. For instance, the thermal stabilities of dimer-forming cytochrome cʹ, which is classified into Ambler’s Class II, proteins clearly depend on interactions between subunits, and a strong subunit–subunit interaction in thermophilic cytochrome cʹ provides a higher thermal stability than that of mesophilic cytochrome cʹ [Citation77,Citation78].
Alterations in amino acid residues correspond to a decreased surface area ratio, increased disulfide bonds [Citation79,Citation80], salt-bridges, and surface charges [Citation81], and rigidification of hydrophobic core packing. Disulfide bonds in thermophilic enzymes function in structural stabilization, though those in mesophilic proteins act as destabilizers [Citation82]. A decrease in disulfide bonds in single or double mutants of cysteine residues in 5′-deoxy-5′-methylthioadenosine phosphorylase II from the hyperthermophile Sulfolobus solfataricus yields decreased thermal stabilities compared with that of the wild-type [Citation79,Citation80].
Some alterations of some amino acid residues alter the secondary structure of the polypeptide. Cytochrome c, which is classified into Ambler’s Class I [Citation83], plays a role as an electron carrier in energy conversion; it contains a heme in a hydrophobic core, and the hydrophobic interaction between apoprotein and heme plays a key role in the structural organization [Citation84]. Cytochrome c555 from the hyperthermophile Aquifex aeolicus has hydrophobic amino acid residues around the heme and an additional α-helix, and it shows an extremely high thermal stability [Citation85]. The importance of the hydrophobic core in monomer proteins has been demonstrated by experiments using homologous proteins from other thermophiles, mesophiles, and psychrophiles and systematic denaturation experiments using different lengths of alkyl urea [Citation86].
Piezophilic cytochrome c exhibits a different adaptation strategy. In seawater environments, Shewanella spp. are distribute not only in shallow sea (normal hydrostatic pressure, ~ 0.1 MPa) but also in deep-sea areas (with higher hydrostatic pressure), and they are separated into piezosensitive and piezophilic (or piezotolerant) types, respectively [Citation87]. The thermal stabilities of cytochrome c5 proteins, which is classified into Ambler’s Class I and play a role of electron carrier protein in respiratory chain, from deep-sea S. violacea and S. benthica are higher than those from piezosensitive psychrophile S. livingstonensis as well as piezosensitive mesophile S. amazonensis [Citation88]. A crystal structure analysis of S. violacea cytochrome c5 showed an additional hydrogen bond between the peptide chain and heme, and reciprocal mutagenesis experiments using S. violacea and S. livingstonensis cytochrome c5 demonstrated the contribution of this hydrogen bond to a high thermal stability [Citation89]. A similar adaptation is observed in cytochrome c551, which is also classified into Ambler’s Class I and may play a role of electron carrier protein in respiratory chain, from deep-sea Pseudomonas sp. strain MT-1. Although the position of additional hydrogen bonds in cytochrome c551 of Pseudomonas sp. strain MT-1 differ from those in cytochrome c5 of S. violacea and S. benthica, it has a higher stability than that of cytochrome c from Pseudomonas aeruginosa strain PAO1 [Citation90]. As described above, it is difficult to discriminate between a piezophilic adaptation mechanism and a thermophilic one because many piezophiles are also thermophiles. However, systematic comparative studies using thermophilic, mesophilic, piezophilic, and psychrophilic proteins would clarify individual adaptation mechanisms.
Energy conversion in acidophiles
Acidic conditions naturally generate a proton gradient across cell membranes because the extracellular and intracellular pH are acidic and neutral, respectively. Indeed, inverted membrane vesicles prepared from the membrane fraction of acidophiles synthesize ATP from ADP and Pi by a pH jump [Citation91]. However, when microbial cells are placed under acidic conditions, a large number of protons will be translocated intracellularly via this ATP synthase, and the intracellular pH will become acidic. Such acidification of the cytosol and distortion of the ATP/ADP balance would influence the maintenance of cellular homeostasis.
This ATP synthesis is catalyzed by membrane F-type ATP synthase (ATPase). The acidophilic iron- and sulfur-oxidizing bacterium Acidithiobacillus ferrooxidans has the F-type ATPase gene. ATP hydrolysis activity of this ATPase indicates neutralophilic activity, corresponding to the organization of the catalytic part (F1-part) on the cytoplasmic side of the cell membrane. Interestingly, this ATP hydrolysis activity increased with sulfite, which is an intermediate in sulfur oxidation [Citation92]. However, the F-type ATP synthase from acidophiles cannot function as ATP synthase in a neutralophilic Escherichia coli, which is authentic ATP synthase deficient strain. Therefore, the regulatory mechanism underlying ATP synthesis/hydrolysis by the F-type ATPase remains unclear.
