ABSTRACT
Madecassoside is a major pentacyclic triterpene saponin from Centella asiatica with multiple pharmaceutical activities. In this study, we focused on its Propionibacterium acnes related anti-inflammation and skin hydration activities, both of which play important roles in skin homeostasis and barrier function. Madecassoside significantly inhibited the pro-inflammatory cytokine IL-1β, TLR2 and nuclear translocation of NF-κB in P. acnes stimulated THP-1 human monocytic cells. In addition, madecasssoside exhibited significant effects on enhancement of skin hydration through increasing the key moisturizing contributors of aquaporin-3, loricrin and involucrin in HaCaT keratinocytes as well as hyaluronan (HA) secretion in human dermal fibroblasts. The upregulation of HA synthases (HAS1, HAS2, HAS3) and inhibition to ROS formation accounted for the increment of HA content. Together, the in vitro study implied the potential medical and cosmetic application of madecassoside in skin protection.
Graphical Abstract
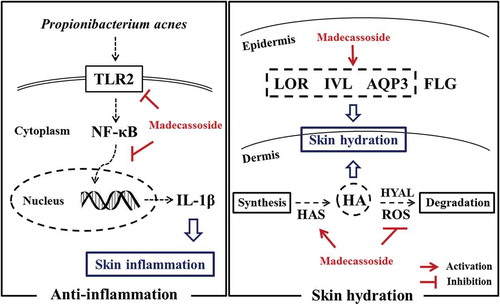
Propionibacterium acnes related anti-inflammation and skin hydration activities of madecassoside.
Skin functions as the first defense line from deleterious environmental invasion and excessive water loss [Citation1]. To keep skin homeostasis and aesthetically attractive, skincare products are widely used for anti-aging, hydration, anti-melanogenesis, etc. Among these, anti-inflammation and hydration are considered as the most crucial skin care step for skin overall homeostasis and barrier function, especially for the young people.
The integrity of skin is a precondition for skin protection. However, acne inflammation, a chronic dermatologic disorder prevalent in teenagers and young adults, results in inflammatory lesions of variable severity and thus is the biggest threat to skin homeostasis and attractive appearance [Citation2,Citation3]. Propionibacterium acnes (P. acnes) proliferation in the pilosebaceous unit plays a critical role in eliciting inflammatory response, in addition to two other actors of the pathophysiology of acne (hyperseborrhoea and abnormal follicular keratinization) [Citation4]. And recent studies have revealed that only specific P. acnes strains are associated with acne [Citation5]. The underlying inflammatory mechanism is largely due to the activation of Toll-like receptor (TLR)-2 on innate immune cells to secrete pro-inflammatory cytokines like interleukin (IL)-1β, tumor necrosis factor (TNF)-α and interleukin (IL)-8 via induction of signaling pathways including nuclear factor (NF)-κB [Citation3,Citation6].
Besides, as extrinsic and intrinsic factors like dry environment, surfactant-induced damages and aging would result in skin dryness, cell dysfunction and poor barrier function, skin hydration is regarded as the basic skin care step for skin homeostasis [Citation1]. Skin hydration depends on many proteins and humectants in epidermis and dermis. In epidermis, aquaporin-3 (AQP3), filaggrin (FLG), loricrin (LOR) and involucrin (IVL) are the four most studied proteins expressed by keratinocytes and play important roles in hydration as their moisturizing and/or barrier enhancing activities. All these four proteins have been demonstrated to change sensitively in HaCaT, an immortal cell line with similar differentiation properties as human keratinocytes [Citation7,Citation8]. And in dermis, hyaluronan (HA), mainly synthesized by dermal fibroblasts, is considered as a key humectant in skin hydration for its outstanding water capacity [Citation9,Citation10]. HA synthesis is performed by HA synthases (HAS) [Citation9]. While HA degradation is carried out by hyaluronidases (HYAL) and non-enzymatic reactions including reactive oxygen species (ROS) [Citation11,Citation12].
