ABSTRACT
Doxorubicin (Dox) is an anthracycline antibiotic that has been used to treat different cancers. Dox-induced cardiotoxicity is common in clinical practice, while its mechanism is unknown. It has been proved that lncRNA FOXC2-AS1 may promote doxorubicin resistance and WNT1-inducible signaling pathway protein-1 (WISP1) blocks doxorubicin-induced cardiomyocyte death. Our study aimed to investigate the involvement of lncRNA FOXC2-AS1 and WISP1 in doxorubicin-induced cardiotoxicity and to explore their interactions. In our study we observed that FOXC2-AS1 and WISP1 mRNA were downregulated in heart tissues of mice with Dox-induced cardiotoxicity. FOXC2-AS1 and WISP1 mRNA expression were positively correlated in mice with Dox-induced cardiotoxicity but not in healthy mice. Overexpression of FOXC2-AS1 promoted to viability of mice cardiomyocytes under Dox treatment and also increased the expression level of WISP1. In contrast, WISP1 overexpression showed no significant effect on FOXC2-AS1. We therefore conclude that lncRNA FOXC2-AS1 may upregulate WISP1 to protect cardiomyocytes from doxorubicin-induced cardiotoxicity.
Graphical Abstract
lncRNA FOXC2-AS1 may upregulate WISP1 to protect cardiomyocytes from doxorubicin-induced cardiotoxicity.
KEYWORDS:
Doxorubicin (Dox) is an anthracycline antibiotic that has been widely used to treat different types of cancers [Citation1,Citation2]. However, the clinical application of doxorubicin (Dox) in the treatment of cancers is challenged by its cardiotoxicity and how to reduce cardiotoxicity during the use of doxorubicin has attracted more and more attention [Citation3]. It has been clearly proved that DOX induces aberrant calcium signaling, cardiomyocyte death, progressive cardiomyopathy, myocardial contractile dysfunction and even heart failure [Citation4]. DOX affect cardiac function mainly by activating oxidative stress-dependent and independent signaling, as well as intrinsic and extrinsic pro-apoptotic signaling in cardiomyocytes to induce cell death [Citation5]. Therefore, inhibition of the death of cardiomyocytes under treatment of Dox is considered to be a therapeutic target for Dox-induced cardiotoxicity.
Clinical studies have proved that the development of Dox resistance during cancer treatment is closely correlated with altered expression of certain long non-coding RNAs (lncRNAs) [Citation6,Citation7], which is a subgroup of non-coding RNAs with pivotal roles in both physiological processes and pathological changes [Citation8]. LncRNA FOXC2-AS1 recently has been proved to be upregulated in doxorubicin resistant osteosarcoma cell lines [Citation9], indicating its possible involvement in the development of doxorubicin resistance. It has been reported that WNT1-inducible signaling pathway protein-1 (WISP1) blocks doxorubicin-induced cardiomyocyte death by activating diverse cell survival pathways [Citation10]. In our study we observed that lncRNA FOXC2-AS1 may protect cardiomyocytes from doxorubicin-induced cardiotoxicity through activation of WISP1.
Material and methods
Mice model
A total of 40 C57BL/6 male mice (8 weeks old) were purchased from SLAC (Shanghai Laboratory Animal Center). Among those mice, 20 were randomly selected and injected with DOX at a dose of 2.5 mg/kg every 2 days for 6 times. The other 20 mice were injected with the equivalent volume of sterile saline solution. Mice were raised in separated cage with free access to food and water. After injection, altered left ventricular end-diastolic pressure (LVEDP), left ventricular systolic pressure (LVSP), and ± dp/dt max parameters were observed in model mice compared with control mice, indicating the impaired cardiac function. Mice were sacrificed under anesthesia to collect heart tissues. Heart tissues were stored in liquid nitrogen before use. This study has been approved by the animal ethics committee of the first affiliated hospital of Zhengzhou university hospital.
Cell culture and transfection
Cardiomyocytes were collected from the other 8 healthy male adult (8 weeks) C57Bl/6 mice (SLAC) and processes using the same method described in a previous study [Citation10]. Cardiomyocytes were cultured under resting conditions in a humidified incubator for at least 24 h before experimentation. Full FOXC2-AS1 cDNA surrounded by EcoRI cutting sites was amplified by PCR. This DNA fragment was inserted into EcoRI linearized pIRSE2 vector (Clontech, Palo Alto, CA, USA) to construct FOXC2-AS1 expression vector. Lipofectamine 2000 was used to transfect 10 nM vector into 5 × 105 cells. Cells were culture for 12 h before subsequent experiments. Cardiomyocytes without transfection were control cells. Cells with empty pIRSE2 vector transfection were negative control cells.
