ABSTRACT
This study was undertaken to determine the effects of enzyme-treated Zizania latifolia (ETZL) and of its major compound tricin on skin photo-aging and to investigate the mechanisms involved. It was found ETZL and tricin suppressed matrix metalloproteinase (MMP) production and increased type I-procollagen production in UVB-irradiated human dermal fibroblasts (HDFs). Furthermore, ETZL and tricin significantly up-regulated the expressions of the antioxidant enzymes HO-1 and SOD1, reduced UVB-induced reactive oxygen species (ROS) generation and mitogen-activated protein kinase (MAPK) induction by ROS and thereby attenuated activator protein-1 (AP-1) expression. In addition, ETZL and tricin both reduced the phosphorylations of IκBα and IKKα/ß and κB blocked the nuclear translocation of nuclear factor-κB (NF-κB) p65. These results show that ETZL have skin protective effects against UVB and suggest tricin as major efficacious material in ETZL protecting skin photoaging.
Graphical Abstract
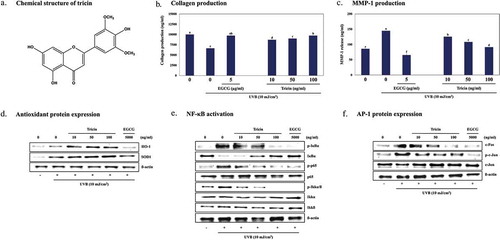
Tricin in enzyme-treated Z. latifolia inhibits MMPs production by regulating antioxidant proteins expression and NF-kB signaling in UVB-irradiated human dermal fibroblasts.
KEYWORDS:
Exposure of skin to ultraviolet B (UVB) radiation can cause wrinkling, sagging, and dryness, the main characteristics of photoaging, by inducing oxidative cell damage [Citation1]. UVB promotes the generation of intracellular reactive oxygen species (ROS), which in turn lead to the activation of oxidative stress-sensitive signaling pathways, such as, the MAPK (mitogen-activated protein kinase) and nuclear factor-kB (NF-kB) pathways, and activate upstream signaling molecules of transcription factors activator protein-1 (AP-1) [Citation2]. In so doing UVB activates the MAPK cascade, which involves ERK (extracellular signal-regulated kinase), JNK (c-Jun N-terminal kinase), and p38 kinase, and increases the production of MMPs (matrix metalloproteinases), which are regulated by NF-kB and AP-1 in the epidermis and dermis [Citation3,Citation4]. It is also known UV-induced ROS initiate the production and release of MMPs, including MMP-1, −2, −3, −9, and/or −13, which cause skin photo damage and photoaging [Citation5].
Furthermore, ROS can cause the MMP activation-dependent degradation of collagenous extracellular matrix (ECM) by breaking down collagen and elastin in skin connective tissue [Citation6]. Therefore, inhibitors of ROS-induced MMP activation can block histological changes in the ECM of photoaged skins, thereby preserve collagen and elastin. Type I collagen is synthesized from type I procollagen, a soluble precursor, which is secreted by human dermal fibroblasts (HDFs) to form insoluble collagen fibers (the main structural protein in skin connective tissue) [Citation7]. Tissue inhibitors of metalloproteinases (TIMPs), which maintain MMPs in an inactive state during physiological conditions, and various collagenase inhibitors, such as, epigallocatechin gallate (EGCG) and quercetin have been reported to protect skin against UVB-induced photodamage and wrinkling [Citation8]. When exposed to UVB, MMP-1 (collagenase-1) initiates the breakdown of fibrillary collagens, and then MMP-2 and −9 (gelatinases) further degrade the collagen fragments formed, whereas MMP-3 might induce HDFs and keratinocytes to secrete MMP-1, 2, and −9. Furthermore, MMP-1 and −9 (both gelatinases) activate MMP-13 (collagenase-3), and thus to the degradation of ECM components.
Zizania latifolia Turcz (Poaceae, known as wild rice) is a perennial aquatic plant native to East Asia found at the margins of rivers and lakes. Several recent studies have demonstrated that Z. latifolia grains have various biological effects, for example, they suppress hyperlipidemia and oxidative stress [Citation9], reduce blood glucose levels, improve insulin resistance [Citation10], and have anti-obesity [Citation11] effects. A small number of studies on the aerial part of Z. latifolia have shown it inhibits H2O2-induced apoptosis in Neuro2A cells [Citation12] and angiotensin-converting enzyme and has anti-oxidative effects [Citation13]. In a previous study, we reported the methanol extract of the aerial part of Z. latifolia and five tricin (4′,5,7-trihydroxy-3′,5′-dimethoxyflavone) derivatives identified in this extract inhibited inflammation in LPS-stimulated RAW264.7 macrophages and allergy in antigen-treated RBL-2H3 mast cells [Citation14]. In a more recent study, we found tricin and nine tricin derivatives identified in an ethanol extract of Z. latifolia suppressed melanin production [Citation15]. Furthermore, tricin was found to be the major compound in this extract and its extraction yield was observed to increase after treating the extract with pectinase. The present study was performed to investigate the protective effects of the hydrolyzed ethanolic extract of the aerial parts of Z. latifolia and of tricin on UVB-induced photo-damage in HDFs.