Catabolic sulfur-oxidizing pathway in acidophiles
I and co-workers have evaluated the catabolic sulfur-oxidizing pathway, which produces sulfate, thereby resulting in the acidification of the environment. Oxidation pathways of reduced sulfur compounds are highly versatile, and a universal mechanism does not exist. Neutralophilic sulfur-oxidizing bacteria prefer a sulfur-oxidizing (SOX) system in the periplasm, and this system does not adapt to acidic conditions. Acidophilic sulfur-oxidizing bacteria prefer a tetrathionate-forming pathway. However, little is known about the sulfur oxidation system of the acidophilic iron- and sulfur-oxidizing bacterium Acidithiobacillus ferrooxidans, which is a model microorganism for studies of the bioleaching of useful metals from ores.
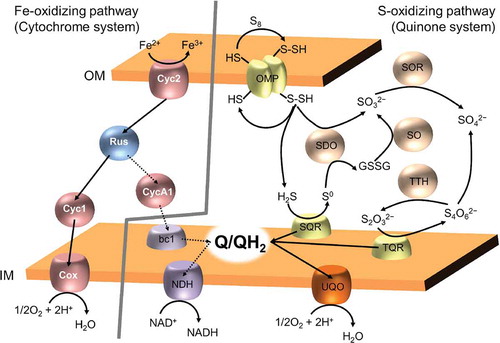
We proposed a novel sulfur-oxidizing system that uses ubiquinone and sulfide: quinone oxidoreductase as an electron carrier and key enzyme, respectively, based on biochemical experiments using various types of respiration inhibitors and molecular genetic experiments [Citation93,Citation94]. This proposed pathway is accepted as part of the oxidation pathway for reduced sulfur compounds together with thiol-bearing outer-membrane proteins, sulfur dioxygenase, sulfite-oxidoreductase, and thiosulfate oxidase, rhodanase, and tetrathionate hydrolase [Citation95,Citation96]. Additionally, iron oxidation by A. ferrooxidans involves the electron transfer chain, which consists of two cytochrome types, rusticyanin, and cytochrome c oxidase. Therefore, iron- and sulfur-oxidizing A. ferrooxidans has at least two types of electron transfer systems and uses them depending on electron donors as follows. When the electron donor is ferrous ion, the electron transport chain with cytochrome-rusticyanin is used, and when electron donors are reduced inorganic sulfur compounds, the electron transport chain with ubiquinone is used (). Accordingly, A. ferrooxidans has various applications in the fields of bioremediation and biochemical production in addition to conventional bioleaching [Citation95].
Figure 4. Fe- and S-oxidizing pathways in acidophilic iron- and sulfur oxidizing bacterium A. ferrooxidans.
Fe-oxidizing pathway in A. ferrooxidans consists of two cytochromes (Cyc2 and Cyc1), rusticyanin (Rus), and aa3-type chtochrome c oxidase. This pathway connect iron-oxidation on outer membrane (OM) to terminal oxidase on inner membrane (IM). From this electron transfer chain, a part of electrons flow into uphill electron pathway, which includes in one cytochrome (CycA1), cytochrome bc1 complex, quinone pool, and NADH dehydrogenase (NDH). This uphill electron pathway is for reduction of NAD+ to NADH, and dashed arrows represent this. In contrast, S-oxidizing pathway use quinone system, which various enzymes (OMP: thiol-bearing outer membrane protein, SDO: sulfur dioxygenase, SOR: sulfur oxygenase reductate, SO: sulfur oxygenase, and TTH: tetrathionate hydrolase) for oxidative conversions of the reduced sulfur compounds, substrate:quinone oxidoreductases (SQR: sulfide:quinone oxidoreductase and TQR: thiosulfate:quinone oxidoreductase), quinone pool, and (bd-type) ubiquinol oxidase.
Adaptation of enzymes against pH
Intracellular pH in acidophiles is neutral [Citation4], and intracellular enzymes do not adapt to acidic conditions. Therefore, little is known about the mechanisms of adaptation for acidophilic enzymes compared with those for thermophilic, piezophilic, and psychrophilic enzymes. Similarly, mechanisms of adaptation against alkaline pH are also unclear, for the same reasons. However, extracellular enzymes, including both acidophilic and alkaliphilic enzymes, must adapt to acidic and alkaline environments owing to direct exposure to such solvents. Therefore, extensive studies have evaluated enzyme function on extracellular substrates, long chain compounds, such as starch, cellulose, and protein, and insoluble compounds [Citation3,Citation4]. For instance, many cellulases, endoglucanases, and exoglucanases are acidic enzymes with optimal activities under acidic conditions. Biochemical insights related to these enzymes are focused on industrial applications, whereas little is known about their structural and biophysical properties.
Exposure of negative charge residues on the enzyme surface is strategy to adapt to acidic conditions [Citation97,Citation98]. A negative surface charge contributes to the prevention of insolubilization by the aggregation and neutralizing excess amounts of proton. In addition, Michaux et al. reported fewer salt bridges and hydrogen bonds compared with those of alkaliphilic homologous enzymes [Citation97]. Uniquely, it has been reported that tetrathionate hydrolase of A. ferrooxidans is folded depending on exposure to acidic conditions [Citation99]. Although folding mechanism has not yet been clarified, protein chemistry of such enzyme would help to understand acidophilic enzymes.