RNA extraction and qRT-PCR
TRIzol® reagent kit (Thermo Fisher Scientific., Inc.) was used to extract total RNA from heart tissues and in vitro cultured cardiomyocytes with all operations performed in strict accordance with manufacturer’s instructions. cDNA was synthesized using SuperScript III reverse transcriptase kit (Thermo Fisher Scientific, Inc.) through following thermal conditions: 52°C for 25 min and 80°C for 10 min. SYBR® Green Real-Time PCR Master Mix (Thermo Fisher Scientific, Inc.) was used to carried out all PCR reactions through following thermal conditions: 95°C for 1 min 15s, followed by 40 cycles of 95°C for 32 s and 59°C for 27 s. Primers used in PCR reactions were: 5′-TTCATCGGCTGCGTATTCG-3′ (forward) and 5′-TTGCCTTCTAGTCGCCTCC-3′ (reverse) for FOXC2-AS1; 5′-CTGGACAGAAAAGGGCATGT-3′ (forward) and 5′- AGGAAGGAGGGGAAATCTCA −3′(reverse) for WISP1; 5ʹ-CCCACTCCTCCACCTTTGAC-3ʹ (forward) and 5ʹ-ATGAGGTCCACCACCCTGTT-3ʹ (reverse) for human GAPDH. Data normalization was performed using 2−∆∆Ct method.
MTT assay
After transfection, expression of FOXC2-AS1 was detected by qRT-PCR and an overexpression rate above 200% was reached. Cardiomyocytes were then collected and cell suspensions with a cell density of 4 × 104 cells/mL were prepared. Each well of a 96-well plate was filled with 4 × 103 cells in 0.1 mL cell suspension. Dox was added into each well to a final concentration of 0, 1, 10 and 100 ng/mL. Cells were cultured in an incubator (37°C, 5% CO2) for 6 h, followed by addition of 10 ul MTT into each well. After incubation for another 4 h at 37°C, formazan was dissolved by adding dimethyl sulfoxide. OD values (570 nm) were measured using a microtiter plate reader.
Western-blot
RIPA solution (Thermo Fisher Scientific) was used for total protein extraction. Protein conceration was measued by BCA assay. After denaturing at 95°C for 5 min, protein samples were subjected to SDS-PAGE gel (10%) electrophoresis with 20 µg protein per lane. After gel transfer to PVDF membranes, membranes were blocked in 5% skimmed milk for 1 h at room temperature. After that, membranes were first incubated with rabbit anti human primary antibodies of WISP1 (1: 1200, ab60114, Abcam) and GAPDH antibody (1: 1000, ab9485, Abcam) overnight at 4°C, and then were further incubated with anti-rabbit IgG-HRP secondary antibody (1:1000, MBS435036, MyBioSource) for 2 h at room temperature. Signal development was performed using ECL™ Start Western Blotting Detection Reagent (Sigma-Aldrich, USA). Image J software was used to normalize grey scales.
Statistical analysis
All data were analyzed by Graphpad Prism 6 software. Data were expressed as mean ± standard deviation. One-way analysis of variance followed by LSD test was used for comparisons among multiple groups. Unpaired test was used for comparisons between 2 groups. Correlation analyses were performed by Pearson correlation analysis. p < 0.05 was considered to be statistically significant.
Results
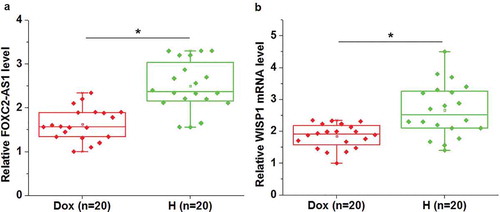
FOXC2-AS1 and WISP1 mRNA expression were downregulated in mice with Dox-induced cardiotoxicity than in healthy mice
Expression of FOXC2-AS1 and WISP1 mRNA in heart tissues of both mice with Dox-induced cardiotoxicity (Dox) and healthy mice (H) was detected by qRT-PCR. As shown in , compared with healthy mice, significantly reduced expression levels of FOXC2-AS1 were observed in heart tissues of mice with Dox-induced cardiotoxicity (p < 0.05). Similarly, WISP1 mRNA expression was significantly downregulated in mice with Dox-induced cardiotoxicity than in healthy mice (, p < 0.05).