Materials and methods
Plant material and preparation of ETZL
The aerial parts of Z. latifolia were purchased from the Pureunsan Agricultural Association Corporation (Dongdaemun-gu, Seoul, Korea) and ETZL was provided from BTC corporation (Sangnok-gu, Ansan 15588, Korea). Briefly, the dried leaves of Z. latifolia (1 kg) was incubated with a mixed hydrolysis enzyme (cellulase, hemicellulase and pectinase) at 35°C for 16 h in H2O, and enzymes were inactivated with heat. the extracted solution was filtered and acquired. After the extraction of the residual with 70% ethanol at 80°C for 6 h, the filtered extracted solution was mixed with the first enzyme extract, and total enzyme extract was concentrated and dried to produce ETZL (17.45 g, a powder).
Reagents
MTT (3-(4,5-dimethylthiazol-2-yl)2-,5-diphenyltetrazolium bromide) and DCFH-DA (2ʹ,7ʹ-dichlorofluorescein diacetate) were purchased from Sigma Chemicals (St. Louis, MO). Antibodies against superoxide dismutase 1 (SOD 1, #2770), heme oxygenase-1 (HO-1, #70081), Akt (#9272), phospho-Akt (#4060), phospho-SAPK/JNK (Thr183/Thr185) (#4668), SAPK/JNK (Thr183/Thr185) (#9252), phospho-p44/42 MAPK (Erk1/2) (#4370), p44/42 MAPK (Erk1/2) (#9102), phospho-p38 (#4511), p38 (#9212), phospho-IκBα (Ser32/36) (#9246), IκBα (#4814), phospho-NF-κB p65 (Ser536) (#3031), NF-κB p65 (#8242), phospho-IκB kinase (IKK) α/ß (Ser176/180) (#2694), IKKα (#2682), IKKβ (#2684), phospho-c-Jun (Ser243) (#3270), c-Jun (#9165), c-Fos (#2250), ß-actin (#3700), horseradish peroxidase-linked anti-rabbit secondary antibody (#7074), and anti-mouse secondary antibody (#7076) were purchased from Cell Signaling Technology (Beverly, MA, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were from the American Type Culture Collection (Manassas, VA, USA). All other chemicals and reagents used were of analytical grade.
Cell culture
HDFs were purchased from Seoulin BioScience (Bundang-gu, Sungnam-si, Korea). Cells were cultured in DMEM supplemented with penicillin (120 units/mL), streptomycin (120 units/mL), and 10% FBS in a 5% CO2 atmosphere at 37°C. HDFs were maintained until 80% confluent and then pre-treated with ETZL (10, 25, 50, 100, or 250 μg/mL) or tricin (10, 50, or 100 ng/mL) for 1 h. Culture media were then discarded and cells were exposed to UVB (10 mJ/cm2) produced using a 312 nm light source (VL-6.LM; Vilber Lourmat, Marne-la Vallee Cedex 1, France). UVB exposure was measured using a Waldman UV meter (model 585100, Waldman, Wheeling, IL, USA). Cells were then treated with ETZL (10, 25, 50, 100, or 250 μg/mL) or tricin (10, 50, or 100 ng/mL) in serum-free medium.
Cell viability assay
HDF viabilities were determined using a MTT colorimetric assay. Briefly, 1 × 105 cells per well were seeded in a 24-well cell culture plate and then pre-treated with various concentrations of ETZL (10, 25, 50, 100, or 250 μg/mL) or tricin (10, 50, or 100 ng/mL) for 1 h. To examine the protective effects against UVB-damage in HDFs, cells were exposed to UVB (10 mJ/cm2) through a thin layer of PBS for 30 seconds and treated with ETZL (10, 25, 50, 100, or 250 μg/mL) or tricin (10, 50, or 100 ng/mL) for 24 h in serum-free medium. UVB strength was measured with a UV meter. MTT (0.5 mg/mL) was then added to cell media and incubated for 3 h. The formazan crystals produced were dissolved in 500 μL of DMSO, and absorbances were measured by spectrophotometry using a microplate reader (Bio-Tek Instruments, Winoosk, VT, USA) at 550 nm.