Recently, an increasing number of plant-derived agents have been included in formulations of skincare products [Citation13]. Triterpenes, constituting the majority of the components of many traditional medicinal plants, are among a class of compounds with multiple biological activities on skin cells including anti-inflammation, hydration and anti-aging [Citation14,Citation15]. Madecassoside ()) is one of the most important pentacyclic triterpene saponins of Centella asiatica, whose extract is widely used in medicines, foods and cosmetics. Centella asiatica extract has been demonstrated moisturizing and anti-inflammatory effects in vivo [Citation16]. However, the active components of the extract and the underlying mechanism were poorly understood. Our study was conducted under the hypothesis that madecassoside was one of the contributing agents of the hydration and anti-inflammation effect of Centella asiatica extract. This work aimed to explore the P. acnes related anti-inflammation and skin hydration effects of madecassoside in vitro. Thus, the present study might cast new light on the medical and cosmetic application of madecassoside in skin care.
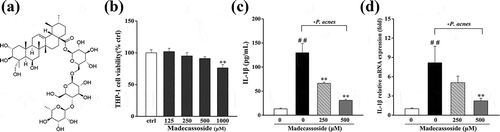
Figure 1. Effects of madecassoside on THP-1 cell viability and IL-1β in P. acnes stimulated THP-1 cells. (a) Chemical structure of madecassoside. (b) Effect of madecassoside on THP-1 cell viability was monitored by MTT. (c) Production of IL-1β was assayed by a Human IL-1β ELISA kit. (d) The mRNA expression of IL-1β was measured by qRT-PCR. Each value represents the mean ± SD of triplicate experiments. (##) P < 0.01 compared with control group; (**) P < 0.01 compared with only P. acnes stimulated group.
Materials and methods
Materials
Madecassoside (purity> 98%) was provided by Shanghai Inoherb Cosmetics Co., Ltd. (Shanghai, China). BCA Protein Assay Kit and MTT assay kit was purchased from Biotech well (Shanghai, China). HAS2, HAS3 and IVL antibodies were purchased from ABclonal (Wuhan, China). HAS1 and β-actin antibodies were purchased from Proteintech (Wuhan, China). NF-κB p65 antibody was got from Cell Signaling Technology (Boston, USA). GAPDH and Lamin B antibodies were purchased from Biotech well (Shanghai, China) and Boster (Wuhan, China) respectively. HRP-conjugated secondary antibodies of Goat anti-rabbit IgG and Goat anti-mouse IgG were purchased from Signalway Antibody (Shanghai, China).
Cell culture and bacteria preparation
Human dermal fibroblasts (HDF) were purchased from ScienCell Research Laboratories (Shanghai, China). HaCaT and human monocytic cell line THP-1 were purchased from China Center for Type Culture Collection (Shanghai, China). HDF and HaCaT were cultured in DMEM medium. THP-1 cells were cultured in RPMI-1640 medium. Medium was all added with 10% (v/v) Fetal bovine serum (FBS) (ExCell Bio, Taicang, China) and cells were all cultured in a carbon dioxide incubator with constant temperature (5% CO2, 37°C). P. acnes (ATCC6919, Xiangfu biotech, Shanghai, China) was cultured under anaerobic conditions in Cooked Meat Medium. Then it was harvested and resuspended in FBS-free RPMI-1640 medium before its immediate application for stimulating THP-1 cells (P. acnes: THP-1 = 100:1) [Citation17].
Cell viability assay
HDF or HaCaT cells were digested and adjusted to about 6000 cells/well, and then the cell suspension was added in 96-well plates. After 12 hours, the medium were replaced by DMEM of various concentrations of madecassoside. THP-1 cells (6000 cells/well) were similarly seeded and treated with madecassoside. The cell viability was quantified by MTT assays. Briefly, cells were first treated with 0.5 mg/mL MTT for 4 hours. Then the supernatant was carefully removed and replaced by 150 μL DMSO to dissolve the formazan. The absorbance at 570 nm and 630 nm were respectively detected as detection and reference wavelengths to determine relative cell viability.
ROS assay
HDF cells (6-well plates, 3 × 105 cells/well) were seeded and pretreated with madecassoside for 24 h and 200 μM H2O2 for 2 more hours [Citation12] to assay the level of ROS by a ROS assay kit (Beyotime, Shanghai). And the data were confirmed by protein contents.