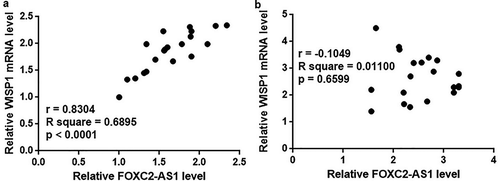
FOXC2-AS1 and WISP1 mRNA expression were positively correlated in mice with Dox-induced cardiotoxicity but not in healthy mice
Correlation between FOXC2-AS1 and WISP1 mRNA expression was analyzed by Pearson correlation coefficient. As shown in , a significantly positive correlation was fund between expression levels of FOXC2-AS1 and WISP1 mRNA in mice with Dox-induced cardiotoxicity. In contrast, no significant correlation was found between FOXC2-AS1 and WISP1 mRNA expression in healthy mice ().
Figure 2. FOXC2-AS1 and WISP1 mRNA expression were positively correlated in mice with Dox-induced cardiotoxicity but not in healthy mice.
Data here show the Pearson correlation coefficient analyses of the correlation between FOXC2-AS1 and WISP1 mRNA expression in mice with Dox-induced cardiotoxicity (a) and in healthy mice (b).
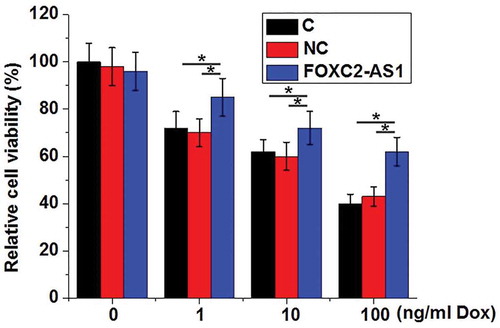
FOXC2-AS1 overexpression increased the viability of cardiomyocytes under Dox treatment
Compared with control cells without Dox treatment (0 ng/mL), Dox significantly reduced the viability of cardiomyocytes in a dose-dependent manner (p < 0.05, ). At each concentration of Dox, compared with controls cells (C) and negative control cells (NC), cells with FOXC2-AS1 overexpression showed significantly increased viability (p < 0.05). In addition, FOXC2-AS1 overexpression showed on effects on viability of cardiomyocytes without Dox treatment (0 ng/mL).
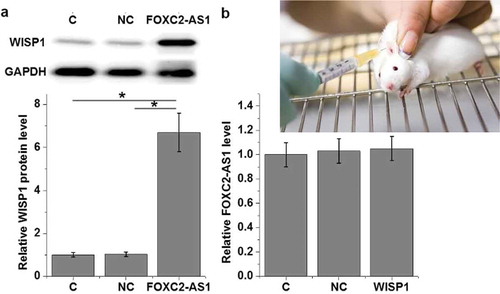
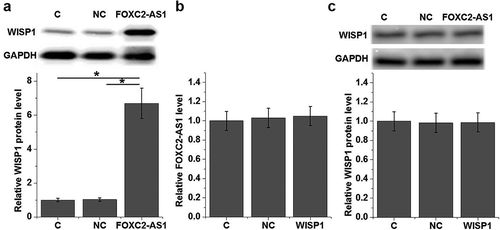
FOXC2-AS1 overexpression is likely an upstream activator of WISP1 in cardiomyocytes
To further investigate the correlation between FOXC2-AS1 and WISP1 expression under Dox treatment (10 ng/mL), cardiomyocytes with FOXC2-AS1 or WISP1 overexpression were constructed and the expression of FOXC2-AS1 and WISP1 protein was detected by qRT-PCR and western blot, respectively. Compared with controls cells (C) and negative control cells (NC), cells with overexpressed FOXC2-AS1 showed significantly increased expression level of WISP1 protein (, p < 0.05). In contrast, no significant changes in expression of FOXC2-AS1 in cardiomyocytes were observed after FOXC2-AS1 overexpression (, p > 0.05). In addition, FOXC2-AS1 overexpression failed to significantly affect WISP1 protein expression without Dox treatment (, p < 0.05).
Figure 4. FOXC2-AS1 overexpression is likely an upstream activator of WISP1 in cardiomyocytes.
Data here show the effects of FOXC2-AS1 overexpression on WISP1 protein expression (a) and the effects of WISP1 overexpression on FOXC2-AS1 expression under Dox treatment (10 ng/mL) (b), as well as the effects of FOXC2-AS1 overexpression on WISP1 protein expression with Dox treatment (c).Notes:*, p < 0.05.