Enzyme-linked immunosorbent assay
HDFs were cultured in a 24-well cell culture plate (1 × 105 cells/well) and pretreated with ETZL (10, 25, 50, 100, or 250 μg/mL) or tricin (10, 50, or 100 ng/mL) for 1 h. After washing with PBS, cells were irradiated with UVB (10 mJ/cm2) through a thin layer of PBS, and then incubated with serum-free DMEM containing ETZL and tricin for 24 h. Cell culture media were collected and type I procollagen and MMP family levels were quantified using a procollagen type I C-peptide enzyme immunoassay kit (MK101; Takara, Shiga, Japan), a human MMP-1 ELISA kit (ab100603; Abcam, USA), a human MMP-2 ELISA kit (ab100603; Abcam, USA), a human MMP-3 ELISA kit (ab100603; Abcam, USA), a human MMP-9 ELISA kit (ab100603; Abcam, USA), or a human MMP-13 ELISA kit (ab100603; Abcam, USA).
Western blot
HDFs were maintained until 80% confluent and then pre-treated with ETZL (10, 25, 50, 100, or 250 μg/mL) or tricin (10, 50, or 100 ng/mL) for 1 h, and then cells were exposed to UVB (10 mJ/cm2) through a thin layer of PBS and treated with ETZL (10, 25, 50, 100, or 250 μg/mL) or tricin (10, 50, or 100 ng/mL) for 24 h in serum-free medium. Cells were harvested by trypsin treating the cells from culture dishes and then collected. HDFs were lysed in RIPA buffer (0.15 M NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 0.05 M Tris-HCl, 0.02 M EDTA, pH 7.5) containing protease inhibitor (P3100_001, GenDEPOT, USA) and phosphatase inhibitor (P3200_001, GenDEPOT, USA) and then incubated on ice for 10 min. Protein concentrations in cell lysates were determined using a BCA protein assay (Thermo Fisher Scientific, Waltham, USA). Proteins (30 μg) were loaded and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to PVDF membranes (Whatman GmbH, Dassel, Germany), blocked with 5% skimmed milk in Tris-buffered saline containing 0.1% Tween-20 for 1 h and then incubated with primary antibodies for 16 h at 4°C. After three washes in Tris-buffered saline containing 0.1% Tween-20, membranes were incubated with horseradish peroxidase-linked secondary antibodies (Cell Signaling Technology, Beverly, MA, USA) for 1 h. Proteins were detected by enhanced chemiluminescence and visualized using image software (UVP Vision Works® LS Image Acquisition & Analysis Software, Upland, CA).
Observation of ROS production
HDFs were pre-treated with ETZL (10, 25, 50, 100, or 250 μg/mL) or tricin (10, 50, or 100 ng/mL) for 1 h, washed with PBS, irradiated with UVB (10 mJ/cm2), and treated with ETZL (10, 25, 50, 100, or 250 μg/mL) or tricin (10, 50, or 100 ng/mL), and stained with 25 µM DCFH-DA for 30 min. Fluorescence signals were observed by fluorescence microscopy (DMIRE2, Leica, USA) using excitation and emission wavelengths of 485 nm and 530 nm, respectively.
Statistical analysis
All experiments were repeated at least three times, and each experiment was performed in triplicate. Results are presented as means ± standard deviations (SD). The results were subjected to analysis of variance (ANOVA) using Tukey’s multiple comparison test to analyze differences, and p < 0.05 was considered to be significant.
Results
Effects on of ETZL and tricin on cell viability, collagen synthesis, and MMP secretion
Treating HDFs with ETZL (10, 25, 50, 100, or 250 μg/mL) or tricin (10, 50, or 100 ng/mL) had no effect on HDF cell viability ()). However, when HDFs were irradiated with UVB (10 mJ/cm2) cell viability reduced by ~23%, but pretreatment with ETZL treatment at 25–250 μg/mL dose-dependently reduced this reduction ()). Tricin also protected HDFs in a dose dependent manner. In our previous study, ETZL was found to contain 1.12 ± 0.079 mg/g of tricin by LC/MS-MS [Citation16]. In the present study, the concentration of tricin used ranged from 10 to 100 ng/mL, which corresponded to 10–100 μg/mL of ETZL. EGCG was selected as a positive control because it is known to have anti-wrinkle activity [Citation17], and in the present study, at 5 μg/mL as was expected it enhanced UV-induced cell damage.