Enzyme linked immunosorbent assay (ELISA)
THP-1 cells seeded in 96-well plates (2 × 104 cells/well) and 6-well plates (4 × 105 cells/well) were treated with madecassoside for 4 h, and then stimulated by P. acnes for 24 h. HDF cells (96-well plates, 6000 cells/well) and HaCaT cells (6-well plates, 3 × 105 cells/well) were treated with medium containing various concentration of madecassoside for 24 hours. A Human IL-1β ELISA kit (Biotech well, Shanghai, China) and a Human HA ELISA kit (ExCell Bio, Taicang, China) were used to analyze IL-1β and HA contents of the cell-free supernatants collected from the 96-well plates. A TLR2 ELISA kit (Biotech well, Shanghai, China), a Human AQP3 ELISA kit and a Human LOR ELISA kit (ExCell Bio, Taicang, China) were respectively used to analyze TLR2, AQP3 and LOR protein levels of the total protein extracted from the 6-well plates. And the data were confirmed by protein contents. N-acetylglucosamine (GlcNAc), a substrate for HA biosynthesis and demonstrated to promote HA production in fibroblast, was used as a positive control [Citation18]. Retinoic acid and CaCl2 were respectively used as positive controls in AQP3 and LOR analysis [Citation19,Citation20].
RNA isolation and quantitative real time polymerase chain reaction (qRT-PCR)
Total RNA of HDF, HaCaT and THP-1 cells (6-well plates, 3 × 105 cells/well) was isolated using RNA extraction kit (KeyGEN BioTECH, Nanjing, China). Next, cDNA was synthesized from total RNA and qRT-PCR was performed by PrimeScript RT reagent kit and SYBR® Premix Ex Taq™ II with gDNA Eraser (Takara, Beijing, China) respectively under the manufacture’s instruction. The sequences of primers used for qRT-PCR in HDF, HaCaT and THP-1 cells were as follows: GAPDH, forward: 5ʹ-TCC ACTGGCGTCTTCACC-3ʹ, reverse: 5ʹ-GGCAGAGATGATGACCCTTTT-3ʹ; HAS1, forward: 5ʹ-GCGATACTGGGTAGCCTTCA-3ʹ, reverse: 5ʹ-GGTTGTACCAGGCCTCAAGA-3ʹ; HAS2, forward: 5ʹ-CCTCATCATCCAAAGCCTGT-3ʹ, reverse: 5ʹ-AAACAGTTGCCCTTTGCATC-3ʹ; HAS3, forward: 5ʹ-GTCATGTACACGGCCTTCAA-3ʹ, reverse: 5ʹ-CCTACTTGGGGATCCTCCTC-3ʹ; HYAL1, forward: 5ʹ-GTGCTGCCCTATGTCCAGAT-3ʹ, reverse: 5ʹ-ATTTTCCCAGCTCACCCAGA-3ʹ; HYAL2, forward: 5ʹ-TCTACCATTGGCGAGAGTG-3ʹ, reverse: 5ʹ-AGCAGCCGTGTCAGGTAAT-3ʹ; HYAL3, forward: 5ʹ-CCTCCAGTGCCCTCTTCC-3ʹ, reverse: 5ʹ-CTGTCCCAGGATGACCTTGT-3ʹ; AQP3, forward: 5ʹ-CATCTACACCCTGGCACAGA-3ʹ, reverse: 5ʹ-GGCTGTGCCTATGAACTGGT-3ʹ; LOR, forward: 5ʹ-CATGATGCTACCCGAGGTTT-3ʹ, reverse: 5ʹ-ACTGGGGTTGGGAGGTAGTT-3ʹ; FLG, forward: 5ʹ-GTTACAATTCCAATCCTGTTGTTTTC-3ʹ, reverse: 5ʹ-CGTTGCATAATACCTTGGATGATC-3ʹ; IVL, forward: 5ʹ-GTGGGGGAGAGAGGGAATTA-3ʹ, reverse: 5ʹ-CTCACCTGAGGTTGGGATTG-3ʹ; β-actin, forward: 5ʹ-CGTGGACATCCGCAAAGA-3ʹ, reverse: 5ʹ-GAAGGTGGACAGCGAGGC-3ʹ; IL-1β, forward: 5ʹ-AAGCTGAGGAAGATGCTG-3ʹ, reverse: 5ʹ-ATCTACACTCTCCAGCTG-3ʹ; TLR2, forward: 5ʹ-CAGGAGCTCTTAGTGACCAAGTGAA-3ʹ, reverse: 5ʹ-CACAAAGTATGTGGCATTGTCCAG-3ʹ. The parameter of qRT-PCR was conducted as a previous research [Citation21]. Expression levels were normalized to those of GAPDH or β-actin mRNA levels.