Electrotrophy – a third biological energy acquisition system
Recently, the acidophilic iron- and sulfur-oxidizing bacterium A. ferrooxidans has been evaluated as an electron eater in the field of electromicrobiology. A. ferrooxidans can be cultivated with ferrous ion and reduced sulfur compounds, though it can grow using only electrons from electrodes, without organic and inorganic substances [Citation100]. An absence of useful energy compounds in the closed system is another extreme environment, according to the concept of habitability. Such an energy acquisition system based on the direct electron uptake from a solid electron source is classified as electrolithotrophy. Phototrophic and chemotrophic systems are biological energy conversion systems from light and chemical energy, respectively. Therefore, the electrotrophic system was proposed as a third energy acquisition system, although a common definition of electrotrophy as a third system has not been adopted worldwide. Irrespective, the electrotrophic system is a focus of research in electromicrobiology, particularly in studies of microbial fuel cells and the microbiologically influenced corrosion of metal materials.
Nakamura et al. reported the electric current flow across a chimney in a deep-sea hydrothermal vent [Citation101]. In addition to the established microbial ecosystem in deep-sea hydrothermal vents maintained by chemolithoautotrophs, the concept of a novel microbial ecosystem consisting of electrolithoautotrophs and an electric current flow as a primary energy source has been proposed ()). To use electrons in the solid-state substrate, an extracellular electron transfer apparatus that enables to flow to cellular membrane components from the extracellular substrate is essential. For instance, A. ferrooxidans has outer membrane cytochrome (Cyc2), periplasmic blue copper protein (rusticyanin), periplasmic cytochrome (Cyc1), and membrane embedded cytochrome c oxidase (). In addition, Deng et al. has recently described extracellular electron transfer systems that consist of periplasmic cytochromes, outer membrane multi heme cytochromes, or a nanowire structure, and transmembrane complexes in the metallic iron-corroding sulfate reducing bacterium Desulfovibrio ferrophilus IS5 [Citation102].
Figure 5. Deep-sea electrotrophic ecology and electrochemical reactions in microbiologically influenced corrosion.
In deep-sea hydrothermal vent, electrochemical cell is formed by high-temperature fluids with reduced compounds such as hydrogen sulfide, electroconductive deposits involving in chalcopyrite (CuFeS2) and pyrite (FeS2), and surround low-temperature sea-water (a). The potential gradient across the electroconductive deposits serves as a driving force for electrical current generation. Generated electrons would be used by various chemical or biological reactions. Similarly, in the corrosion of metal materials, utilization of cathodic electron flow by microorganisms has been focused. Under anaerobic condition, corrosion rate of metallic iron is very slow (b). In contrast, energy acquisition system of some microorganisms couples to cathodic reaction (c-d). In old model of microbiologically influenced corrosion, it is expected that hydrogen consuming sulfate reducing bacterium may accelerate the corrosion by consuming molecular hydrogen generated on the cathode (c). Recently, iron-corrosive sulfate-reducing bacterium (d), methanogen (e), and nitrate-reducing bacterium (f) have been isolated as true corrosive agents. These microorganisms probably possess electron up-take system from insoluble electron donors in addition to electron transfer from soluble electron donors.
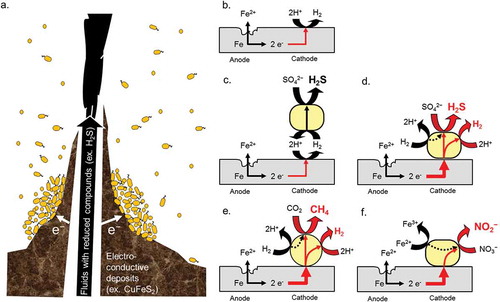
Here, I focus specifically on the mechanism underlying the corrosion of metallic iron by the iron-corrosive methane producing archaea Methanococcus maripaludis strains KA1 and Mic1c10 [Citation103,Citation104]. Under anaerobic condition, metallic iron slowly corrodes and generates trace amounts of molecular hydrogen ()). In contrast, some microorganisms accelerate dissolution of ferrous/ferric ions from metallic iron. Such accelerated corrosion is referred to microbiologically influenced corrosion. Iron-corrosive sulfate reducing bacterium and iron-corrosive methanogens are known as causative agents (). The iron-corrosive methanogens can grow on metallic iron as a sole electron donor and carbon dioxide as a carbon source and can produce methane using electrons from metallic iron during corrosion ()). Since the type culture strain JJT of M. maripaludis does not corrode metallic iron, the iron-corrosive ability is a unique feature in M. maripaludis strains. In contrast to the iron-corrosive sulfate-reducing bacterium D. ferrophilus, genus Methanococcus do not have cytochrome c as electron carrier proteins. Therefore, the extracellular electron transfer system of these iron-corrosive methanogens would be clearly different from those of D. ferrophilus IS5. Based on a comparative genome analysis, a unique iron-corrosive gene island has been found in the genome sequence of iron-corrosive methanogens (unpublished). In the near future, we will examine the function of the gene products. Furthermore, co-workers have isolated the iron-corrosive nitrate-reducing bacterium Prolixibacter denitrificans strain MIC1-1 from crude oil stored in an oil tank [Citation105]. This strain is also expected to possess a direct electron uptake system because it cannot utilize molecular hydrogen, and trace amounts of molecular hydrogen are generated by dissolving ferrous ion from metallic iron during abiotic, chemical corrosion ()). To date, an extracellular electron transfer system in the phylum Bacteroidetes, which includes Prolixibacter denitrificans [Citation106], has not been reported. Therefore, the taxon is a good representative for studies of the distribution and diversity of extracellular electron transfer systems.