Figure 1. Effects of ETZL and tricin on collagen synthesis and the expressions of MMP family members in UVB-irradiated human dermal fibroblasts (HDFs). (a) Viability of HDFs treated with ETZL and tricin, (b) Cell viability of UVB exposed HDFs treated with ETZL and tricin, (c) Effects of ETZL and tricin on collagen synthesis, (d) MMP-1, (e) MMP-2, (f) MMP-3, (g) MMP-9, and (h) MMP-13 in UVB exposed HDFs. EGCG was used as a positive control. Results are expressed as means ± SD (% control) of three independent experiments. Values with different superscript letters are significantly different (p < 0.05) by Tukey’s multiple comparison test (n = 3).
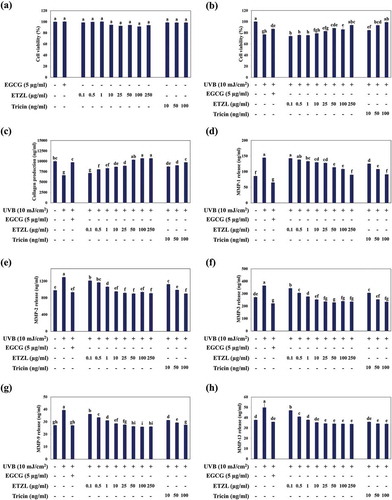
We next examined the effects of ETZL and tricin on collagen synthesis and on the secretions of MMPs by measuring type I-procollagen levels and MMP secretions into UVB-irradiated HDF media. Both ETZL and tricin significantly increased type I procollagen levels in media (by 1.56-fold at an ETZL concentration of 50 μg/ml and by 1.36-fold at a tricin concentration of 50 ng/mL) ()). The secretions of MMP-1, −2, −3, −9, and −13 were increased by 168.6%, 132.0%, 134.4%, 145.2%, and 130.2%, respectively, by UVB. These increases were down-regulated dose dependently by ETZL (by 132.7%, 92.2%, 84.7%, 97.1%, and 90.2% for MMP-1, −2, −3, −9, −13 at 50 μg/mL, respectively) and by tricin (by 126.0%, 101.0%, 93.5%, 107.9%, and 90.5% for MMP-1, −2, −3, −9, −13 at 50 ng/mL, respectively) compared with each MMP values in not-UVB-irradiated groups ()). Kim et al. [Citation18] also reported that the secretion of MMP-1 in UVB-irradiated HDF cells was increased in 1.5-fold than the control cells.
The results demonstrated that UVB up-regulated collagen-degrading MMPs and that ETZL and tricin efficiently inhibited these up-regulations. The positive control EGCG also strongly inhibited MMP up-regulations and significantly enhanced collagen synthesis in UVB-irradiated HDFs.
Effects of ETZL and tricin on ROS generation and antioxidant protein expressions
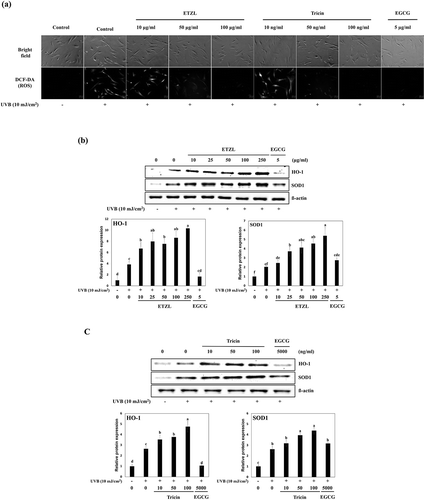
To investigate the effects of ETZL and tricin on intracellular ROS generation induced by UVB exposure in HDFs, changes in ROS levels were assessed by fluorescence staining. As shown in ), ETZL and tricin both dose-dependently inhibited UVB-induced intracellular ROS generation, which suggested tricin might be the major active component in ETZL. We also examined their effects on the expressions of the antioxidant proteins HO-1 and SOD1, which are known to protect cells against oxidative damage. Relative protein levels were normalized versus actin. The levels of HO-1 and SOD1 in UVB-treated HDFs were higher than in the normal control cells, and these levels were further increased by ETZL (by 1.9- and 2.0-fold, respectively, at 50 μg/mL) and by tricin (by 1.4- and 1.5-fold, respectively, at 50 ng/mL) as compared with of UVB-irradiated controls ()).EGCG, the reference substance, decreased HO-1 expression and increased SOD1 expression compared to the UVB control in our experimental condition. HO-1 protein level was also expected to increase by EGCG treatment, but HO-1 expression enhanced by UVB irradiation was reduced by EGCG.
Figure 2. Effects of ETZL and tricin on antioxidant enzyme expressions in UVB-induced HDFs. (a) HO-1 and SOD1 expressions in ETZL treated UVB-irradiated HDFs, (b) HO-1 and SOD1 expressions in tricin treated UVB-irradiated HDFs. β-Actin was used as the internal control for Western blotting, and EGCG was used as the positive control. Results are presented as the means ± SD of percentages calculated with respect to control levels of three independent experiments. Values with different superscript letters are significantly different (p < 0.05) by Tukey’s multiple comparison test (n = 3).
