ABSTRACT
Aluminum (Al) toxicity is a primary limiting factor for crop production in acid soils. Callose deposition, an early indicator and likely a contributor to Al toxicity, is induced rapidly in plant roots under Al stress. SbGlu1, encoding a β-1,3-glucanase for callose degradation, showed important roles in sorghum Al resistance, yet its regulatory mechanisms remain unclear. The STOP1 transcription factors mediate Al signal transduction in various plants. Here, we identified their homolog in sweet sorghum, SbSTOP1, transcriptionally activated the expression of SbGlu1. Moreover, the DNA sequence recognized by SbSTOP1 on the promoter of SbGlu1 lacked the reported cis-acting element. Complementation lines of Atstop1 with SbSTOP1 revealed enhanced transcription levels of SbGlu1 homologous gene and reduced callose accumulation in Arabidopsis. These results indicate, for the first time, that SbSTOP1 is involved in the modulation of callose deposition under Al stress via transcriptional regulation of a β-1,3-glucanase gene.
Graphical Abstract
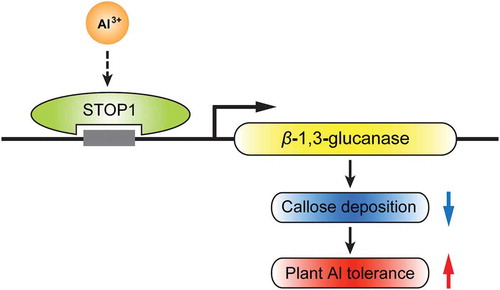
SbSTOP1 directly activities the transcription of a β-1,3-glucanase gene, which confers reduced callose deposition and increased Al tolerance in plants.
Acid soil covers nearly 50% of the potential arable land worldwide [Citation1]. Additionally, human activities, such as improper crop-cultivating methods, further promote soil acidification [Citation2,Citation3]. Aluminum (Al) toxicity is a primary limiting factor for crop production in acid soils. At soil pH below 5, Al ions (the most abundant form, Al3+) are released from clay minerals and absorbed by plant roots, leading to the rapid inhibition of root elongation and restriction of water and nutrient uptake [Citation4–Citation6].
Plants have evolved a series of mechanisms for Al resistance that involve multiple genes. Transcription factors have received widespread attention due to their central roles in Al signal perception and transduction. These transcription factors, such as sensitive to proton rhizotoxicity 1 (STOP1), Al resistance transcription factor 1 (ART1), abscisic acid, stress, and ripening 5 (ASR5), WRKY46 (and WRKY22), and calmodulin-binding transcription activator 2 (CAMTA2), were identified from various plant species, especially Arabidopsis and rice [Citation6–Citation12]. Among them, STOP1-like transcription factors, a type of C2H2 zinc finger protein, were found to play the key roles in the regulation of tolerance mechanisms to Al and/or proton stress. Transcriptome analyses showed that AtSTOP1 regulates the expression of multiple Al-responsive genes (and also H+-responsive genes) [Citation13]. For instance, AtALMT1 (Al-activated malate transporter)-mediated malate exudation is a critical Al tolerance mechanism in Arabidopsis, and AtSTOP1 positively regulates the expression of AtALMT1 [Citation7,Citation14]. AtSTOP1 is also essential for Al-induced expression of AtMATE (Al-activated citrate transporter), which is involved in citrate exudation in Al tolerance [Citation15]. ART1, a rice homolog of AtSTOP1, however, specifically regulates the expression of Al tolerance genes. Thirty-one genes regulated directly or indirectly by ART1 are probably involved in both internal and external detoxification of Al at different cellular levels. For example, OsSTAR1 (sensitive to Al rhizotoxicity1) and OsSTAR2 were proposed to mediate the efflux of UDP-glucose into the cell wall, thereby altering the cell wall composition and alleviating Al toxicity [Citation16]. Most of these 31 genes, such as OsSTAR1 and OsSTAR2, possess a cis-acting element as GGN(T/g/a/C)V(C/A/g)S(C/G) in their promoter, which can be directly bound by ART1 [Citation8,Citation17]. We previously isolated and characterized the first STOP1-like protein, SbSTOP1 (SbSTOP1d, Sb03g041170.1), in sweet sorghum (Sorghum bicolor L.). However, compared with AtSTOP1 and OsART1, few homologs of SbSTOP1-regulated Al tolerance genes have been identified in sorghum, with the exception of SbMATE and SbSTAR2 [Citation18].
Callose (β-1,3-glucan), a cell wall-associated polysaccharide, plays important roles during multiple processes in plant development and/or in response to a number of biotic and abiotic stresses [Citation19]. Al-induced callose deposition is a sensitive indicator of Al toxicity and has been used as a convenient and rapid screening parameter for Al injury in addition to root elongation measurement [Citation20–Citation22]. Moreover, several lines of evidence show that callose deposition at the plasmodesmata can block symplastic transport and possibly inhibit apoplastic flow of high-molecular weight solutes, which suggest that Al-induced callose deposition is not just an early consequence of Al stress, but is also likely to be a contributor to Al toxicity [Citation23,Citation24]. Callose is degraded by β-1,3-glucanases (BGs, EC: 3.2.1.39) and most of β-1,3-glucanases are regulated at the transcription level during stresses [Citation25]. Genes encoding β-1,3-glucanase precursors (or β-1,3-glucanase-like protein) are positively regulated by Al treatment in wheat, rice and Arabidopsis [Citation26–Citation28]. We previously also isolated a gene encoding a β-1,3-glucanase protein in sweet sorghum, named SbGlu1, that shows high similarity to OsGlu (β-1,3-glucanase in rice) with 71% identity. Further studies indicated that heterologous expression of SbGlu1 in Arabidopsis reduced callose deposition and improved plant Al tolerance, which established a link between β-1,3-glucanase and Al toxicity [Citation29].