Although causative microorganisms for microbiologically influenced corrosion have long been unknown, electromicrobiology research and the development of isolation methods for novel iron-corrosive microorganisms have contributed to substantial advances in this area. I have proposed that the microbiologically influenced corrosion of metal materials is similar to infectious diseases by microorganisms [Citation107]. However, preventive care, diagnosis, and treatment strategies have not yet been developed for this microbial corrosion, unlike for infectious diseases in humans. This cannot simply be attributed to a lack of knowledge about the causative microorganisms and mechanisms. Recent novel insights in electromicrobiology, electrotrophy, and extracellular electron transfer, and information about microbiologically influenced corrosion improve our understanding of the underlying mechanisms and may contribute to the development of diagnostic and preventive technologies.
Conclusion
In this review, I briefly summarize current knowledge of extreme environments and extremophilic microorganisms as well as energy conversion mechanisms in some extremophiles based on my research. More detailed descriptions of the adaptation strategies of extremophilic enzymes have been described in the literature [Citation40,Citation55,Citation108–Citation112]. Although our understanding of the adaptation strategies of extremophilic enzymes has continually progressed by mutagenesis experiments and structural biology studies, the correlation between the functional mechanisms of such protein machineries and their structure has not been determined. It is essential to accumulate information about the relationships among protein structure, the substrate binding state, and solution interactions during catalytic reactions; this would enable the de novo design of artificial enzymes. In addition to understanding the molecular mechanisms, explorations of the “limits of life” and energy conversion in extremophiles would contribute to studies of “the origin of life” and, more broadly, to the definition of living organisms.
Acknowledgments
These studies were carried out at The Hiroshima University, Okayama University, and National Institute of Technology and Evaluation (NITE). I am most grateful to Prof. Yoshihiro Sambongi (Hiroshima University), Prof. Kazuo Kamimura (Okayama University), Prof. Shigeaki Harayama (Chuo University) for their great support, critical advice, and helpful discussions. I am also grateful to Prof. Tsuyoshi Sugio (Okayama University), Prof. Tadayoshi Kanao (Okayama University), Prof. Shunn-ichi Kidokoro (Nagaoka University of Technology), Prof. Kaoru Nakasone (Kinki University), Prof. Makoto Suzuki (Tohoku University), Dr. Kimio Ito (Nippon Steel and Sumitomo Metal Corporation) for their support and helpful discussion. I thank all the co-workers for their cooperation. Finally, I would like to thank the Japan Society for Bioscience, Biotechnology and Agrochemistry for the JSBBA Awards for the Encouragement of Young Scientists.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Demirjian DC, Morís-Varas F, Cassidy CS. Enzymes from extremophiles. Curr Opin Chem Biol. 2001;5:144–151.
- Morozkina EV, Slutskaya ES, Fedorova TV, et al. Extremophilic microorganisms: biochemical adaptation and biotechnological application. Appl Biochem Microbiol. 2010;46:1–14.
- Sarethy IP, Saxena Y, Kapoor A, et al. Alkaliphilic bacteria: applications in industrial biotechnology. J Ind Microbiol Biotechnol. 2011;38:769–790.
- Sharma A, Kawarabayasi Y, Satyanarayana T. Acidophilic bacteria and archaea: acid stable biocatalysts and their potential applications. Extremophiles. 2012;16:1–19.
- Bischoff JL, Rosenbauer JR. Liquid-vapor relations in the critical region of the system NaCl-H2O from 380 to 415ºC: A refined determination of the critical point and two-phase boundary of seawater. Geochim Cosmochim Acta. 1988;52:2121–2126.
- Koschinsky A, Garbe-Schönberg D, Sander S, et al. Hydrothermal venting at pressure-temperature conditions above the critical point of seawater, 5 S on the Mid-Atlantic Ridge. Geology. 2008;36:615–618.
- Heine M, Chandra SB. The linkage between reverse gyrase and hyperthermophiles: a review of their invariable association. J Microbiol. 2009;47:229–234.
- Takai K, Nakamura K, Toki T, et al. Cell proliferation at 122 degrees C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc Natl Acad Sci U S A. 2008;105:10949–10954.
- Takai K, Gamo T, Tsunogai U, et al. Geochemical and microbiological evidence for a hydrogen-based, hyperthermophilic subsurface lithoautotrophic microbial ecosystem (HyperSLiME) beneath an active deep-sea hydrothermal field. Extremophiles. 2004;8:269–282.
- Feller G. Protein stability and enzyme activity at extreme biological temperatures. J Phys Condens Matter. 2010;22:323101.