Western blot analysis
After treated with madecassoside for 24 hours, the cells (6-well plates, 4 × 105 cells/well) were lysed to collect the total protein. THP-1 cells were treated with madecassoside for 4 hours and stimulated by P. acnes for 1 hour before using a nuclear protein and cytoplasmic protein extraction kit (KeyGEN BioTECH, Nanjing, China) to get nuclear and cytoplasmic proteins. Protein samples (20 μg) were separated by SDS-PAGE before transferred to PVDF membranes (Millipore, Bedford, MA, USA). After blocking with 5% BSA for 2 hours, HAS1, HAS2, HAS3, IVL, NF-κB p65 (1:1000), Lamin B (1:200), GAPDH (1:2000) and β-actin (1:6000) were incubated with primary antibodies overnight and HRP-conjugated antibodies for 2 hours. The immune complexes of antibody were visualized with an ECL system (Biotech well, Shanghai, China). Band intensity was quantified by Smart View Bio-electrophoresis Image Analysis System (Furi, Shanghai, China).
Immunofluorescence assay
THP-1 cells (6-well plates, 4 × 105 cells/well), either treated or untreated with madecassoside, stimulated or unstimulated with P. acnes, were harvested and rinsed. Next, cells were treated as previous described [Citation21] and then slides were sealed by anti-fluorescence mounting medium and observed with a fluorescent microscope.
Statistical analysis
All data were presented as the mean ± standard errors. One-way analysis of variance (ANOVA) and LSD were employed to analyze the significant differences between groups with IBM SPSS V22.0. P < 0.05 was considered statistically significant.
Results
Effects of madecassoside on IL-1β in P. acnes-stimulated THP-1 cells
The level of IL-1β, a representative pro-inflammatory cytokines and possible therapeutic targets in acne [Citation3], was analyzed by ELISA in P. acnes induced THP-1 cells. Madecassoside (up to 500 μM) exhibited slight effect on the viability of THP-1 cells (> 90%; )). Similar to our previous research [Citation6], secretion of IL-1β was low in untreated groups while drastically increased in groups incubated with live P. acnes (P. acnes: THP-1 = 100:1). When madecassoside (250 μM and 500 μM) was added in the system of cocultivation, the release of IL-1β was significantly inhibited ()). We further analyzed the mRNA levels of IL-1β by qRT-PCR. As shown in ), madecassoside (500 μM) significantly inhibited the transcription of IL-1β in P.acnes-stimulated THP-1 cells.
Effects of madecassoside on TLR2 in P. acnes-stimulated THP-1 cells
TLR2 signaling is a target for suppressing inflammatory cytokine response inflammatory conditions and has been regarded to lead to a partial induction of IL-1β [Citation3]. Here we studied the effect of madecassoside on TLR2 signaling by qRT-PCR and ELISA. The data (,b)) revealed that TLR2 transcription and protein levels were both significantly increased after P. acnes stimulation, while markedly decreased after the treatment of madecassoside at concentration of 500 μM.
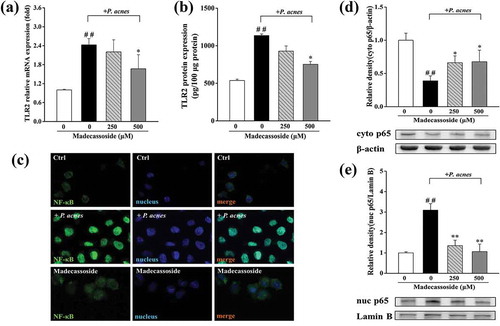
Figure 2. Effects of madecassoside on TLR2 and the nuclear translocation of NF-κB in P. acnes-stimulated THP-1 cells. (a) The mRNA expression of TLR2 was measured by qRT-PCR. (b) The protein expression of TLR2 was measured by ELISA. The translocation of NF-κB was detected by immunofluorescence (c) and western blot (d, e). The control group was incubated without madecassoside and bacteria. Each value represents the mean ± SD of triplicate experiments. (##) P < 0.01 compared with control group; (*) P < 0.05 and (**) P < 0.01compared with only P. acnes stimulated group.