Although β-1,3-glucanases play important roles in plant Al resistance, their regulatory mechanisms remain unclear. STOP1, as the key transcription factor in various plants, regulates multiple Al resistance genes that belong to different biological processes, however, the transcriptional regulation roles for β-1,3-glucanase genes has never been elucidated [Citation8,Citation13,Citation30,Citation31]. The present study showed that SbSTOP1 regulated the transcriptional expression of SbGlu1 by directly binding to its promoter, and the target DNA sequence of SbSTOP1 on the SbGlu1 promoter lacked the reported cis-acting element GGN(T/g/a/C)V(C/A/g)S(C/G). Transgenic lines expressing SbSTOP1 showed enhanced β-1,3-glucanase gene expression and reduced callose accumulation. These results indicated for the first time that SbSTOP1 participates in the modulation of callose deposition under Al stress, thus providing novel insights into the molecular mechanisms of Al tolerance in plants.
Materials and methods
Plant materials and culture conditions
Sweet sorghum ((Sorghum bicolor L.) seeds POTCHETSTRM was used for DNA extraction and promoters cloning of SbGlu1 (Sb03g045630.1) [Citation29]. The promoter sequence of SbGlu1 was obtained from NCBI reference sequence: NC_012872.2. For quantitative real-time PCR analysis and callose content measurement, Arabidopsis thaliana ecotype Columbia (Col-0) as wild type (WT), the Atstop1 mutant (SALK 114108), and two independent complemented lines (T4 generation) of Atstop1 with SbSTOP1 as previously described were used [Citation18]. After vernalized at 4°C in darkness for 3 d, seeds were surface sterilized with 1% (v/v) NaClO for 10 min and rinsed with deionized water five times, then germinated on solid MS medium vertically for 7 d. Uniform seedlings were transferred to 0.5 mM CaCl2 at pH 5.0, without or with 15 μM AlCl3 for 3 h or 6 h, then indicated assays were performed. The seedlings were grown in an environmentally controlled growth chamber at 22°C with a 16 h light (150 μmol m−2 s−1)/8 h dark photoperiod and 70% relative humidity.
HEK293 coexpression system and dual-luciferase reporter assay
The reporter plasmid (pSbGlu1::LUC-SV40::REN), encodes two luciferases, the Renilla luciferase (REN) controlled by the SV40 promoter, and the firefly luciferase (LUC) controlled by the SbGlu1 promoter which is fused upstream to a mini promoter. SbGlu1 promoter (−2000 bp to −1 bp, Figure S1) was cloned into the HindIII site of the modified vector pNL2.2 [Citation32]. The effector plasmid (CMV::SbSTOP1-Myc) was constructed by inserting the coding region of SbSTOP1 into BamHI site of the vector pCMV under the control of the cytomegalovirus (CMV) promoter.
HEK293 (human embryonic kidney) cells transfection, and dual-luciferase reporter assay were performed as previously described [Citation18]. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with FBS (10%) and penicillin/streptomycin (1%) and incubated in a 37°C incubator with 5% CO2 in air. When the cell count reaches 2 × 107, cells are ready for transfection. The above reporter and effector were co-transfected into the cells using the calcium phosphate transfection method as reported [Citation33]. After 30–48 h, the transfected cells were ready for the dual-luciferase reporter assay.
The dual-luciferase reporter assay was performed according to the technical manual of the Dual-luciferase Reporter Assay System (Promega, E1910). The transfected cells were gently rinsed with 1× PBS (pH 7.2, Thermo, 20012050) to remove the residual culture medium, lysed in 1× Passive Lysis Buffer (PLB). 20 μL/well of the PLB lysate was plated in a 96-well plate. The LUC activity was measured by adding 100 μL of Luciferase Assay Reagent II to generate a luminescent signal that was measured with a luminometer (Berthold LB960). This reaction was quenched, and the REN reaction is simultaneously initiated by adding 100 μL of Stop & Glo® Reagent. REN-induced luminescent signal were measured as an internal control.
DNA transfer to protoplasts
The reporter plasmid and effector plasmid were constructed as described with minor modification [Citation34]. The reporter plasmid (CaMV 35S::REN-pSbGlu1-CaMV 35S (−46)::LUC), encodes two luciferases, the REN controlled by the cauliflower mosaic virus 35S (CaMV 35S) promoter, and the LUC controlled by the indicated SbGlu1 promoter. CaMV 35S minimal promoter (−46) was synthesized [Citation35], and inserted into the HindIII/BamHI sites of the vector pGreen-0800-LUC. The indicated SbGlu1 promoter was cloned into the HindIII site, which fused upstream to the CaMV 35S minimal promoter (−46). The effector plasmid (CaMV 35S::Myc-SbSTOP1) was prepared by cloning the coding region of SbSTOP1 into the EcoRI and XhoI sites of the vector pEGAD-Myc under the control of the CaMV 35S promoter. Arabidopsis protoplasts were isolated from 4-week-old plants. Leaves were cut and transferred into the enzyme solution [1% (w/v) cellulase R10, 0.25% (w/v) macerozyme R10, 0.4 M D-mannitol, 20 mM KCl, 20 mM MES pH 5.7 and 10 mM CaCl2] for 3 h digestion in darkness. Protoplasts were filtered through a 200-micronnylon mesh and centrifuged, rinsed with ice-cold W5 buffer [154 mM NaCl, 125 mM CaCl2, 5 mM KCl and 2 mM MES, pH 5.7], and re-suspended in MMg buffer [0.4 M mannitol, 15 mM MgCl2, 4 mM MES, pH 5.7]. Afterward, the protoplasts were transformed using PEG-mediated protoplast transformation method. Each 100 μL protoplasts were mixed with 20 mg each of plasmids, and 120 μL PEG solution [40% (w/v) PEG4000, 0.2 M mannitol, 100 mM CaCl2]. The protoplast/DNA mixture was incubated at in darkness for 15 min, rinsed with W5 buffer, and incubated in darkness at room temperature for 12–16 h. Then the transformed protoplasts were ready for dual-luciferase reporter assay as described above.