Effects of ETZL and tricin on the NF-κB signaling pathway
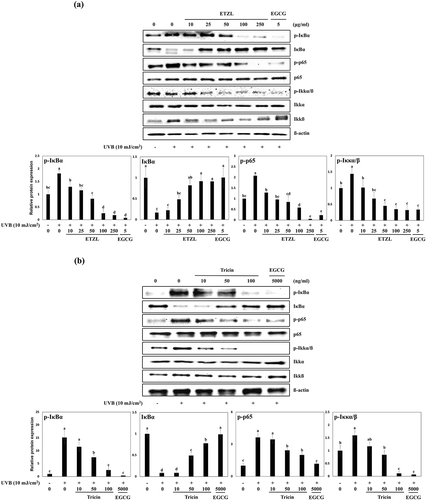
NF-κB has been implicated in the control of MMP up-regulations in UVB-irradiated HDFs [Citation19]. When HDFs are exposed to UVB, IκB, IKKα/β and p65, which are normally complexed with NF-κB p65 and p50 in cytosol, are phosphorylated and as a result NF-κB is translocated from cytosol to nuclei, where it regulates the transcriptional activation of MMPs [Citation20]. Thus, blocking the phosphorylations of IκB, IKKα/β and NF-κB p65 inhibits the productions of MMPs and their secretions. To examine the inhibitory effects of ETZL or tricin on NF-κB signaling in UVB-exposed HDFs, we examined their effects on the phosphorylations of IκBα, p65 subunit and IKKα/β and the degradation of IκB by Western blot. We found the UVB-induced phosphorylations of IκBα, p65, and IKKα/β were inhibited by ETZL (by 45.4%, 40.8%, and 31.3% at 50 μg/mL, respectively) and by tricin (by 49.4%, 66.9%, and 52.5% at 50 ng/mL, respectively) compared to UVB-irradiated control. In addition, UVB degraded IκB level to 16.8% of the control level, and this inhibited by 50 µg/mL ETZL to 81.8% of the control level and by 50 ng/ml tricin to 49.3% of the normal control ()).
Figure 3. Effects of ETZL and tricin on the NF-κB signaling pathway in UVB-irradiated HDFs. (a) Effect of ETZL on the expressions of IκBα, p65, and IKKα/β in UVB-irradiated HDFs, (b) Effect of tricin on the expressions of IκBα, p65, and IKKα/β in UVB-irradiated HDFs. β-Actin was used as the internal control for Western blotting and EGCG was used as the positive control. Results are presented as the means ± SD of percentages calculated with respect to control levels of three independent experiments. Values with different superscript letters are significantly different (p < 0.05) by Tukey’s multiple comparison test (n = 3).
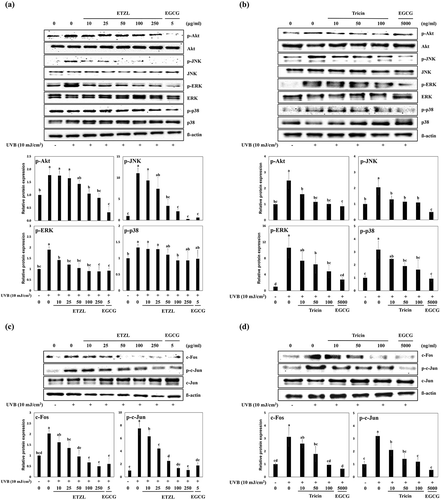
Effects of ETZL and tricin on the MAPK and AP-1 signaling pathways
ROS is known to induce the activations of NF-κB and AP-1, and these activations increase the expressions of MMPs [Citation21]. AP-1 is composed of c-Jun and c-Fos family members, and increased transcription of AP-1 is known to suppress the synthesis of collagens I and III (major dermal collagens). In addition, Akt, JNK, ERK, and p38 MAPK signaling pathways are also stimulated by UVB, and these activations result in the activations of c-Jun and c-Fos or NF-κB [Citation5,Citation21].
ETZL or tricin treatments lowered the phosphorylations of Akt (by 80.7% at 50 μg/mL and 43.9% at 50 ng/mL), JNK (by 43.6% at 50 μg/mL and 55.8% at 50 ng/mL), ERK (by 55.2% at 50 μg/mL and 60.5% at 50 ng/mL), and of p38 (83.3% at 50 µg/mL and 60.1% at 50 ng/mL) versus UVB-irradiated HDFs, which suggested the inhibitory effects of ETZL or tricin on the UVB-induced expressions of MMPs might be mediated by the suppression of MAPK signaling ()). Treatment with ETZL or tricin also inhibited the expression of c-Fos protein (by 47.2% at 50 μg/mL and 57.5% at 50 ng/mL) and the phosphorylation of c-Jun (by 33.1% at 50 μg/mL and 36.3% at 50 ng/mL) as compared with UVB-irradiated control HDFs, which showed both ETZL and tricin inhibited UVB-induced AP-1 transcription ()).