- Lavire C, Normand P, Alekhina I, et al. Presence of Hydrogenophilus thermoluteolus DNA in accretion ice in the subglacial Lake Vostok, Antarctica, assessed using rrs, cbb and hox. Environ Microbiol. 2006;8:2106–2114.
- Feller G. Life at low temperatures: is disorder the driving force? Extremophiles. 2007;11:211–216.
- Mykytczuk NC, Foote SJ, Omelon CR, et al. Bacterial growth at −15ºC; molecular insights from the permafrost bacterium Planococcus halocryophilus Or1. ISME J. 2013;7:1211–1226.
- Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583.
- Kato C, Li L, Tamaoka J, et al. Molecular analyses of the sediment of the 11,000-m deep Mariana Trench. Extremophiles. 1997;1:117–123.
- Kusube M, Kyaw TS, Tanikawa K, et al. Colwellia marinimaniae sp. nov., a hyperpiezophilic species isolated from an amphipod within the Challenger Deep, Mariana Trench. Int J Syst Evol Microbiol. 2017;67:824–831.
- Fang J, Zhang L, Bazylinski DA. Deep-sea piezosphere and piezophiles: geomicrobiology and biogeochemistry. Trends Microbiol. 2010;18:413–422.
- Zobell CE, Cobet AB. Filament formation by Escherichia coli at increased hydrostatic pressures. J Bacteriol. 1964;87:710–719.
- Kimura K, Morimatsu K, Inaoka T, et al. Injury and recovery of Escherichia coli ATCC25922 cells treated by high hydrostatic pressure at 400-600 MPa. J Biosci Bioeng. 2017;23:698–706.
- Kushner DJ. Growth and nutrition of halophilic bacteria. In: Vreeland RH, Hochstein L, editors. The biology of halophilic bacteria. Boca Raton, FL: CRC Press; 1993. p. 87–103.
- Bowers KJ, Wiegel J. Temperature and pH optima of extremely halophilic archaea: a mini-review. Extremophiles. 2011;15:119–128.
- van der Wielen PW, Bolhuis H, Borin S, et al. Biodeep scientific party. The enigma of prokaryotic life in deep hypersaline anoxic basins. Science. 2005;307:121–123.
- Hallsworth JE, Yakimov MM, Golyshin PN, et al. Limits of life in MgCl2-containing environments: chaotropicity defines the window. Environ Microbiol. 2007;9:801–813.
- Schleper C, Pühler G, Kühlmorgen B, et al. Life at extremely low pH. Nature. 1995;375:741–742.
- Schleper C, Puehler G, Holz I, et al. Picrophilus gen. nov., fam. nov.: a novel aerobic, heterotrophic, thermoacidophilic genus and family comprising archaea capable of growth around pH 0. J Bacteriol. 1995;177:7050–7059.
- Czop M, Motyka J, Sracek O, et al. Geochemistry of the hyperalkaline Gorka pit lake (pH> 13) in the Chrzanow region, southern Poland. Water Air Soil Pollution. 2011;214:423–434.
- Roadcap GS, Sanford RA, Jin Q, et al. Extremely alkaline (pH > 12) ground water hosts diverse microbial community. Ground Water. 2006;44:511–517.
- Takai K, Moser DP, Onstott TC, et al. Alkaliphilus transvaalensis gen. nov., sp. nov., an extremely alkaliphilic bacterium isolated from a deep South African gold mine. Int J Syst Evol Microbiol. 2001;51:1245–1256.
- Horikoshi K. Alkaliphiles: some applications of their products for biotechnology. Microbiol Mol Biol Rev. 1999;63:735–750.
- Harrison JP, Gheeraert N, Tsigelnitskiy D, et al. The limits for life under multiple extremes. Trends Microbiol. 2013;21:204–212.
- Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–280.
- Hoehler TM. An energy balance concept for habitability. Astrobiology. 2007;7:824–838.
- Oren A. Life at high salt concentrations, intracellular KCl concentrations, and acidic proteomes. Front Microbiol. 2013;4:315.
- Médicis ED, Paquette J, Gauthier JJ, et al. Magnesium and manganese content of halophilic bacteria. Appl Environ Microbiol. 1986;52:567–573.
- George P, Witonsky RJ, Trachtman M, et al. “Squiggle-H2O”. An enquiry into the importance of solvation effects in phosphate ester and anhydride reactions. Biochim Biophys Acta. 1970;223:1–15.
- de Meis L. Role of water in the energy of hydrolysis of phosphate compounds – energy transduction in biological membranes. Biochim Biophys Acta. 1989;973:333–349.
- Saint-Martin H, Ortega-Blake I, Leś A, et al. The role of hydration in the hydrolysis of pyrophosphate. A Monte Carlo simulation with polarizable-type interaction potentials. Biochim Biophys Acta. 1994;1207:12–23.
- Hong J, Yoshida N, Chong SH, et al. Elucidating the molecular origin of hydrolysis energy of pyrophosphate in water. J Chem Theory Comput. 2012;8:2239–2246.
- Romero PJ, de Meis L. Role of water in the energy of hydrolysis of phosphoanhydride and phosphoester bonds. J Biol Chem. 1989;264:7869–7873.