Protein preparation
SbSTOP1DD (91–425 aa, containing dimerization domain and DNA-binding domain) was inserted into NdeI site of a vector derived from pGEX4T-2, which contains N-terminal GST tag. Protein purification was conducted as previously described [Citation36]. The recombinant protein was overexpressed in Escherichia coli JM109 (DE3) cells. The cells were grown in LB medium at 37°C until OD600 nm = 0.8. The expression of recombinant protein was then induced with IPTG at a final concentration of 0.2 mM at 18°C for 16 h. The cells were harvested and resuspended in purification buffer (50 mM Tris–HCl pH 8.0, 300 mM NaCl) supplemented with 1 mM PMSF, 10 mM MgCl2 and 150 U/ mL DNaseI. The cells were lysed by sonication and the lysate was centrifuged at 13000 rpm for 45 min. The GST-tag fusion proteins in the supernatant were bound to GST agarose (TransGen, J029) pre-equilibrated with the purification buffer. After washed, the protein was eluted using purification buffer supplemented with 10 mM GSH. The protein purity was greater than 95% as judged by SDS-PAGE.
Electrophoretic mobility shift assay (EMSA)
EMSA were performed as described with minor modification [Citation37]. 6-carboxyfluorescein (6-FAM) was used for labelling oligonucleotides at the 5ʹ ends. Both single stranded DNA, 5ʹ-FAM-probe 4 and 5ʹ-FAM-probe 6 were synthesized (Shengon, Shanghai, China). The double-stranded probes were prepared as described [Citation38]. Oligos were dissolved in deionized water. Oligo pairs were mixed at 1:1 molar ratio, incubated for 10 min at 95 °C, and annealed by slow cooling to room temperature.
To estimate the Kd value (dissociation constant) between SbSTOP1 protein and probe, different concentrations of SbSTOP1 proteins ranging from 0.27 μM to 64.8 μM, as indicated, were prepared in 50 mM Tris-HCl (pH 8.0). The protein/FAM-labeled probe mixture, containing 0.5 μM FAM-labeled probe and indicated SbSTOP1 protein, was incubated at room temperature for 30 min, and then mixed with 2 μL loading buffer without SDS (50% Glycerol, 0.25% Bromophenol blue). The mixture was subjected to electrophoresis at 4 °C with a 4% native PAGE [containing 2.5% glycerol and 0.5× TB buffer (45 mM Tris, 45 mM boric acid)] in 0.5× TB buffer. The fluorescence imaging system was used to detect the signals in the gel.
For competition assay, DNA-binding reaction contained 50 mM Tris-HCl (pH 8.0), 1.8 μM FAM-labeled probe and 30 μM SbSTOP1 protein, in the absence or presence of competitor (non-FAM-labeled probe). After incubation at room temperature for 30 min, the mixture was subjected to electrophoresis as described above.
RNA isolation and quantitative real-time PCR
Total RNA isolation, cDNA preparation and quantitative real-time PCR (qRT-PCR) were performed as previously described [Citation39]. The gene-specific primers were designed using Primer 5.0 software. The house-keeping gene Actin 8 was used as an internal control. Primer sequences were listed in Table SI. The qRT-PCR was performed using SYBR Premix ExTaq (Takara) in an Mx3005P qPCR system (Stratagene, United States). The relative expression level of the genes was calculated using the 2−ΔΔCT method [Citation40]. The experiment was conducted using three biological replicates.
Callose content measurement in Arabidopsis roots
Callose was extracted from Arabidopsis roots according to previously described [Citation29,Citation41], with minor modification. In brief, 30 roots of Arabidopsis were fixed in dehydrated ethanol for at least 12 h at 4°C. The root was then suction-dried and homogenized in 1 M NaOH for 2 min, and subsequently transferred to 1.5 mL of Eppendorf tubes. To dissolve the callose, the homogenate was incubated in a water bath (80 °C, 15 min) and then centrifuged (12000 rpm, 15 min) at room temperature. The 200 μL supernatant including callose was mixed with 400 μL of 0.1% (w/v) aniline blue water solution, resulting in a violet red color. After the addition of 210 μL of 1 M HCl, the color changes to deep blue, indicating neutral to acidic pH values. The final pH value was adjusted by the addition of 590 μL 1 M glycine/NaOH buffer (pH 9.5) and the tubes were mixed vigorously. During the following incubation in a water bath (50 °C, 20 min) and further 30 min at room temperature, the aniline blue mixture becomes almost completely decolorized. The callose was quantified by fluorescence spectrophotometry using an excitation wavelength of 400 nm and an emission wavelength of 500 nm using laminarin (LE) as a standard callose source.