Figure 4. Effects of ETZL and tricin on the MAPK and AP-1 signaling pathways in UVB-irradiated HDFs. (a) Effect of ETZL on MAPK/AP-1 in UVB-irradiated HDFs, (b) Effect of tricin on MAPK/AP-1 signaling in UVB-irradiated HDFs. β-Actin was used as the internal control for Western blotting and EGCG was used as the positive control. Results are presented as means ± SD of percentages calculated with respect to control levels of three independent experiments. Values with different superscript letters are significantly different (p < 0.05) by Tukey’s multiple comparison test (n = 3).
Discussion
Tricin (4′,5,7-trihydroxy-3′,5′-dimethoxyflavone) is found in various plants and has been reported to inhibit the growths of colorectal carcinoma [Citation22] and breast cancer cells [Citation23] and to inhibit influenza virus activity [Citation24]. Furthermore, several studies have reported tricin isolated from Njavara rice has anti-inflammatory and antioxidative effects [Citation25,Citation26]. Tricin and its derivatives were previously identified as the major components in Z. latifolia, which inhibits allergy and inflammation [Citation14], however, the effects of tricin on skin have not been previously explored. Z. latifolia (Gramineae) is a perennial aquatic plant found in lakes, ponds, and wetlands, and it is cultivated in Russia, Korea, Japan, and China [Citation27]. Its culms and rhizomes have long been used to treat heart, kidney, and liver disorders in China [Citation28]. Nevertheless, few studies have investigated the biological efficacy of the aerial part of this plant. In a previous study, we reported Z. latifolia extract suppresses ß-hexosaminidase release, a marker enzyme of mast cell degranulation, by RBL-2H3 cells [Citation29], suggesting it possesses immunoregulatory properties. Recently, Z. latifolia and tricin (a major component in its ethanoic extracts) were shown to inhibit melanin production in α-melanocyte stimulated hormone-treated B16-F0 melanoma cells. Furthermore, because it has been reported the tricin content in EtOH extracts is markedly enhanced when extracts are treated with the enzyme mixture, we used the hydrolyzed extract of Z. latifolia in the present study, which was conducted to explore the ability of tricin to block UVB-induced changes in HDFs.
Long-term repeated exposure to UV radiation can lead to skin aging and skin cancer. UVB is the most harmful component of UV radiation and causes damage at the molecular level and photosensitive reactions, which are largely due to excessive ROS production. Resulting ROS can then induce the secretions of MMPs, including MMP-1, −2, −3, −9, and −13, in skin fibroblasts and keratinocytes [Citation5], and metalloproteinases degrade collagen and other ECM proteins, impair collagen synthesis, and cause wrinkle formation and skin photoaging [Citation6]. Many studies have shown various phenolics, such as, catechin [Citation30], resveratrol [Citation31], apigenin [Citation32], and baicalin [Citation33], protect against photoaging by suppressing ROS generation, and thus, inhibiting MMP production. In addition, antioxidant-rich natural extracts have been shown to block UV-stimulated MMP production and secretion in skin [Citation34,Citation35].
In the present study, tricin and ETZL were found to markedly downregulate the UVB-induced secretions of MMPs and to restore collagen synthesis. Furthermore, tricin enhanced the UVB-induced expressions of HO-1 and SOD1, and thus, protected HDFs from ROS-induced damage. These results suggest the beneficial effects of tricin and ETZL on skin cells are due, at least in part, to the inhibition of UVB-induced ROS generation and the inductions of antioxidant proteins.
Activation of the NF-κB pathway by UVB-induced ROS upregulates MMP production and the expressions of MMPs in dermal fibroblasts [Citation2], and the suppression of NF-κB activation is known to prevent UVB-mediated photoaging [Citation3,Citation4]. In fact, brazilin targets NF-κB to inhibit the expressions of MMPs in UV-irradiated HDFs [Citation36]. In the present study, tricin and ETZL both reduced the phosphorylations of IκB and NFκB p65 in UVB irradiated HDFs, and thus, prevented the nuclear translocation of phosphorylated IκB and NFκB p65.