- Madern D, Ebel C, Zaccai G. Halophilic adaptation of enzymes. Extremophiles. 2000;4:91–98.
- Kastritis PL, Papandreou NC, Hamodrakas SJ. Haloadaptation: insights from comparative modeling studies of halophilic archaeal DHFRs. Int J Biol Macromol. 2007;41:447–453.
- Siglioccolo A, Paiardini A, Piscitelli M, et al. Structural adaptation of extreme halophilic proteins through decrease of conserved hydrophobic contact surface. BMC Struct Biol. 2011;11:50.
- Miyashita Y, Ohmae E, Nakasone K, et al. Effects of salt on the structure, stability, and function of a halophilic dihydrofolate reductase from a hyperhalophilic archaeon, Haloarcula japonica strain TR-1. Extremophiles. 2015;19:479–493.
- Takashina T, Hamamoto T, Otozai K, et al. Haloarcula japonica sp. nov., a new triangular halophilic archaebacterium. Syst Appl Microbiol. 1990;13:177–181.
- Ozawa K, Harashina T, Yatsunami R, et al. Gene cloning, expression and partial characterization of cell division protein FtsZ1 from extremely halophilic archaeon Haloarcula japonica strain TR-1. Extremophiles. 2005;9:281–288.
- Onodera M, Yatsunami R, Tsukimura W, et al. Gene analysis, expression, and characterization of an intracellular α-amylase from the extremely halophilic archaeon Haloarcula japonica. Biosci Biotechnol Biochem. 2013;77:281–288.
- Ohshida T, Hayashi J, Satomura T, et al. First characterization of extremely halophilic 2-deoxy-D-ribose-5-phosphate aldolase. Protein Expr Purif. 2016;126:62–68.
- Wakai S, Abe A, Fujii S, et al. Pyrophosphate hydrolysis in the extremely halophilic archaeon Haloarcula japonica is catalyzed by a single enzyme with a broad ionic strength range. Extremophiles. 2017;21:471–477.
- Boujelben I, Gomariz M, Martínez-García M, et al. Spatial and seasonal prokaryotic community dynamics in ponds of increasing salinity of Sfax solar saltern in Tunisia. Antonie Van Leeuwenhoek. 2012;101:845–857.
- Mancinelli R, Botti A, Bruni F, et al. Hydration of sodium, potassium, and chloride ions in solution and the concept of structure maker/breaker. J Phys Chem B. 2007;111:13570–13577.
- Karan R, Capes MD, DasSarma S. Function and biotechnology of extremophilic enzymes in low water activity. Aquat Biosyst. 2012;8(1):4.
- Frolow F, Harel M, Sussman JL, et al. Insights into protein adaptation to a saturated salt environment from the crystal structure of a halophilic 2Fe-2S ferredoxin. Nat Struct Biol. 1996;3:452–458.
- McMillan LJ, Hepowit NL, Maupin-Furlow JA. Archaeal inorganic pyrophosphatase displays robust activity under high-salt conditions and in organic solvents. Appl Environ Microbiol. 2015;82:538–548.
- Madern D, Ebel C. Influence of an anion-binding site in the stabilization of halophilic malate dehydrogenase from Haloarcula marismortui. Biochimie. 2007;89:981–987.
- Zhang G, Ge H. Protein hypersaline adaptation: insight from amino acids with machine learning algorithms. Protein J. 2013;32:239–245.
- Taupin CM, Härtlein M, Leberman R. Seryl-tRNA synthetase from the extreme halophile Haloarcula marismortui–isolation, characterization and sequencing of the gene and its expression in Escherichia coli. Eur J Biochem. 1997;243:141–150.
- Marg BL, Schweimer K, Sticht H, et al. A two-alpha-helix extra domain mediates the halophilic character of a plant-type ferredoxin from halophilic archaea. Biochemistry. 2005;44:29–39.
- Evilia C, Hou YM. Acquisition of an insertion peptide for efficient aminoacylation by a halophile tRNA synthetase. Biochemistry. 2006;45:6835–6845.
- Wolfenden R. Degrees of difficulty of water-consuming reactions in the absence of enzymes. Chem Rev. 2006;106:3379–3396.
- Wakai S, Kidokoro S, Masaki K, et al. Constant enthalpy change value during pyrophosphate hydrolysis within the physiological limits of NaCl. J Biol Chem. 2013;288:29247–29251.
- Morin PE, Freire E. Direct calorimetric analysis of the enzymatic activity of yeast cytochrome c oxidase. Biochemistry. 1991;30:8494–8500.
- Todd MJ, Gomez J. Enzyme kinetics determined using calorimetry: a general assay for enzyme activity? Anal Biochem. 2001;296:179–187.
- Bianconi ML. Calorimetric determination of thermodynamic parameters of reaction reveals different enthalpic compensations of the yeast hexokinase isozymes. J Biol Chem. 2003;278:18709–18713.
- Hau HH, Gralnick JA. Ecology and biotechnology of the genus Shewanella. Annu Rev Microbiol. 2007;61:237–258.
- Nogi Y, Kato C, Horikoshi K. Taxonomic studies of deep-sea barophilic Shewanella strains and description of Shewanella violacea sp. nov. Arch Microbiol. 1998;170:331–338.