Results
Transcriptional regulation of SbGlu1 by SbSTOP1
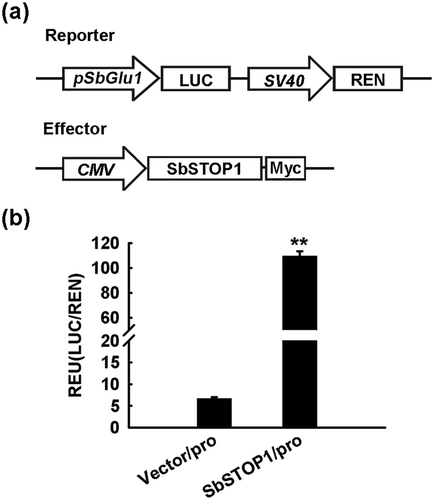
As stated, SbSTOP1 positively regulates the expression of SbMATE and SbSTAR2 [Citation18]. To identify the candidate transcription factors for SbGlu1 (encoding a β-1,3-glucanase) in sorghum, we first tested if SbSTOP1 works on it using the HEK293 coexpression system with a dual-luciferase reporter assay, as previously described [Citation18]. The SbGlu1 promoter (−2000 bp to −1 bp from the translation start site) was cloned (Figure S1), and inserted into a reporter vector to drive LUC reporter gene. Expression of REN reporter gene driven by the SV40 promoter was used as the internal control. SbSTOP1 was inserted into the effector vector under the control of the CMV promoter ()). Both the reporter and effector were co-transfected into HEK293 cells, and a dual-luciferase reporter assay was performed. As shown in ), the SbGlu1 promoter-driven reporter exhibited significantly higher luciferase activity in the presence of the SbSTOP1 effector compared to the vector-only control (P < 0.01). The result indicated that SbSTOP1 interacted with the SbGlu1 promoter in HEK293 cells, thus transcriptionally regulated the expression of SbGlu1.
Figure 1. Transcriptional regulation of SbGlu1 by SbSTOP1 in HEK293 cells.
(a) Schematic diagram of the reporter and effector used in the HEK293 coexpression system. pSbGlu1, SbGlu1 promoter (−2000 bp to −1 bp); LUC, firefly luciferase reporter; REN, Renilla luciferase reporter as an internal control; SV40 and cytomegalovirus (CMV), two promoters commonly used in mammalian expression vectors to drive gene expression; Myc, protein tag. (b) Relative luciferase activity of SbGlu1 promoter-driven reporter regulated by SbSTOP1. Luciferase activity of reporter (LUC) driven by the SbGlu1 promoter (pro) was normalized to the internal control reporter (REN). Data represent the means ± SD from three independent biological replicates. Asterisks (**) represent significant differences from the vector-only control at P < 0.01.
Identification of the potential cis-acting sequence on the SbGlu1 promoter in plants
To confirm the above result, a dual-luciferase reporter assay was performed in plants. The LUC reporter gene was driven by SbGlu1 promoter (−2000 bp to −1 bp). The expression of REN reporter gene driven by CaMV 35S was used as the internal control. The effector contained SbSTOP1 under the control of the CaMV 35S promoter ()). Both the reporter and effector were transiently introduced into Arabidopsis protoplasts. Significant differences in the luciferase activity of the SbGlu1 promoter-driven reporters were observed between the presence of the SbSTOP1 effector and control, further supporting the role for SbSTOP1 in the transcriptional regulation of SbGlu1 in plants ()). Since SbGlu1 encodes a β-1,3-glucanase for callose degradation [Citation29], this result suggested for the first time that STOP1-like proteins may participate in the modulation of callose deposition under Al stress.
Figure 2. Identification of the DNA-binding region of SbSTOP1 on the SbGlu1 promoter in Arabidopsis protoplasts.
(a) Schematic diagram of the reporter and effector in the transient transformation assay. 35S, cauliflower mosaic virus 35S promoter; 35S (−46), cauliflower mosaic virus 35S minimal promoter; pSbGlu1, SbGlu1 promoter (−2000, −1349, −1112, −500, or −373 bp to −1 bp from the translation start codon). (b) Relative luciferase activity of various regions of SbGlu1 promoter-driven reporters regulated by SbSTOP1. Luciferase activity of reporter (LUC) driven by the different promoters of SbGlu1 was normalized to the internal control reporter (REN). Data represent the means ± SD from three independent biological replicates. Asterisks (**) represent significant differences from the vector-only control at P < 0.01.
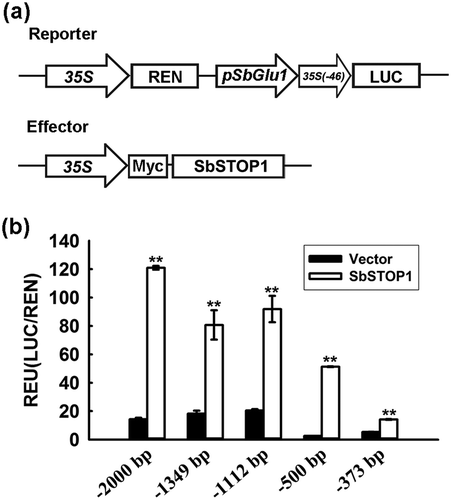
To further identify the cis-acting region targeted by SbSTOP1, reporters with the SbGlu1 promoter containing a region between −1349, −1112, −500, or −373 bp to −1 bp from the translation start codon were transformed respectively with effectors into plant protoplasts. All of the putative cis-acting regions of the SbGlu1 promoter showed high luciferase activity, suggesting a widespread distribution of cis-acting elements on the promoter. The minimum promoter region (−373 bp to −1 bp)-driven reporter also revealed higher luciferase activity compared to the vector-only control ()), indicating the presence of the cis-acting sequence recognized by SbSTOP1 within the region between −373 bp to −1 bp, which was investigated in detail.