Discussion
LncRNA FOXC2-AS1 recently has been proved to be upregulated in doxorubicin resistant osteosarcoma cell lines [Citation9], indicating its possible involvement in the development of doxorubicin resistance. Our study revealed a novel potential protective role of FOXC2-AS1 in Dox-induced cardiotoxicity. We also provided experimental evidence that FOXC2-AS1 can protect cardiomyocytes under Dox treatment and upregulate WISP1 protein, which is also critical for the survival of cardiomyocytes [Citation10].
Dox globally affects gene expression and interact with multiple signaling pathways to not only inhibit tumor cell growth but also affect the functions of major organs [Citation11,Citation12]. A recent study also revealed that the use of Dox also induces the altered expression of a large set of lncRNAs [Citation13], indicting the involvement of lncRNAs in the Dox-induced cytotoxicity. How to increase Dox resistance in major organs during cancer treatment is a major challenge for the clinical use of Dox. LncRNA FOXC2-AS1 is upregulated in breast cancer and the overexpression of FOXC2-AS1 is proved to be significantly correlated with disease progression and poor survival [Citation14]. In another study Zhang et al proved that lncRNA FOXC2-AS1 is upregulated in doxorubicin resistant osteosarcoma cell lines compared with doxorubicin sensitive osteosarcoma cell lines [Citation9]. In our study we observed significantly downregulated expression of FOXC2-AS1 in mice with Dox-induced cardiotoxicity, indicating the possible involvement of FOXC2-AS1 in this disorder.
WISP1 as newly identified mediator linking development and disease participates in the regulation of cytoprotection, cell proliferation, as well as extracellular matrix production [Citation15]. Wisp1 protects cardiomyocytes and activation of this signaling is considered as a promising target for treatment of cardiac diseases [Citation16,Citation17]. Our study observed significantly downregulated WISP1 mRNA expression in mice with Dox-induced cardiotoxicity than in healthy mice, further confirming the involvement of WISP1 in this disease. WISP1 achieves its bilogical roles through the interactions with multiple pathways, such as Wnt signaling [Citation18], Notch1 [Citation19] and TGF-β [Citation20]. However, interactions between lncRNAs and WISP1 still haven’t been reported. In our study we observed a significant positive correlation between the expression of WISP1 and lncRNA FOXC2-AS1. Our in vitro cardiomyocytes experiments also proved that FOXC2-AS1 is likely an upstream activator of WISP1 in cardiomyocytes due to the facts that:1) FOXC2-AS1 overexpression upregulated WISP1 expression; 2) WISP1 overexpression showed no significant effects on FOXC2-AS1 expression. However, our study only provided evidence of a FOXC2-AS1-WISP1 sequential signaling in cardiomyocytes. Whether this interaction is direct or indirect is unknown. Our speculation is that there should be Dox-related intermediators between FOXC2 and AS1-WISP1 due to the lack of significant correlation between FOXC2-AS1 and WISP1 in heart tissues of normal mice and the unaffected WISP1 expression after FOXC2-AS1 overexpression without Dox treatment.
Dox induces death of cardiomyocytes [Citation21]. In the present study we also observed reduced viability if cardiomyocytes after treatment of different doses of Dox. FOXC2-AS1 overexpression increased cell viability after Dox treatment. However, the protective effect of OXC2-AS1 overexpression on cardiomyocytes seems to be stress- (Dox) specific because no improvement of cell viability was observed after FOXC2-AS1 overexpression in cardiomyocytes without Dox expression. In addition, it has been reported that WISP1 signaling blocks doxorubicin-induced cardiomyocyte death by activating multiple survival pathways [Citation10]. Therefore, FOXC2-AS1 may directly activate survival pathways though WISP1.
Conclusion
In conclusion, FOXC2-AS1 is downregulated in heart tissues of mice after Dox treatment. FOXC2-AS1 overexpression may reduce Dox-induced cardiotoxicity by increasing cardiomyocytes viability and upregulating WISP1 expression. However, all our experiments were performed on animal model. Clinical studies are needed to further confirm our conclusions.
Authors contribution
Shenwei Zhang, Yiqiang Yuan, Zheng Zhang and Chunguang Qiu designed and carried out the study. Shenwei Zhang, Yiqiang Yuan, Zheng Zhang, Jing Guo, Jing Li, Kui Zhao and Yanping Qin participated in experiments and statistical analysis. Shenwei Zhang and Yiqiang Yuan wrote the manuscript. Zheng Zhang and Chunguang Qiu revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Consent for publication
All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Zhang M, Jiang L. Doxorubicin hydrochloride-loaded mesoporous silica nanoparticles inhibit non-small cell lung cancer metastasis by suppressing VEGF-mediated angiogenesis[J]. J Biomed Nanotechnol. 2016;12(11):1975–1986.
- Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance)[J]. J Clin Oncol. 2015;33(1):13–21.
- Tahover E, Patil YP, Gabizon AA. Emerging delivery systems to reduce doxorubicin cardiotoxicity and improve therapeutic index: focus on liposomes[J]. Anticancer Drugs. 2015;26(3):241–258.
- Ferreira ALA, Matsubara LS, Matsubara BB. Anthracycline-induced cardiotoxicity[J]. Cardiovasc Hematol Agents Med Chem. 2008;6(4):278–281.
- Salvatorelli E, Guarnieri S, Menna P, et al. Defective one- or two-electron reduction of the anticancer anthracycline epirubicin in human heart. Relative importance of vesicular sequestration and impaired efficiency of electron addition[J]. J Biol Chem. 2006;281(16):10990–11001.
- Zhang CL, Zhu KP, Shen GQ, et al. A long non-coding RNA contributes to doxorubicin resistance of osteosarcoma[J]. Tumour Biol. 2016;37(2):2737–2748.
- Jiang M, Huang O, Xie Z, et al. A novel long non-coding RNA-ARA: adriamycin resistance associated[J]. Biochem Pharmacol. 2014;87(2):254–283.
- Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function[J]. Nat Rev Genet. 2016;17(1):47–62.
- Zhang CL, Zhu KP, Ma XL. Antisense lncRNA FOXC2-AS1 promotes doxorubicin resistance in osteosarcoma by increasing the expression of FOXC2[J]. Cancer Lett. 2017;396:66–75.
- Venkatesan B, Prabhu SD, Venkatachalam K, et al. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death[J]. Cell Signal. 2010;22(5):809–820.
- Ayers M, Symmans WF, Stec J, et al. Gene expression profiles predict complete pathologic response to neoadjuvant paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide chemotherapy in breast cancer[J]. J Clin Oncol. 2004;22(12):2284–2293.
- Folgueira MAAK, Carraro DM, Brentani H, et al. Gene expression profile associated with response to doxorubicin-based therapy in breast cancer[J]. Clin Cancer Res. 2005;11(20):7434–7443.
- Zhu KP, Zhang CL, Shen GQ, et al. Long noncoding RNA expression profiles of the doxorubicin-resistant human osteosarcoma cell line MG63/DXR and its parental cell line MG63 as ascertained by microarray analysis[J]. Int J Clin Exp Pathol. 2015;8(8):8754–8773.
- Yang H, Chen T, Xu S, et al. Long non-coding RNA FOXC2-AS1 predicts poor survival in breast cancer patients and promotes cell proliferation[J]. Oncol Res. 2018. DOI:10.3727/096504018X15213126075068.
- Berschneider B, Königshoff M. WNT1 inducible signaling pathway protein 1 (WISP1): a novel mediator linking development and disease[J]. Int J Biochem Cell Biol. 2011;43(3):306–309.
- Li L, Guleria RS, Thakur S, et al. Thymosin β4 prevents angiotensin II‐induced cardiomyocyte growth by regulating Wnt/WISP signaling[J]. J Cell Physiol. 2016;231(8):1737–1744.
- Wright LH, Herr DJ, Brown SS, et al. Angiokine Wisp-1 is increased in myocardial infarction and regulates cardiac endothelial signaling[J]. JCI Insight. 2018;3(4):e95824.
- Maeda A, Ono M, Holmbeck K, et al. WNT1-induced secreted protein-1 (WISP1), a novel regulator of bone turnover and Wnt signaling[J]. J Biol Chem. 2015;290(22):14004–14018.
- Shao H, Cai L, Moller M, et al. Notch1-WISP-1 axis determines the regulatory role of mesenchymal stem cell-derived stromal fibroblasts in melanoma metastasis[J]. Oncotarget. 2016;7(48):79262–79273.
- Berschneider B, Ellwanger DC, Baarsma HA, et al. miR-92a regulates TGF-β1-induced WISP1 expression in pulmonary fibrosis[J]. Int J Biochem Cell Biol. 2014;53:432–441.
- Carvalho FS, Burgeiro A, Garcia R, et al. Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy[J]. Med Res Rev. 2014;34(1):106–135.