- Venkateswaran K, Dollhopf ME, Aller R, et al. Shewanella amazonensis sp. nov., a novel metal-reducing facultative anaerobe from Amazonian shelf muds. Int J Syst Bacteriol. 1998;48:965–972.
- Kuribayashi TA, Fujii S, Masanari M, et al. Difference in NaCl tolerance of membrane-bound 5ʹ-nucleotidases purified from deep-sea and brackish water Shewanella species. Extremophiles. 2017;21:357–368.
- Nealson KH, Rowe AR. Electromicrobiology: realities, grand challenges, goals and predictions. Microb Biotechnol. 2016;9:595–600.
- Amend JP, Plyasunov AV. Carbohydrates in thermophile metabolism: calculation of the standard molal thermodynamic properties of aqueous pentoses and hexoses at elevated temperatures and pressures. Geochim Cosmochima Acta. 2001;65:3901–3917.
- Amend JP, Shock EL. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol Rev. 2001;25:175–243.
- Goto E, Kodama T, Minoda Y. Growth and taxonomy of thermophilic hydrogen bacteria. Agric Biol Chem. 1978;42:1305–1308.
- Wakai S, Masanari M, Ikeda T, et al. Oxidative phosphorylation in a thermophilic, facultative chemoautotroph, Hydrogenophilus thermoluteolus, living prevalently in geothermal niches. Environ Microbiol Rep. 2013;5:235–242.
- Jetten MS, Stams AJ, Zehnder AJ. Isolation and characterization of acetyl-coenzyme A synthetase from Methanothrix soehngenii. J Bacteriol. 1989;171:5430–5435.
- Kumari S, Tishel R, Eisenbach M, et al. Cloning, characterization, and functional expression of acs, the gene which encodes acetyl coenzyme A synthetase in Escherichia coli. J Bacteriol. 1995;177:2878–2886.
- Mayer F, Küper U, Meyer C, et al. AMP-forming acetyl coenzyme A synthetase in the outermost membrane of the hyperthermophilic crenarchaeon Ignicoccus hospitalis. J Bacteriol. 2012;194:1572–1581.
- Vieille C, Zeikus GJ. Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol Mol Biol Rev. 2001;65:1–43.
- Fujii S, Oki H, Kawahara K, et al. Structural and functional insights into thermally stable cytochrome c’ from a thermophile. Protein Sci. 2017;26:737–748.
- Kato Y, Fujii S, Kuribayashi TA, et al. Thermal stability of cytochrome c’ from mesophilic Shewanella amazonensis. Biosci Biotechnol Biochem. 2015;79:1125–1129.
- Cacciapuoti G, Porcelli M, Bertoldo C, et al. Purification and characterization of extremely thermophilic and thermostable 5ʹ-methylthioadenosine phosphorylase from the archaeon Sulfolobus solfataricus. Purine nucleoside phosphorylase activity and evidence for intersubunit disulfide bonds. J Biol Chem. 1994;269:24762–24769.
- Cacciapuoti G, Fuccio F, Petraccone L, et al. Role of disulfide bonds in conformational stability and folding of 5ʹ-deoxy-5ʹ-methylthioadenosine phosphorylase II from the hyperthermophilic archaeon Sulfolobus solfataricus. Biochim Biophys Acta. 2012;1824:1136–1143.
- Fukuchi S, Nishikawa K. Protein surface amino acid compositions distinctively differ between thermophilic and mesophilic bacteria. J Mol Biol. 2001;309:835–843.
- Chan CH, Yu TH, Wong KB. Stabilizing salt-bridge enhances protein thermostability by reducing the heat capacity change of unfolding. PLoS ONE. 2011;6(6):e21624.
- Ambler RP. Sequence variability in bacterial cytochromes c. Biochim Biophys Acta. 1991;1058:42–47.
- Kang X, Carey J. Role of heme in structural organization of cytochrome c probed by semisynthesis. Biochemistry. 1999;38:15944–15951.
- Yamanaka M, Masanari M, Sambongi Y. Conferment of folding ability to a naturally unfolded apocytochrome c through introduction of hydrophobic amino acid residues. Biochemistry. 2011;50:2313–2320.
- Kobayashi S, Fujii S, Koga A, et al. Pseudomonas aeruginosa cytochrome c551 denaturation by five systematic urea derivatives that differ in the alkyl chain length. Biosci Biotechnol Biochem. 2017;81:1274–1278.
- Kato C, Nogi Y. Correlation between phylogenetic structure and function: examples from deep-sea Shewanella. FEMS Microbiol Ecol. 2001;35:223–230.
- Masanari M, Wakai S, Ishida M, et al. Correlation between the optimal growth pressures of four Shewanella species and the stabilities of their cytochromes c5. Extremophiles. 2014;18:617–627.
- Masanari M, Fujii S, Kawahara K, et al. Comparative study on stabilization mechanism of monomeric cytochrome c5 from deep-sea piezophilic Shewanella violacea. Biosci Biotechnol Biochem. 2016;80:2365–2370.