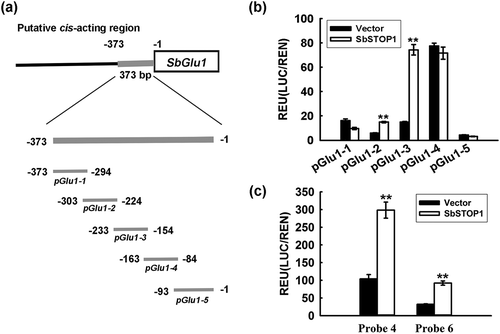
To narrow this region (−373 bp to −1 bp), five shorter putative cis-acting regions (10 bp neighbor overlap) were designed ()), and the same dual-luciferase reporter assays were performed in plants. ) showed the different transcriptional effects of SbSTOP1 on various putative cis-acting regions. Among them, pGlu1-2 (−303 bp to −224 bp)- and pGlu1-3 (−233 bp to −154 bp)-driven reporters displayed significant differences at P < 0.01 compared with the vector-only control ()), therefore, these two target regions of SbSTOP1 were further narrowed into 10 probes (Figure S1). Each probe was a 20-bp-length fragment containing a 6-bp neighbor overlap. As shown in ) and Figure S2, probe 4- and probe 6-driven reporters displayed over two-fold higher luciferase activity compared to the vector-only control. Interestingly, probe 4 and 6 did not contain any reported cis-acting elements as GGN(T/g/a/C)V(C/A/g)S(C/G) in their sequences, indicating that in plants, SbSTOP1 can recognize DNA sequences lacking the reported typical cis-acting element.
Figure 3. Further characterization of the cis-acting sequence recognized by SbSTOP1 on SbGlu1 promoter in plants.
(a) Five putative cis-acting regions of the SbGlu1 promoter designed for the transient transformation assay in Arabidopsis protoplasts. (b) Transcriptional regulation of five regions of SbGlu1 promoter-driven reporters by SbSTOP1 in plant cells. (c) Binding of the SbSTOP1 protein to different probes (20-bp-length fragment) on the SbGlu1 promoter in plant. Data represent the means ± SD from three independent biological replicates. Asterisks (**) represent significant differences from the vector-only control at P < 0.01.
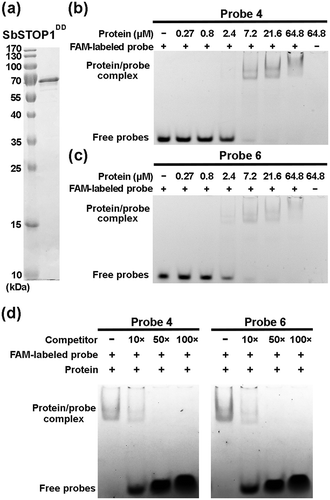
SbSTOP1 binds SbGlu1 promoter sequences directly in vitro
To determine the direct interaction between SbSTOP1 and the above identified DNA sequences on the SbGlu1 promoter, electrophoretic mobility shift assays (EMSA) were performed. We purified recombinant SbSTOP1DD protein (91–425 aa, containing dimerization domain and DNA-binding domain) from Escherichia coli cells ()) and synthesized 20-bp double-stranded DNA (probe 4 and probe 6) labeled with FAM fluorophore or not. The SbSTOP1DD protein was able to directly bind both probe 4 and probe 6 in a similar dose-dependent manner. The Kd value (dissociation constant) was estimated to be 2.4 μM (,c)), suggesting a pretty tight interaction between SbSTOP1DD and probe 4/probe 6 that strong enough to take place physiologically in plants. To verify specific interaction between SbSTOP1DD and probe 4/probe 6, a competition assay was performed as shown in ). SbSTOP1/FAM-labeled probe 4 (or probe 6) complex band was yield in the absence of competitor (non-FAM-labeled probe). Presence of competitor weakened (or eliminated) the band abundance with increasing concentration (10×, 50×, 100× in molar excess), indicating the direct interaction between SbSTOP1 protein and probe 4/probe 6 in vitro.
Figure 4. SbSTOP1 protein interacts with identified DNA sequences on SbGlu1 promoter tightly in vitro.
(a) Purification of SbSTOP1DD protein (91–425 aa, containing dimerization domain and DNA-binding domain) from Escherichia coli cells. The protein purity was judged by SDS-PAGE. (b, c) Tight interaction between SbSTOP1 protein and FAM-labeled DNA in electrophoretic mobility shift assay (EMSA). A series of concentrations of SbSTOP1 proteins were mixed individually with FAM-labeled probe 4 (b) and FAM-labeled probe 6 (c), incubated at room temperature for 30 min, and then subjected to electrophoresis with a 4% native PAGE. (d) A competitive EMSA showing specific interaction of SbSTOP1 with FAM-labeled probe 4/probe 6 in the absence and presence of increasing amount of non-FAM-labeled probe as a competitor (10×, 50×, 100× in molar excess).