Effects of madecassoside on NF-κB signaling pathway in P. acnes-stimulated THP-1 cells
An immunofluorescence assay and western blot analysis were used to figure out the effects of madecassoside on the NF-κB translocation. After incubation with P. acnes, NF-κB translocated from the cytoplasm to the nucleus. And the fluorescence intensity increased, indicating that the level of NF-κB also increased ()). Compared to that in the stimulated group, the P. acnes-induced translocation of NF-κB was markedly suppressed under the pretreatment of 250 and 500 μM madecassoside for 4 hours (,e)).
Effects of madecassoside on LOR, IVL, FLG and AQP3 in HaCaT
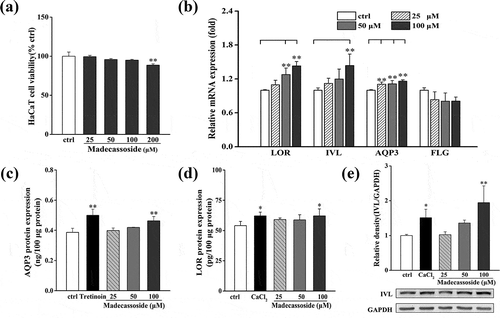
Madecassoside at a dosage of 0–100 μM exhibited slight effect on the viability of HaCaT (> 90%; )). Quantified RT-PCR and ELISA were applied to elucidate whether madecassoside affected moisturizing contributors (LOR, IVL, AQP3 and FLG) of epidermis. Compared with the untreated groups, the mRNA expression of LOR, IVL and AQP3 were significantly upregulated in a dose-dependent manner ()) while FLG insignificantly changed. To confirm the results, following detections of protein expression of AQP3, LOR and IVL were conducted and significant promotion was observed in the group treated with 100 μM madecassoside (-e)).
Figure 3. Effects of madecassoside on the HaCaT cell viability and AQP3, FLG, LOR, IVL expression in HaCaT. (a) Effect of madecassoside on HaCaT cell viability was monitored by MTT. (b) Effects of madecassoside on mRNA expression of AQP3, FLG, LOR and IVL were analyzed by qRT-PCR. (c, d) Effects of madecassoside on protein expression of AQP3 and LOR of HaCaT cells were measured by ELISA. (e) Effect of madecassoside on protein expression of IVL of HaCaT cells was measured by western blot. Each value represents the mean ± SD of triplicate experiments. (*) P < 0.05 and (**) P < 0.01 as compared with control group.
Effects of madecassoside on HA content in HDF
HDF cells treated with madecassoside at a dosage of 0–50 μM exhibited slight effect on cell viability (> 90%; )). ELISA was adopted to detect the effect of madecassoside on the content of HA. Compared with the untreated group, hyaluronan produced by madecassoside treated HDF was upregulated in a dose-dependent manner ()). HDF treated with 50 μM madecassoside produced 33.37 ± 2.20 ng/mL HA, a bit higher than the group treated with GlcNAc (32.66 ± 1.91 ng/mL).
Figure 4. Effect of madecassoside on the HDF cell viability and HA content. (a) Effect of madecassoside on HDF cell viability was monitored by MTT. (b) HA content was measured by ELISA. (c) Effects of madecassoside on mRNA expression of HA related enzymes were analyzed by qRT-PCR. (d) HAS1, HAS2 and HAS3 protein levels were analyzed by western blot respectively. (e) The ROS changes were monitored by using ROS assay kit and the result was confirmed by protein contents. Each value represents the mean ± SD of triplicate experiments. (*) P < 0.05 and (**) P < 0.01 as compared with control group; (##) P < 0.01 compared with only H2O2 stimulated group.