Oxidative stress-induced ROS production can also stimulate the MAPK signaling pathway via the phosphorylations of REK, JNK, and p38 MAPK and by inducing AP-1 activity [Citation37,Citation38]. Furthermore, UVB-induced MMP-1 expression is inhibited by inhibitors of MAPK proteins [Citation39,Citation40], indicating that MAPKs act upstream of MMPs in skin cells. In addition, blocking of ROS production inhibits MAPK signaling, which reduces the activations of NFκB and AP-1 and inhibits the up-regulations of MMPs [Citation41].
These findings indicate ETZL and its major component tricin block UVB-induced MAPK inductions, and thereby, inhibit the activities of NFκB and AP-1 activity and the productions of MMPs in HDFs. Although the effects of ETZL and tricin on other biomarkers of photoaging were not determined, it would appear ROS scavenging by antioxidant proteins importantly determines the anti-photoaging efficacies of candidate materials. Further studies are required to explore more fully the anti-photoaging effect of ETZL in vivo and to identify the mechanisms responsible for these effects.
Author Contributions
S-H Park, M-H Bang, K-H Jhee, S-A Yang designed the study. S-H Park, S-S Lee, SK Jo performed the experiments and data analysis, S-H Park wrote manuscript, S-A Yang supervised the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Fisher GJ, Kang S, Varani J, et al. Mechanism of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470.
- Yaar M, Gilchrest BA. Photoaging: mechanism, prevention and therapy. Br J Dermatol. 2007;157:874–887.
- Rittié L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002;1:705–720.
- Muthusamy V, Piva TJ. The UV response of the skin: a review of the MAPK, NFκB and TNFα signal transduction pathways. Arch Dermatol Res. 2010;302:5–17.
- Fisher GJ, Datta SC, Talwar HS, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339.
- Pillai S, Oresajo C, Hayward J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation – a review. Int J Cosmet Sci. 2005;27:17–34.
- Gelse K, Pöschi E, Aigner T. Collagen – structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546.
- Nagle DG, Ferreira D, Zhou YD. Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry. 2006;67:1849–1855.
- Zhang H, Cao P, Agellon LB, et al. Wild rice (Zizania latifolia (Griseb) Turcz) improves the serum lipid profile and antioxidant status of rats fed with a high fat/cholesterol diet. Br J Nutr. 2009;102:1723–1727.
- Han S, Zhang H, Qin L, et al. Effects of dietary carbohydrate replaced with wild rice (Zizania latifolia (Griseb) Turcz) on insulin resistance in rats fed with a high-fat/cholesterol diet. Nutrients. 2013;5:552–564.
- Han SF, Zhang H, Zhai CK. Protective potentials of wild rice (Zizania latifolia (Griseb) Turcz) against obesity and lipotoxicity induced by a high-fat/cholesterol diet in rats. Food Chem Toxicol. 2012;50:2263–2269.
- Park WH, Cha YY. Inhibition effect of Zizania latifolia on apoptosis induced by H2O2 in Neuro2A cell. J Physiol Pathol Korean Med. 2005;19:1062–1067.
- Qian B, Luo Y, Deng Y, et al. Chemical composition, angiotensin-converting enzyme-inhibitory activity and antioxidant activities of few-flower wild rice (Zizania latifolia Turcz.). J Sci Food Agric. 2012;92:159–164.
- Lee SS, Baek YS, Eun CS, et al. Tricin derivatives as anti-inflammatory and anti-allergic constituents from the aerial part of Zizania latifolia. Biosci Biotechnol Biochem. 2015;79:700–706.
- Lee SS, Baek NI, Baek YS, et al. New flavonolignan glycosides from the aerial parts of Zizania latifolia. Molecules. 2015;20:5616–5624.
- Moon JM, Park SH, Jhee KH, et al. Protection against UVB-induced wrinkle formation in SKH-1 hairless mice: efficacy of tricin isolated from enzyme-treated Zizania latifolia extract. Molecules. 2018;23:2254.
- Thring TS, Hili P, Naughton DP. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement Altern Med. 2009;9:27.
- Kim MS, Park YG, Lee HJ, et al. Youngiasides A and C isolated from Youngia denticulatum inhibit UVB-induced MMP expression and promote type I procollagen production via repression of MAPK/AP-1/NF-κB and activation of AMPK/Nrf2 in HaCaT cells and human dermal fibroblast. J Agric Food Chem. 2015;63:5428–5438.
- Berneburg M, Plettenberg H, Krutmann J. Photoaging of human skin. Photodermatol Photo. 2000;16:239–244.
- Jung YR, Kim DH, Kim SR, et al. Anti-wrinkle effect of magnesium lithospermate B from Salvia miltiorrhiza BUNGE: inhibition of MMPs via NF-κB signaling. Plos One. 2014;9:e102689.