SbSTOP1 overexpression enhances Al tolerance of Arabidopsis associated with elevated β-1,3-glucanase gene expression and reduced callose deposition in roots
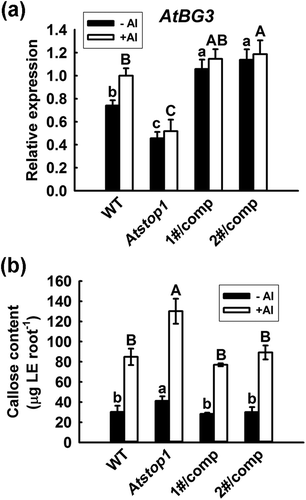
As we previously reported, heterologous expression of SbSTOP1 in Arabidopsis Atstop1 mutant enhanced the Al tolerance of transgenic lines [Citation18]. However, there are no indications that SbSTOP1, or even its homologs in other plant species, are involved in the modulation of callose deposition under Al stress. We investigated this possibility using complemented lines as mentioned above, since SbSTOP1 up-regulated the expression of a β-1,3-glucanase (SbGlu1) gene for callose degradation. AtBG3 (β-1,3-glucanase 3, At3g57240.1), a homolog of SbGlu1 in Arabidopsis, shares high similarity to SbGlu1, with 49.2% identity. Quantitative real-time PCR was used to determine the transcript levels of AtBG3 in wild-type (WT), the Atstop1 mutant and two independent SbSTOP1 complemented lines under Al stress or not. As shown in ), AtBG3 expression was significantly blocked in the Atstop1 mutant compared with WT, while its expression in transgenic lines was similar (or even higher) to that in WT under Al treatment, suggesting that SbSTOP1 may enhanced the Al tolerance of transgenic lines via enhancing β-1,3-glucanase gene expression. Similar expression levels of AtBG3 in two SbSTOP1 complemented lines with or without Al stress were showed due to the constitutive expression of SbSTOP1 under the control of the CaMV 35S promoter. Callose accumulation was also determined in transgenic lines with or without Al treatment. All plants showed lower callose content without Al treatment. Under Al stress, the Atstop1 mutant showed an obviously higher callose content in roots compared to WT, whereas the heterologous expression of SbSTOP1 greatly reduced the callose content in complemented lines ()). These results suggested that SbSTOP1 enhanced the Al tolerance of plants associated with transcriptional activation of the β-1,3-glucanase gene and callose degradation.
Figure 5. Complementation lines of Atstop1 with SbSTOP1 shows enhanced β-1,3-glucanase gene expression and reduced callose accumulation.
(a) Quantitative real-time PCR analysis of the expression of SbGlu1 homolog (AtBG3) in WT (Col-0), Atstop1 and two independent SbSTOP1 complemented lines. Seven-day-old seedlings were pre-cultured on solid MS medium vertically, then transferred to liquid medium containing 0.5 mM CaCl2 at pH 5.0 without AlCl3 or with 15 μM AlCl3 treatment for 3 h. (b) Callose content in the roots of WT (Col-0), Atstop1 and two independent SbSTOP1 complemented lines without or with 15 μM AlCl3 treatment for 6 h. Data represent the means ± SD from three independent biological replicates. Columns with different letters are significantly different at P < 0.05.
Discussion
STOP1-like transcription factors regulate multiple genes that are implicated in Al tolerance in various plant species. These genes are involved with a number of biological processes, such as cell wall maintenance, organic acid transport, metabolism and detoxification [Citation6,Citation8,Citation13,Citation30,Citation42–Citation44]. Compared with the numerous genes regulated by AtSTOP1 and OsART1, only two genes, SbMATE and SbSTAR2, have been characterized being modulated by their homolog in sorghum, SbSTOP1 [Citation18]. These studies suggest two possible regulatory pathways for SbSTOP1 in response to Al stress. First, SbSTOP1 may contribute to SbMATE-dependent citrate excretion in Al stress, which is a primary Al tolerance mechanism in sorghum [Citation18,Citation45]. Second, SbSTOP1 is also likely involved in SbSTAR2-mediated modification of cell wall components under Al stress [Citation16,Citation18]. The present study indicated a novel SbSTOP1 mediated pathway for Al tolerance in sorghum.
SbGlu1, encoding a β-1,3-glucanase, was identified and further confirmed to be effective for Al tolerance [Citation29]. However, the regulatory pathways of SbGlu1 remain unknown. We examined SbGlu1 transcriptional regulation by SbSTOP1 in both HEK293 cells and plant cells ( and ). This is the first piece of evidence that STOP1-like protein can regulates a β-1,3-glucanase gene in plants, implying that STOP1-like proteins, at least SbSTOP1, are involved in callose modulation under Al stress.
ART1 in rice interacts with a cis-acting element [GGN(T/g/a/C)V(C/A/g)S(C/G)] present in the promoter of 29 out of the 31 genes [Citation17]. There are two and three such motifs in the promoters of AtALMT1 and AtALS3 which are regulated by AtSTOP1 [Citation31]. The same cis-acting element (GGNVS) in the promoter of CcMATE is essential for CcSTOP1 binding in pigeonpea [Citation46]. In this study, we identified the target DNA sequence of SbSTOP1 on the promoter of SbGlu1, however, to our surprise, the 20-bp-length probes recognized by SbSTOP1 did not include the above reported cis-acting element ( and ; Figure S1). This result signifies the existence of novel transcriptional regulatory mechanisms of STOP1 protein in sorghum differ from the other plant species. VuSTOP1 in Vigna umbellate could also interacted with a DNA sequence (~ 300 bp) lacking reported GGNVS cis-acting element, whereas the specific DNA binding sequence was not identified [Citation31]. The detailed cis-acting element recognized by SbSTOP1 needs to be investigated further. EMSA revealed that the purified SbSTOP1 protein directly bound the identified DNA. And to eliminate the dosage effects of transient transformation in plant protoplasts, a series of protein at different concentrations were used in EMSA. The concentration of SbSTOP1 protein that bound half of the tested DNA was approximately 2.4 μM (Kd value), which means the interactions of SbSTOP1 and the DNA probes are tight enough to be physiologically relevant in plants ().