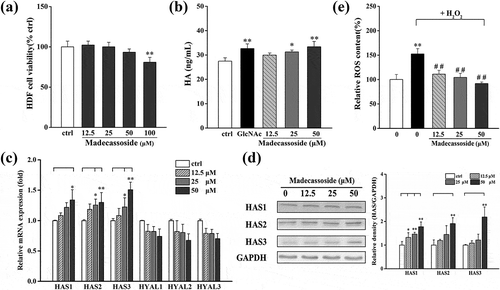
Effects of madecassoside on HA synthesis and degradation in HDF
Then the mRNA expression of some HA related genes (HA synthases and hyaluronidase) and ROS, a nonenzymatic factors, were closely analyzed to elucidate the mechanism of HA upregulation. The results ()) showed that three HA synthases (HAS1, HAS2, HAS3) were significantly upregulated with a dose-dependent pattern while hyaluronidases (HYAL1, HYAL2, HYAL3) exhibited no significant changes in mRNA expression. Further study on HAS protein expression ()) was basically in accordance with the mRNA expression. But a marked suppression with a dose-dependent pattern on H2O2-stimulated ROS expression ()) was observed.
Discussion
Madecassoside is one of the most studied terpenoids saponin in Centella asiatica with multiple biological activities in different types of cells [Citation22–Citation25]. And recent literature has highlighted its skin beneficial activities including wound healing, UV induced inflammation, antioxidation, etc [Citation23,Citation26,Citation27]. Our in vitro study exhibited the skin protective effect (anti-inflammation and hydration) of madecassoside and thereby madecassoside might be a contribution component of moisturizing and anti-inflammatory properties of Centella asiatica demonstrated by a previous in vivo study [Citation16].
Acne inflammation is the biggest threat to skin integrity for the young people and adverse to skin homeostasis [Citation2]. Some other bacterial strains (e.g., Staphylococcus aureus, Staphylococcus epidermidis) could also partly exacerbate acne inflammatory event, but are far less significant than P. acnes [Citation28]. Here we have certified that madecassoside could significantly inhibit the production of IL-1β released by P. acnes stimulated THP-1 cells, which suggested a suppressed P. acnes-induced inflammatory response. As a wide variety of studies have unravelled the roles of TLR2 and NF-κB in P. acnes stimulated inflammation [Citation3,Citation6], we assessed whether madecassoside could affect TLR2 and NF-κB in P. acnes-stimulated THP-1 cells. The decreased TLR2 expression and the inhibited activation of the NF-κB signaling pathway were observed. NF-κB directly bound to the IL-1β gene promoter and activated its transcription [Citation29], so the inhibited translocation of NF-κB might also account for the downregulation of IL-1β mRNA expression. And these indicated that madecassoside could suppress inflammation activities of P. acnes via a mechanism of diminished NF-κB activation and TLR2-mediated signaling pathways, similar to a previous study on Rosmarinus officinalis extract [Citation30].
Hydration is also vital in skin care as water endows skin with continuous deformation, suppleness and overall homeostasis. Skin moisturization relies on many hydration-related components of epidermis and dermis. In this study, the marked upregulation expression of AQP3 was observed in madecassoside treated HaCaT, which was quite similar to the results of a study on A. houstonianum ethanolnolic extract [Citation7]. Induction of AQP3 expression is concluded as a potential therapy for dry skin conditions [Citation31] and our results thus suggested increased skin hydration effect as AQP3 facilitates water and glycerol transport. In addition, a marked increased expression of two major cornified envelope components (LOR and IVL) suggested an enhanced barrier function and thus less skin moisture loss [Citation8]. The mRNA expression of filaggrin was not increased and it might disclose an inactive effect of madecassoside on NMF increment. Thereby, the increased expression of AQP3 and two envelope proteins might be concluded as three epidermal hydration targets of madecassoside.
Another well-known water binding substance in skin is HA. HA regulates water balance and osmotic pressure, promotes cell motility and proliferation, participates in wound healing, etc [Citation9]. Our data suggested that madecassoside could increase the production of HA in HDF, thereby contributing to the hydration of dermis layer due to the increment of HA-bound water. As skin hydration is considered to be the key step of anti-aging care, our findings might also partially account for in vivo results of the improvement of madecassoside in skin aging state [Citation32]. Many researches on hydration or aging included the analysis of HAS, HYAL and ROS to elucidate changes in HA level [Citation8,Citation12,Citation33]. And the upregulation of three HA synthases and inhibition to ROS formation observed could partially account for the increment of HA content. The findings were also in accord with a previous study, which showed it was HAS instead of HYAL that play a major role in hyaluronan production by human fibroblasts [Citation34]. As it was reported that transforming growth factor-β1 (TGF-β1), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), and platelet-derived growth factor-BB (PDGF-BB) could upregulate HAS genes expression, further studies are needed to explain the mechanism underlying madecassoside-induced changes in HAS1, HAS2 and HAS3 mRNA expression [Citation35]. Owing to the unique wound healing properties of madecassoside [Citation23] and the important role that HA played in wound healing [Citation9], there might be some connections between our findings about HA and wound healing activities of madecassoside.