- Fujii S, Masanari-Fujii M, Kobayashi S, et al. Commonly stabilized cytochromes c from deep-sea Shewanella and Pseudomonas. Biosci Biotechnol Biochem. 2018;82:792–799.
- Apel WA, Dugan PR, Tuttle JH. Adenosine 5ʹ-triphosphate formation in Thiobacillus ferrooxidans vesicles by H+ ion gradients comparable to those of environmental conditions. J Bacteriol. 1980;142:295–301.
- Wakai S, Ohmori A, Kanao T, et al. Purification and biochemical characterization of the F1-ATPase from Acidithiobacillus ferrooxidans NASF-1 and analysis of the atp operon. Biosci Biotechnol Biochem. 2005;69:1884–1891.
- Wakai S, Kikumoto M, Kanao T, et al. Involvement of sulfide: quinoneoxidoreductase in sulfur oxidation of an acidophilic iron-oxidizing bacterium, Acidithiobacillus ferrooxidans NASF-1. Biosci Biotechnol Biochem. 2004;68:2519–2528.
- Wakai S, Tsujita M, Kikumoto M, et al. Purification and characterization of sulfide: quinoneoxidoreductase from an acidophilic iron-oxidizing bacterium, Acidithiobacillus ferrooxidans. Biosci Biotechnol Biochem. 2007;71:2735–2742.
- Zhang S, Yan L, Xing W, et al. Acidithiobacillus ferrooxidans and its potential application. Extremophiles. 2018;22:563–579.
- Quatrini R, Appia-Ayme C, Denis Y, et al. Extending the models for iron and sulfur oxidation in the extreme acidophile Acidithiobacillus ferrooxidans. BMC Genomics. 2009;10:394.
- Michaux C, Pouyez J, Mayard A, et al. Structural insights into the acidophilic pH adaptation of a novel endo-1,4-β-xylanase from Scytalidium acidophilum. Biochimie. 2010;92:1407–1415.
- Huang Y, Krauss G, Cottaz S, et al. A highly acid-stable and thermostable endo-beta-glucanase from the thermoacidophilic archaeon Sulfolobus solfataricus. Biochem J. 2005;385:581–588.
- Kanao T, Matsumoto C, Shiraga K, et al. Recombinant tetrathionate hydrolase from Acidithiobacillus ferrooxidans requires exposure to acidic conditions for proper folding. FEMS Microbiol Lett. 2010;309:43–47.
- Ishii T, Kawaichi S, Nakagawa H, et al. From chemolithoautotrophs to electrolithoautotrophs: CO2 fixation by Fe(II)-oxidizing bacteria coupled with direct uptake of electrons from solid electron sources. Front Microbiol. 2015;6:994.
- Nakamura R, Takashima T, Kato S, et al. Electrical current generation across a black smoker chimney. Angew Chem Int Ed Engl. 2010;49:7692–7694.
- Deng X, Dohmae N, Nealson KH, et al. Multi-heme cytochromes provide a pathway for survival in energy-limited environments. Sci Adv. 2018;4:eaao5682.
- Uchiyama T, Ito K, Mori K, et al. Iron-corroding methanogen isolated from a crude-oil storage tank. Appl Environ Microbiol. 2010;76:1783–1788.
- Mori K, Tsurumaru H, Harayama S. Iron corrosion activity of anaerobic hydrogen-consuming microorganisms isolated from oil facilities. J Biosci Bioeng. 2010;110:426–430.
- Iino T, Ito K, Wakai S, et al. Iron corrosion induced by nonhydrogenotrophic nitrate-reducing Prolixibacter sp. strain MIC1-1. Appl Environ Microbiol. 2015;81:1839–1846.
- Iino T, Sakamoto M, Ohkuma M. Prolixibacter denitrificans sp. nov., an iron-corroding, facultatively aerobic, nitrate-reducing bacterium isolated from crude oil, and emended descriptions of the genus Prolixibacter and Prolixibacter bellariivorans. Int J Syst Evol Microbiol. 2015;65:2865–2869.
- Wakai S. Metal materials suffer from infectious disease: microbiologically influenced corrosion. Kagakutoseibutsu. 2015;53: 515–520. Japanese.
- Adams MW, Perler FB, Kelly RM. Extremozymes: expanding the limits of biocatalysis. Nat Biotechnol. 1995;13(7):662–668.
- Stetter KO. Extremophiles and their adaptation to hot environments. FEBS Lett. 1999;452:22–25.
- Feller G, Gerday C. Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol. 2003;1:200–208.
- Dubnovitsky AP, Kapetaniou EG, Papageorgiou AC. Enzyme adaptation to alkaline pH: atomic resolution (1.08 Å) structure of phosphoserine aminotransferase from Bacillus alcalophilus. Protein Sci. 2005;14:97–110.
- Graziano G, Merlino A. Molecular bases of protein halotolerance. Biochim Biophys Acta. 2014;1844:850–858.
- Oren A. Halobacterium sodomense sp. nov., a Dead Sea halobacterium with an extremely high magnesium requirement. Int J Syst Bacteriol. 1983;33:381–386.