Inhibition of root elongation and induction of callose formation are both Al sensitive markers for various plants, such as soybean (Glycine max), wheat (Triticum aestivum), maize (Zea mays) and sorghum (Sorghum bicolor) [Citation20,Citation21,Citation47,Citation48]. Callose increases in roots as early as 30 min to a few hours after application of Al treatment, thus contributes to Al toxicity [Citation23,Citation47,Citation49,Citation50]. Root growth inhibition by Al can also be assessed after 90 min to 24 h [Citation29,Citation51,Citation52]. Numerous screening assays showed significantly different levels of resistance to Al between sensitive and tolerant cultivars within 24 h [Citation20,Citation29,Citation47,Citation53]. These studies suggest that most plant species possess certain rapid response mechanisms to Al stress. In sorghum, it is reported that long Al exposure is needed to fully induce its Al tolerance (4–6 days), and this slow induction of Al resistance is due to the slow regulatory mechanism of Al-induced SbMATE-dependent high rates of root citrate exudation over several days [Citation45,Citation48]. However, as investigated previously [Citation29], compared with a sensitive cultivar of sweet sorghum, a tolerant cultivar showed significantly greater Al tolerance within 24 h of Al treatment. The present study also revealed that SbSTOP1 up-regulated the expression of SbGlu1 homolog in Arabidopsis and reduced callose accumulation in plants within 3 h or 6 h of Al treatment (). These results suggested that SbSTOP1 and SbGlu1-meditated modulation of callose deposition may play a role in the early mechanisms of Al tolerance in sweet sorghum.
In summary, the present study suggests that SbSTOP1 activates the transcription of a β-1,3-glucanase gene to reduce callose deposition under Al toxicity, which provides a novel pathway for Al tolerance in plants.
Author contributions
ZY designed the research; JG, SY, MZ, KG, YW performed the experiments; JG, SY and HY analyzed the data; JG wrote the manuscript; ZY revised the manuscript.
supplementary_material-final_submission.docx
Download MS Word (2.6 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary data for this article can be accessed here.
Additional information
Funding
References
- Uexküll HRV, Mutert E. Global extent, development and economic impact of acid soils. Plant Soil. 1995;171:1–15.
- Guo JH, Liu XJ, Zhang Y, et al. Significant acidification in major Chinese croplands. Science. 2010;327:1008–1010.
- Kochian LV, Piñeros MA, Hoekenga OA. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil. 2005;274:175–195.
- Kochian LV, Hoekenga OA, Piñeros MA. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annual Rev Plant Biol. 2004;55:459–493.
- Ma JF. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int Rev Cytol. 2007;264:225–252.
- Kochian LV, Piñeros MA, Liu J, et al. Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annu Rev Plant Biol. 2015;66:571–598.
- Iuchi S, Koyama H, Iuchi A, et al. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc Natl Acad Sci USA. 2007;104:9900–9905.
- Yamaji N, Huang CF, Nagao S, et al. A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell. 2009;21:3339–3349.
- Arenhart RA, Lima JC, Pedron M, et al. Involvement of ASR genes in aluminium tolerance mechanisms in rice. Plant Cell Environ. 2013;36:52–67.
- Ding ZJ, Yan JY, Xu XY, et al. WRKY46 functions as a transcriptional repressor of ALMT1, regulating aluminum-induced malate secretion in Arabidopsis. Plant J. 2013;76:825–835.
- Tokizawa M, Kobayashi Y, Saito T, et al. Sensitive to proton rhizotoxicity1, calmodulin binding transcription activator 2, and other transcription factors are involved in aluminum-activated malate transporter1 expression. Plant Physiol. 2015;167:991–1003.
- Li GZ, Wang ZQ, Yokosho K, et al. Transcription factor WRKY22 promotes aluminum tolerance via activation of OsFRDL4 expression and enhancement of citrate secretion in rice (Oryza sativa). New Phytol. 2018;219:149–162.
- Sawaki Y, Iuchi S, Kobayashi Y, et al. STOP1 regulates multiple genes that protect Arabidopsis from proton and aluminum toxicities. Plant Physiol. 2009;150:281–294.
- Hoekenga OA, Maron LG, Piñeros MA, et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci USA. 2006;103:9738–9743.
- Liu J, Magalhaes JV, Shaff J, et al. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009;57:389–399.
- Huang CF, Yamaji N, Mitani N, et al. A bacterial-type ABC transporter is involved in aluminum tolerance in rice. Plant Cell. 2009;21:655–667.
- Tsutsui T, Yamaji N, Ma JF. Identification of a cis-acting element of ART1, a C2H2-type zinc-finger transcription factor for aluminum tolerance in rice. Plant Physiol. 2011;156:925–931.
- Huang S, Gao J, You J, et al. Identification of STOP1-like proteins associated with aluminum tolerance in sweet sorghum (Sorghum bicolor L.). Front Plant Sci. 2018;9:258.
- Chen XY, Kim JY. Callose synthesis in higher plants. Plant Signal Behav. 2009;4:489–492.
- Horst WJ. uschel A-KP, Schmohl N. Induction of callose formation is a sensitive marker for genotypic aluminium sensitivity in maize. Plant Soil. 1997;192:23–30.
- Yang ZM, Sivaguru M, Horst WJ, et al. Aluminium tolerance is achieved by exudation of citric acid from roots of soybean (Glycine max). Physiol Plant. 2000;110:72–77.
- Hirano Y, Walthert L, Brunner I. Callose in root apices of European chestnut seedlings: A physiological indicator of aluminum stress. Tree Physiol. 2006;26:431–440.
- Sivaguru M, Fujiwara T, Samaj J, et al. Aluminum-induced 1-3-beta-D-glucan inhibits cell-to-cell trafficking of molecules through plasmodesmata. A new mechanism of aluminum toxicity in plants. Plant Physiol. 2000;124:991–1005.
- Sivaguru M, Horst WJ, Eticha D, et al. Aluminum inhibits apoplastic flow of high-molecular weight solutes in root apices of Zea mays L. J Plant Nutr Soil Sci. 2006;169:679–690.
- Zavaliev R, Ueki S, Epel BL, et al. Biology of callose (beta-1,3-glucan) turnover at plasmodesmata. Protoplasma. 2011;248:117–130.