In conclusion, this study exhibited that madecassoside could enhance skin hydration through upregulating key moisturizing contributors (AQP3, LOR, IVL and HA) in skin and inhibiting P. acne-related inflammation to keep skin healthy. The in vitro findings of this study supported further exploration of madecassoside as ingredient of moisturizers with protective effect against acne inflammation.
Author contributions
Xueqing Shen, Yanhua Lu, Zhi Lu and Dan Liu conceived and designed the study. Xueqing Shen, Miaomiao Guo and Haiyuan Yu performed the experiments. Xueqing Shen wrote and edited the manuscript.
Disclosure statement
The authors have no conflicts of interest to declare.
Additional information
Funding
References
- Bonté F. Skin moisturization mechanisms: new data. Ann Pharm Fr. 2011 May;69(3):135–141.
- Rocha MA, Bagatin E. Skin barrier and microbiome in acne. Arch Dermatol Res. 2018 Apr;310(3):181–185.
- Qin M, Pirouz A, Kim MH, et al. Propionibacterium acnes Induces IL-1beta secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014 Feb;134(2):381–388.
- Dreno B. What is new in the pathophysiology of acne, an overview. J Eur Acad Dermatol Venereol. 2017 Sep;31(Suppl 5):8–12.
- Dréno B, Pécastaings S, Corvec S, et al. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. J Eur Acad Dermatol Venereol. 2018;32:5–14.
- Huang WC, Tsai TH, Huang CJ, et al. Inhibitory effects of wild bitter melon leaf extract on Propionibacterium acnes-induced skin inflammation in mice and cytokine production in vitro. Food Funct. 2015 Aug;6(8):2550–2560.
- Shin SY, Lee DH, Gil H-N, et al. Agerarin, identified from Ageratum houstonianum, stimulates circadian CLOCK-mediated aquaporin-3 gene expression in HaCaT keratinocytes. Sci. Rep.-UK. 2017;7(1):11175.
- Lee S, Kim J-E, Suk S, et al. A fermented barley and soybean formula enhances skin hydration. J Clin Biochem Nutr. 2015;57(2):156–163.
- Kavasi RM, Berdiaki A, Spyridaki I, et al. HA metabolism in skin homeostasis and inflammatory disease. Food Chem Toxicol. 2017;101:128–138.
- Stern R, Maibach HI. Hyaluronan in skin: aspects of aging and its pharmacologic modulation. Clin Dermatol. 2008 Mar-Apr;26(2):106–122.
- Stern R, Kogan G, Jedrzejas MJ, et al. The many ways to cleave hyaluronan. Biotechnol Adv. 2007 Nov-Dec;25(6):537–557.
- Wen SY, Chen JY, Weng YS, et al. Galangin suppresses H2O2-induced aging in human dermal fibroblasts. Environ Toxicol. 2017 Dec;32(12):2419–2427.
- Draelos ZD. The science behind skin care: moisturizers. J Cosmet Dermatol. 2018;17(2):138–144.
- Farwick M, Kohler T, Schild J, et al. Pentacyclic triterpenes from terminalia arjuna show multiple benefits on aged and dry skin. Skin Pharmacol Physiol. 2014;27(2):71–81.
- Tan H, Sonam T, Shimizu K. The potential of triterpenoids from loquat leaves (Eriobotrya japonica) for prevention and treatment of skin disorder. Int J Mol Sci. 2017 May;18(5):1030.
- Ratz-Łyko A, Jaak PYTKOWSKA. Moisturizing and antiinflammatory properties of cosmetic formulations containing centella asiatica extract. Indian J Pharm Sci. 2016;78s(1):27–33.