- Brenneisen P, Sies H, Scharffertter-Kochanek K. Ultraviolet-B irradiation and matrix metalloproteinases: from induction via signaling to initial events. Ann N Y Acad Sci. 2002;973:31–43.
- Malvicini M, Gutierrez-Moraga A, Rodriguez MM, et al. A tricin derivative from deschampsia antarctica Desv. inhibits colorectal carcinoma growth and liver metastasis through the induction of a specific immune response. Mol Cancer Ther. 2018;molcanther–0193. DOI:10.1158/1535-7163.MCT-17-0193
- Cai H, Hudson EA, Mann P, et al. Growth-inhibitory and cell cycle-arresting properties of the rice bran constituent tricin in human-derived breast cancer cells in vitro and in nude mice in vivo. Br J Cancer. 2004;91:1364.
- Yazawa K, Kurokawa M, Obuchi M, et al. Anti-influenza virus activity of tricin, 4ʹ,5,7-trihydroxy-3ʹ,5ʹ-dimethoxyflavone. Antivir Chem Chemother. 2011;22:1–11.
- Shalini V, Bhaskar S, Kumar KS, et al. Molecular mechanisms of anti-inflammatory action of the flavonoid, tricin from Njavara rice (Oryza sativa L.) in human peripheral blood mononuclear cells: possible role in the inflammatory signaling. Int Immunopharmacol. 2012;14:32–38.
- Ajitha MJ, Mohanlal S, Suresh CH, et al. DPPH radical scavenging activity of tricin and its conjugates isolated from “Njavara” rice bran: a density functional theory study. J Agric Food Chem. 2012;60:3693–3699.
- Guo HB, Li SM, Peng J, et al. Zizania latifolia Turcz. cultivated in China. Genet Resour Crop Evol. 2007;54:1211–1217.
- Zhai CK, Tang WL, Jang XL, et al. Studies of the safety of Chinese wild rice. Food Chem Toxicol. 1996;34:347–352.
- Lee EJ, Whang EY, Whang K, et al. Anti-allergic effect of Zizania latifolia Turcz extracts. Korean J Food Sci Technol. 2009;41:717–721. Available from: http://www.koreascience.or.kr/article/ArticleFullRecord.jsp?cn=SPGHB5_2009_v41n6_717
- Shin HJ, Kim SN, Kim JK, et al. Effect of green tea catechins on the expression and activity of MMPs and type I procollagen synthesis in human dermal fibroblasts. J Soc Cosmet Sci Korea. 2006;32:117–121. Available from: http://www.koreascience.or.kr/article/ArticleFullRecord.jsp?cn=HJPHBN_2006_v32n2s57_117
- Baxter RA. Anti-aging properties of resveratrol: review and report of a potent new antioxidant skin care formulation. J Cosmet Dermatol. 2008;7:2–7.
- Hwang YP, Oh KN, Yun HJ, et al. The flavonoids apigenin and luteolin suppress ultraviolet A-induced matrix metalloproteinase-1 expression via MAPKs and AP-1-dependent signaling in HaCaT cells. J Dermatol Sci. 2011;61:23–31.
- Zhang JA, Yin Z, Ma LW, et al. The protective effect of baicalin against UVB irradiation induced photoaging: an in vitro and in vivo study. PLoS One. 2014;9:e99703.
- Bravo K, Duque L, Ferreres F, et al. Passiflora tarminiana fruits reduced UVB-induced photoaging in human skin fibroblasts. J Photochem Photobiol. 2017;168:78–88.
- Kim SR, Jung YR, An HJ, et al. Anti-wrinkle and anti-inflammatory effects of active garlic components and the inhibition of MMPs via NF-κB signaling. PLoS One. 2013;8:e73877.
- Legrand-Poels S, Schoonbroodt S, Matroule JY, et al. NF-κB: an important transcription factor in photobiology. J Photochem Photobiol. 1998;45:1–8.
- Schroeder P, Haendeler J, Krutmann J. The role of near infrared radiation in photoaging of the skin. Exp Gerontol. 2008;43:629–632.
- Hwang BM, Noh EM, Kim JS, et al. Curcumin inhibits UVB-induced matrix metalloproteinase-1/3 expression by suppressing the MAPK-p38/JNK pathways in human dermal fibroblasts. Exp Dermatol. 2013;22:371–374.
- Brennan M, Bhatti H, Nerusu KC, et al. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem Photobiol. 2003;78:43–48.
- Kim MS, Lee SR, Rho HS, et al. The effects of a novel synthetic retinoid, seletinoid G, on the expression of extracellular matrix proteins in aged human skin in vivo. Clin Chim Acta. 2005;362:161–169.
- Jenkins G. Molecular mechanisms of skin aging. Mech Ageing Dev. 2002;123:801–810.