- Cruz-Ortega R, Cushman JC, Ownby JD. cDNA clones encoding 1,3-beta- glucanase and a fimbrin-like cytoskeletal protein are induced by Al toxicity in wheat roots. Plant Physiol. 1997;114:1453–1460.
- Goodwin SB, Sutter TR. Microarray analysis of Arabidopsis genome response to aluminum stress. Biologia Plantarum. 2009;53:85–99.
- Tsutsui T, Yamaji N, Huang CF, et al. Comparative genome-wide transcriptional analysis of Al-responsive genes reveals novel Al tolerance mechanisms in rice. Plos one. 2012;7:e48197.
- Zhang H, Shi WL, You JF, et al. Transgenic Arabidopsis thaliana plants expressing a beta-1,3-glucanase from sweet sorghum (Sorghum bicolor L.) show reduced callose deposition and increased tolerance to aluminium toxicity. Plant Cell Environ. 2015;38:1178–1188.
- Ohyama Y, Ito H, Kobayashi Y, et al. Characterization of AtSTOP1 orthologous genes in tobacco and other plant species. Plant Physiol. 2013;162:1937–1946.
- Fan W, Lou HQ, Gong YL, et al. Characterization of an inducible C2H2-type zinc finger transcription factor VuSTOP1 in rice bean (Vigna umbellata) reveals differential regulation between low pH and aluminum tolerance mechanisms. New Phytol. 2015;208:456–468.
- Yang L, Mo W, Yu X, et al. Reconstituting Arabidopsis CRY2 signaling pathway in mammalian cells reveals regulation of transcription by direct binding of CRY2 to DNA. Cell Rep. 2018;24:585–593.e4.
- Gao J, Wang X, Zhang M, et al. Trp triad-dependent rapid photoreduction is not required for the function of Arabidopsis CRY1. Proc Natl Acad Sci USA. 2015;112:9135–9140.
- Liu H, Yu X, Li K, et al. Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science. 2008;322:1535–1539.
- Fang RX, Nagy F, Sivasubramaniam S, et al. Multiple cis regulatory elements for maximal expression of the cauliflower mosaic virus 35S promoter in transgenic plants. Plant Cell. 1989;1:141–150.
- Li H, Wang J, Wang J, et al. Structural mechanism of DNA recognition by the p202 HINa domain: insights into the inhibition of Aim2-mediated inflammatory signalling. Acta Cryst F. 2014;70:21–29.
- Wang K, Gao Y, Peng X, et al. Using FAM labeled DNA oligos to do RNA electrophoretic mobility shift assay. Mol Biol Rep. 2010;37:2871–2875.
- Li H, Wang ZX, Wu JW. Comparative purification and characterization of two HIN domains, hematopoietic interferon-inducible nuclear antigens with a 200-amino-acid repeat, in murine AIM2-like receptors. Biosci Biotechnol Biochem. 2013;77:2283–2287.
- Qin X, Huang S, Liu Y, et al. Overexpression of A RING finger ubiquitin ligase gene AtATRF1 enhances aluminium tolerance in Arabidopsis thaliana. J Plant Biol. 2017;60:66–74.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408.
- Köhle H, Jeblick W, Poten F, et al. Chitosan-elicited callose synthesis in soybean cells as a Ca2+-dependent process. Plant Physiol. 1985;77:544–551.
- Kobayashi Y, Ohyama Y, Kobayashi Y, et al. STOP2 activates transcription of several genes for Al- and low pH-tolerance that are regulated by STOP1 in Arabidopsis. Mol Plant. 2014;7:311–322.
- Sawaki Y, Kobayashi Y, Kihara-Doi T, et al. Identification of a STOP1-like protein in Eucalyptus that regulates transcription of Al tolerance genes. Plant Sci. 2014;223:8–15.
- Che J, Tsutsui T, Yokosho K, et al. Functional characterization of an aluminum (Al)-inducible transcription factor, ART2, revealed a different pathway for Al tolerance in rice. New Phytol. 2018;220:209–218.
- Magalhaes JV, Liu J, Guimarães CT, et al. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet. 2007;39:1156–1161.
- Daspute AA, Kobayashi Y, Panda SK, et al. Characterization of CcSTOP1; a C2H2-type transcription factor regulates Al tolerance gene in pigeonpea. Planta. 2018;247:201–214.
- Zhang G, Hoddinott J, Taylor GJ. Characterization of 1,3-β-D-glucan (callose) synthesis in roots of Triticum aestivum in response to aluminum toxicity. J Plant Physiol. 1994;144:229–234.
- Sivaguru M, Liu J, Kochian LV. Targeted expression of SbMATE in the root distal transition zone is responsible for sorghum aluminum resistance. Plant J. 2013;76:297–307.
- Wissemeier AH, Horst WJ. Effect of calcium supply on aluminium-induced callose formation, its distribution and persistence in roots of soybean (Glycine max (L.)Merr.). J Plant Physiol. 1995;145:470–476.
- Yang JL, Zhu XF, Peng YX, et al. Cell wall hemicellulose contributes significantly to aluminum adsorption and root growth in Arabidopsis. Plant Physiol. 2011;155:1885–1892.
- Llugany M, Massot N, Wissemeier AH, et al. Aluminium tolerance of maize cultivars as assessed by callose production and root elongation. J Plant Nutr Soil Sci. 1994;157:447–451.
- Maron LG, Kirst M, Mao C, et al. Transcriptional profiling of aluminum toxicity and tolerance responses in maize roots. New Phytol. 2008;179:116–128.
- Yang JL, Li YY, Zhang YJ, et al. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol. 2008;146:602–611.