- Guo M, Lu Y, Yang J, et al. Inhibitory effects of Schisandra chinensis extract on acne-related inflammation and UVB-induced photoageing. Pharm Biol. 2016;54(12):2987–2994.
- Polubinska A, Cwalinski J, Baum E, et al. N-Acetylglucosamine modulates function of the skin fibroblasts. Int J Cosmet Sci. 2013 Oct;35(5):472–476.
- Bellemere G, Von Stetten O, Oddos T. Retinoic acid increases aquaporin 3 expression in normal human skin. J Invest Dermatol. 2008 Mar;128(3):542–548.
- Luo X, Jin R, Wang F, et al. Interleukin-15 inhibits the expression of differentiation markers induced by Ca(2+) in keratinocytes. Exp Dermatol. 2016 Jul;25(7):544–547.
- Guo M, An F, Wei X, et al. Comparative Effects of Schisandrin A, B, and C on Acne-Related Inflammation. Inflammation. 2017 Dec;40(6):2163–2172.
- Cao W, Li XQ, Zhang XN, et al. Madecassoside suppresses LPS-induced TNF-alpha production in cardiomyocytes through inhibition of ERK, p38, and NF-kappaB activity. Int Immunopharmacol. 2010 Jul;10(7):723–729.
- Hou Q, Li M, Lu YH, et al. Burn wound healing properties of asiaticoside and madecassoside. Exp Ther Med. 2016 Sep;12(3):1269–1274.
- Bian D, Liu M, Ying L, et al. Madecassoside, a triterpenoid saponin isolated from Centella asiatica herbs, protects endothelial cells against oxidative stress. J Biochem Mol Toxicol. 2012;26(10):399–406.
- Nalinratana N, Meksuriyen D, Ongpipattanakul B. Differences in neuritogenic activity and signaling activation of madecassoside, asiaticoside, and their aglycones in neuro-2a cells. Planta Med. 2018 Nov;84(16):1165–1173.
- Jung E, Lee JA, Shin S, et al. Madecassoside inhibits melanin synthesis by blocking ultraviolet-induced inflammation. Molecules. 2013 Dec;18(12):15724–15736.
- Song J, Xu H, Lu Q, et al. Madecassoside suppresses migration of fibroblasts from keloids: involvement of p38 kinase and PI3K signaling pathways. Burns. 2012 Aug;38(5):677–684.
- Poomanee W, Chaiyana W, Mueller M, et al. In-vitro investigation of anti-acne properties of Mangifera indica L. kernel extract and its mechanism of action against Propionibacterium acnes. Anaerobe. 2018;52:64–74.
- Lawrence T. The nuclear factor NF- κB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651.
- Tsai TH, Chuang LT, Lien TJ, et al. Rosmarinus officinalis extract suppresses Propionibacterium acnes-induced inflammatory responses. J Med Food. 2013 Apr;16(4):324–333.
- Choudhary V, Olala LO, Kagha K, et al. Regulation of the glycerol transporter, aquaporin-3, by histone deacetylase-3 and p53 in keratinocytes. J Invest Dermatol. 2017 Sep;137(9):1935–1944.
- Haftek M, Mac-Mary S, Le Bitoux MA, et al. Clinical, biometric and structural evaluation of the long-term effects of a topical treatment with ascorbic acid and madecassoside in photoaged human skin. Exp Dermatol. 2008 Nov;17(11):946–952.
- Li WH, Wong HK, Serrano J, et al. Topical stabilized retinol treatment induces the expression of HAS genes and HA production in human skin in vitro and in vivo. Arch Dermatol Res. 2017 May;309(4):275–283.
- Terazawa S, Nakajima H, Tobita K, et al. The decreased secretion of hyaluronan by older human fibroblasts under physiological conditions is mainly associated with the down-regulated expression of hyaluronan synthases but not with the expression levels of hyaluronidases. Cytotechnology. 2015 Aug;67(4):609–620.
- Nagaoka A, Yoshida H, Nakamura S, et al. Regulation of hyaluronan (HA) metabolism mediated by HYBID (Hyaluronan-binding protein involved in HA depolymerization, KIAA1199) and HA synthases in growth factor-stimulated fibroblasts. J Biol Chem. 2015 Dec 25;290(52):30910–30923.
